Abstract
Background
Few population-based cohort studies have examined reported food hypersensitivity longitudinally. We investigated prevalence, incidence and remission of perceived food hypersensitivity among schoolchildren from 8 to 12 years of age, and risk factors associated with incidence and remission.
Methods
A population-based cohort including all 7–8 year-old children in three Swedish towns was recruited in 2006. A total of 2,585 (96% of invited) children participated in a parental questionnaire. The children in two of the towns, n = 1,700 (90% of invited) also participated in skin-prick-testing with airborne allergens. The cohort was followed using the same methods at 11–12 years of age. At study follow up, specific IgE to foods was analyzed in a randomized subset of children (n = 652).
Results
The prevalence of perceived food hypersensitivity increased from 21% at 8 years to 26% at 12 years of age. During this four-year-period, the cumulative incidence of food hypersensitivity was high (15%), as was remission (33%). This pattern was particularly evident for hypersensitivity to cow´s milk, while the incidence of hypersensitivity to other foods was lower. Female sex, allergic heredity, current rhinitis and allergic sensitization were associated with the incidence of food hypersensitivity and allergic sensitization was negatively associated with remission. Risk-factor-patterns for both incidence and remission were different for hypersensitivity to milk compared with hypersensitivity to other foods. Generally, the agreement between reported food hypersensitivity and IgE-sensitization to the implicated food was poor.
Conclusions
In this longitudinal, population-based cohort-study perceived food hypersensitivity was common among children between ages 8 and 12, often transient and not well correlated with food-specific IgE. While these findings suggest an overestimated prevalence of food hypersensitivity, the public-health-significance remains high as they reflect the perceived reality to which the children adapt their life and food intakes.
Electronic supplementary material
The online version of this article (doi:10.1186/2045-7022-4-32) contains supplementary material, which is available to authorized users.
Keywords: Food hypersensitivity, Incidence, Remission, Risk factors, Sensitization
Introduction
Food hypersensitivity (FHS) has emerged as a costly health problem in Western countries [1]. FHS is an umbrella term that includes reactions of both immunological (allergies) and non-immunological origin (intolerances) [2]. In a Swedish cohort-study, reported FHS affected 26% of children during the first 8 years of life, but the prevalence was halved when specific IgE and doctor´s diagnosis of food-allergy were required for classification [3]. The prevalence of both FHS and food allergy varies between studies due to differences in methodology, definition, age and ethnicity of the study populations. In two recent meta analyses investigating food allergy in Europe, the pooled life-time and point prevalence of reported food allergy were 17.9% and 5.9% respectively [4] and the lifetime challenge-proven prevalence of allergy to eight common foods varied between 0.1-0.6%. [5]. A meta-analysis including 51 studies investigating allergy towards 5 common foods showed that the prevalence of self-reported food allergy varied between 3-35% while challenge-proven food allergy varied between 1-11% [6]. As over-reporting of FHS is common [7, 8], thorough clinical diagnostics is essential in order to identify children with true FHS [1, 6]. Nevertheless, from a public health perspective it is important to have knowledge about the prevalence of perceived FHS, since experienced FHS can affect the child’s nutritional intake [9, 10] and has a negative impact on quality of life [11].
The natural course of FHS in schoolchildren is a research-area previously lacking of data [3, 12]. Few studies have investigated risk factors for FHS and most studies focus on IgE-mediated allergies [13, 14]. However, factors that have been associated with reported FHS are geographic setting [15, 16], age [3, 16], sex [17–19], eczema [3, 17], rhinitis [3, 17], asthma [3, 15] and allergic heredity [16]. This study was designed to investigate the incidence and remission of reported FHS and its associated risk factors in a Swedish population-based cohort followed from 8 to 12 years of age. We hypothesized that reported FHS would be common and that over this age-range, reported FHS to cow´s milk and hen´s egg would decrease while there would be an increase of reported symptoms to foods containing birch-pollen cross-reacting proteins.
