Abstract
In testing paired serum samples from 40 consecutive cases of African tick bite fever, we detected diagnostic antibodies against spotted fever group rickettsiae in 45% of the patients by immunofluorescence assay (IFA) and in 100% of the patients by Western blotting (WB) (P < 0.01). A specific diagnosis of Rickettsia africae infection could be established in 15% of the patients by IFA and in 73% of the patients by a combination of WB and cross-adsorption assays (P < 0.01).
African tick bite fever (ATBF) is a flu-like illness frequently accompanied by inoculation eschars, headache, and neck myalgia (7). The disease is caused by Rickettsia africae, a recently identified spotted fever group (SFG) rickettsia, and is transmitted by cattle ticks in large parts of rural sub-Saharan Africa (9). ATBF typically occurs in clusters and has recently emerged as a common cause of acute febrile illness in international visitors to the region (2, 4, 8, 13, 14). Serology is the most widely used microbiological method for diagnosing rickettsial infections. Several tests are available, but with the exception of immunofluorescence assay (IFA), Western blotting (WB), and cross-adsorption assay, most are not recommended (10, 11). IFA, which can detect immunoglobulin G (IgG) and IgM antibodies, has a sensitivity rate ranging from 84 to 100% (1, 6, 13, 15). Poor species specificity is a major drawback, but this may be circumvented by using a multiple-antigen IFA where reactions to several species can be compared directly (4, 13). WB detects both early occurring antibodies against nonspecific lipopolysaccharide (LPS) antigens and late occurring antibodies against specific protein antigens (SPAs) located in the rickettsial outer membrane (15). In cases where WB is unable to identify the causative species, a cross-adsorption assay followed by a second WB on the resulting supernatant may be conclusive (11).
(Part of this paper was presented at the 14th European Congress of Clinical Microbiology and Infectious Diseases, Prague, Czech Republic, 1 to 4 May 2004.)
Eighty serum samples collected between days 1 and 45 after the onset of symptoms from 40 consecutive patients with ATBF were obtained from a prospective cohort study of tropical fevers in Norwegian safari travelers to rural sub-Saharan Africa in 1999 to 2001 (8). All cases met the epidemiological and clinical criteria for ATBF, including myalgia and fever commencing no later than 10 days after a safari, and 26 (65%) cases occurred in clusters. Twenty-nine (73%) patients were males, and the mean age was 38.3 years (range, 14 to 57 years). Nineteen (48%) patients were treated with doxycycline, one (3%) had a complicated course (reactive arthritis), and one (3%) was hospitalized.
A multiple-antigen IFA was performed as previously reported (4), using seven SFG rickettsial antigens: R. africae strain ESF-5, R. conorii strain seven (Malish) ATCC VR-613T, Rickettsia sibirica mongolotimonae strain HA-91T, Rickettsia aeschlimannii strain MC16T, Rickettsia massiliae strain Mtu1T, Rickettsia akari strain MK ATCC VR-148T, and Rickettsia felis strain URRWXCal2 ATTC VR-1525. IgG titers of ≥1:64 and/or IgM titers of ≥1:32 against any of the tested species were considered diagnostic for SFG rickettsial infection. We considered IgG and IgM titers against R. africae that were at least 2 dilutions higher than to any of the other species as serological evidence of R. africae infection (9). Conversely, samples with smaller differences in titer dilutions were classified as undetermined SFG rickettsial infection.
WB procedures were performed as described elsewhere (12), using 20 μl of a 1-mg/ml suspension of R. africae, R. conorii, or R. aeschlimannii antigen per lane. We considered the detection of reactions against the broad washboard-like SFG rickettsia LPS in the <60-kDa regions or reactions to SFG rickettsia SPAs in the 110- to 140-kDa region, or both, as serological evidence of SFG rickettsial infection. The presence of antibodies against SPAs only in the 112- to 115-kDa range were considered diagnostic of R. africae infection, whereas demonstration of antibodies against SPAs of more than one species or only against LPS were classified as undetermined SFG rickettsial infection.
The cross-adsorption assay using R. africae and R. conorii antigens and followed by WB on the resulting supernatant was performed as previously described (5). We considered assays where adsorption with R. africae antigen removed both homologous and heterologous antibodies and adsorption with R. conorii antigen removed homologous antibodies only as serological evidence of R. africae infection. If these criteria were not met, the result was classified as undetermined SFG rickettsial infection.
All 80 serum samples were examined with the seven-antigen IFA and the three-antigen WB assay. The cross-adsorption assay was performed on convalescent-phase sera from all cases classified as undetermined SFG rickettsial infection by IFA and WB and with IgG titers of >1:64 by IFA. All data were analyzed using a database software program (SPSS version 11.0; SPSS, Chicago, Ill.). Comparison of variables used the χ2 test or Fisher's exact test where appropriate. Observed differences were considered significant when P < 0.05 for two-tailed tests.
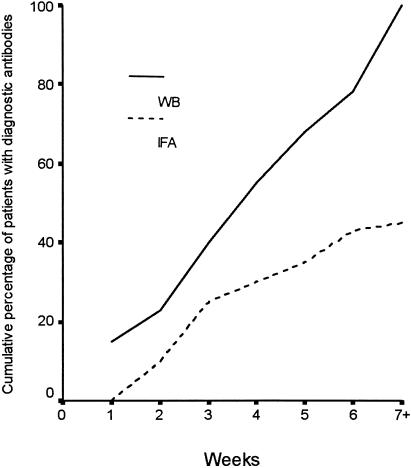
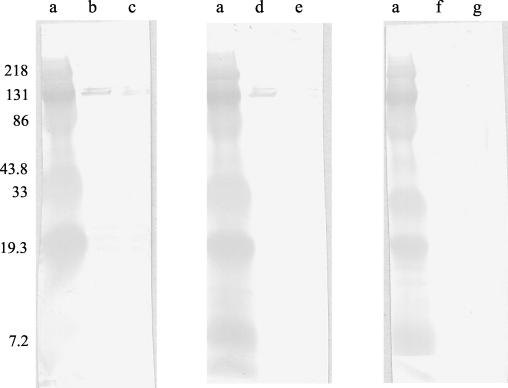
Evidence of SFG rickettsial infection was detected in 18 (45%) patients by IFA and in 40 (100%) patients by WB (P < 0.01) (Fig. 1). All patients had WB reactions directed against SFG rickettsia SPAs, whereas 24 (60%) had antibodies against SFG rickettsia LPS. A specific diagnosis of R. africae infection was established by IFA in 6 (15%) patients and by WB in 22 (55%) patients (P < 0.01). Convalescent-phase samples obtained from 13 patients with undetermined SFG rickettsial infection were tested by the cross-adsorption assay, which confirmed R. africae infection in another seven cases (Fig. 2), resulting in identification of species-specific antibodies by WB in a total of 29 (73%) patients.
FIG. 1.
Cumulative percentages of ATBF patients with diagnostic antibodies against SFG rickettsiae as detected by WB and IFA.
FIG. 2.
Western immunoblotting of serum samples from a patient with ATBF before and after cross-adsorption assay with R. conorii or R. africae. Lanes: a, molecular mass markers; b, d, and f, R. africae antigen; c, e, and g, R. conorii antigen. Serum samples were either untreated (lanes b and c) or absorbed with R. conorii (lanes d and e) or R. africae (lanes f and g). The positions of molecular mass markers (in kilodaltons) are shown to the left of the gel.
The present report constitutes one of the few serological evaluations of consecutive patients with rickettsioses. In contrast to most previous studies, which are retrospective and based on clinical samples submitted to reference laboratories (1, 6, 13, 15), our series of patients contains many cases with uncomplicated and mild presentation (e.g., not necessitating antibiotic therapy or hospitalization). With a sensitivity rate of only 45%, the present data indicate that IFA, the considered reference method in rickettsial infections (10) and a commercially available test used by laboratories worldwide, may miss many cases of ATBF. This limitation, which was also reported from an outbreak among U.S. soldiers deployed to Botswana (14), adds to other recently identified problems associated with IFA, including a poor antibody response in ATBF patients treated with doxycycline (3). In contrast, WB appears to be highly sensitive in evaluating consecutive cases of ATBF and detected antibodies against SFG rickettsia SPAs in all our patients. Thus, if serological diagnosis is regarded important in IFA-negative cases of assumed ATBF, serum samples could be shipped to a reference laboratory for WB. For unknown reasons and in contrast to other reports (11, 15), antibodies against SFG rickettsia LPS were detected in less than two-thirds of our patients. Although the antibodies were directed against nonspecific antigens shared by most rickettsial species, the demonstration of these antibodies is of diagnostic importance, since they are frequently the first serological evidence of a rickettsiosis (15).
A specific serological diagnosis of ATBF vis-à-vis other SFG rickettsioses is sometimes of clinical and epidemiological importance. Whereas the present seven-antigen IFA detected species-specific reactions in only a small proportion of our patients, WB established a diagnosis of R. africae infection in 55% of the cases, with a further increase to 73% if the cross-adsorption assay, a sophisticated serological tool designed to neutralize cross-reacting antibodies, was added (5). The major limitations with the cross-adsorption assay are the large amounts of antigen needed, high costs, and the fact that conclusive reactions are usually only seen in samples with pronounced immune response (e.g., with IgG titers of >1:64) (11).
Acknowledgments
This study was funded by The Norwegian Research Council (grant 136239/320), Research Forum, Aker University Hospital, and Centre for Tropical and Imported Diseases, Ullevål University Hospital, Oslo, Norway.
REFERENCES
- 1.Beati, L., N. Teysseire, and D. Raoult. 1992. Kinetics of specific antibodies in Mediterranean spotted fever determined by Western blotting and microimmunofluorescence. Acta Virol. 36:62-66. [PubMed] [Google Scholar]
- 2.Caruso, G., C. Zasio, F. Guzzo, C. Granata, V. Mondardini, E. Guerra, E. Macri, and P. Benedetti. 2002. Outbreak of African tick-bite fever in six Italian tourists returning from South Africa. Eur. J. Clin. Microbiol. Infect. Dis. 21:133-136. [DOI] [PubMed] [Google Scholar]
- 3.Fournier, P. E., M. Jensenius, H. Laferl, S. Vene, and D. Raoult. 2002. Kinetics of antibody responses in Rickettsia africae and Rickettsia conorii infections. Clin. Diagn. Lab. Immunol. 9:324-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fournier, P. E., V. Roux, E. Caumes, M. Donzel, and D. Raoult. 1998. Outbreak of Rickettsia africae infections in participants of an adventure race in South Africa. Clin. Infect. Dis. 27:316-323. [DOI] [PubMed] [Google Scholar]
- 5.Hechemy, K. E., R. L. Anacker, N. L. Carlo, J. A. Fox, and H. A. Gaafar. 1983. Absorption of Rickettsia rickettsii antibodies by Rickettsia rickettsii antigens in four diagnostic tests. J. Clin. Microbiol. 17:445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helmick, C. G., K. W. Bernard, and L. J. D'Angelo. 1984. Rocky Mountain spotted fever: clinical, laboratory, and epidemiological features of 262 cases. J. Infect. Dis. 150:480-488. [DOI] [PubMed] [Google Scholar]
- 7.Jensenius, M., P. E. Fournier, P. Kelly, B. Myrvang, and D. Raoult. 2003. African tick bite fever. Lancet Infect. Dis. 3:557-564. [DOI] [PubMed] [Google Scholar]
- 8.Jensenius, M., P. E. Fournier, S. Vene, T. Hoel, G. Hasle, A. Z. Henriksen, K. B. Hellum, D. Raoult, and B. Myrvang. 2003. African tick bite fever in travelers to rural sub-Equatorial Africa. Clin. Infect. Dis. 36:1411-1417. [DOI] [PubMed] [Google Scholar]
- 9.Kelly, P. J., L. Beati, P. R. Mason, L. A. Matthewman, V. Roux, and D. Raoult. 1996. Rickettsia africae sp. nov., the etiological agent of African tick bite fever. Int. J. Syst. Bacteriol. 46:611-614. [DOI] [PubMed] [Google Scholar]
- 10.La Scola, B., and D. Raoult. 1997. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J. Clin. Microbiol. 35:2715-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Scola, B., L. Rydkina, J. B. Ndihokubwayo, S. Vene, and D. Raoult. 2000. Serological differentiation of murine typhus and epidemic typhus using cross-adsorption and Western blotting. Clin. Diagn. Lab. Immunol. 7:612-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raoult, D., and G. A. Dasch. 1989. Line blot and Western blot immunoassays for diagnosis of Mediterranean spotted fever. J. Clin. Microbiol. 27:2073-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raoult, D., P. E. Fournier, F. Fenollar, M. Jensenius, T. Prioe, J. J. de Pina, G. Caruso, N. Jones, H. Laferl, J. E. Rosenblatt, and T. J. Marrie. 2001. Rickettsia africae, a tick-borne pathogen in travelers to sub-Saharan Africa. N. Engl. J. Med. 344:1504-1510. [DOI] [PubMed] [Google Scholar]
- 14.Smoak, B. L., J. B. McClain, J. F. Brundage, L. Broadhurst, D. J. Kelly, G. A. Dasch, and R. N. Miller. 1996. An outbreak of spotted fever rickettsiosis in U.S. Army troops deployed to Botswana. Emerg. Infect. Dis. 2:217-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teysseire, N., and D. Raoult. 1992. Comparison of Western immunoblotting and microimmunofluorescence for diagnosis of Mediterranean spotted fever. J. Clin. Microbiol. 30:455-460. [DOI] [PMC free article] [PubMed] [Google Scholar]