Materials and methods
Study population
The pediatric cohort was established in 2006 as part of the OLIN-studies (Obstructive Lung disease In Northern Sweden) [20, 21]. All 2,704 children in first and second grade (age 7–8 years, median 8 year) in three towns in Northern Sweden were invited to a questionnaire-study and 2,585 (96%) participated. The 1,895 children in two of the towns (Kiruna and LuleÅ) were also invited to be skin-prick-tested (SPT) with ten common airborne allergens and 1,700 (90%) participated. In 2010 when the children were 11–12 years old (median 12 year), the cohort was followed using the same methods and 2,378 (89% of participants in 2006) answered the questionnaire. The participation rate was again very high in all areas. At the follow-up, all children in Kiruna and a random sample of children from LuleÅ were also invited to donate a blood-sample for analysis of IgE-antibodies and 652 (71%) children participated.
Both in 2006 and 2010 the studies were performed from February to April. The study was approved by the Regional Ethical Review Board in UmeÅ, Sweden.
Questionnaires
The parental questionnaire included the International Study of Asthma and Allergies in Childhood (ISAAC) core questions [22] with added questions about symptoms, physician diagnosis, medication and possible determinants of asthma, rhinitis and eczema [20]. The questionnaire has been used since 1996 in a previous OLIN-cohort [20, 23]. For the current cohort the questionnaire was somewhat modified and questions regarding FHS were included (Additional file 1). The part of the questionnaire addressing FHS included the questions; “Has your child ever had an allergy/hypersensitivity to food” and “Does your child have an ongoing allergy/hypersensitivity to food”. Children reporting ongoing allergy/hypersensitivity also answered questions concerning the presence of reactions and type of symptoms to cow´s milk, hen´s egg, fish, wheat, soy, kiwi, orange, apple, raw carrots, banana, nuts, peanuts and almonds. The questionnaires were distributed by school-personnel and answered by the children’s parents.
Skin prick test
The SPT followed the European Academy of Allergology and Clinical Immunology (EACCI) recommendations [24]. The panel included birch, timothy, mugwort, cat, dog, horse, two house dust mite extracts (Dermatophagoides pteronyssius and farinae) and two mold extracts (Claudosporium and Alternaria) (Solu-prick, ALK, Denmark). Inclusion of common allergenic foods to the SPT-panel was not possible due to safety reasons, since the SPTs were performed at the children’s schools. The potency of the extracts was 10 HEP except for the two molds, which were 1:20 w/v. Histamine 10 mg/ml and glycerol were used as the positive and negative controls. A positive test was defined as at least one wheal ≥3 mm in diameter, recorded after 15 minutes. The allergen potency and SPT-method were identical in the initial and follow-up study. The correlation between a positive SPT and specific IgE >0.35 kU/L to aeroallergens included in the test panel has been previously validated and was found to be excellent [25].
Analysis of specific IgE
Food-specific IgE was measured in a random sample of children (n = 652) at study follow-up in 2010 at the age of 11–12 years. Specific IgE was measured in serum using Immuno-CAP (ThermoFisher Diagnostics, Uppsala, Sweden). The samples were initially analyzed using a food-screening Immune-CAP (fx5, ThermoFisher Diagnostics, Uppsala, Sweden), including cow´s milk, hen´s egg, cod, soy, wheat and peanut. If the screening test was positive (>0.35 kU/L,) specific IgE to all foods in the screening-test was analyzed separately. Specific IgE >0.35 kU/L was considered positive.
Food hypersensitivity and other definitions
Any FHS: reported symptoms to at least one of the specific foods: cow´s milk, hen´s egg, fish, wheat, soy, kiwi, orange, apple, raw carrots, banana, nuts, peanuts and almonds. Non-milk FHS was defined as symptoms to one or more of the specific foods; hen´s egg, fish, wheat, soy, kiwi, orange, apple, raw carrots, banana, nuts, peanuts and almonds, cow´s milk excluded. Milk FHS was defined as reported symptoms to cow´s milk only (including symptoms of lactose intolerance). Allergic heredity: reported parental asthma, rhinitis and/or eczema. Allergic sensitization: at least one positive SPT. Current asthma, rhinitis and eczema: reported doctor´s diagnosis combined with use of medication or symptoms of disease during the last 12 months. Incidence of FHS was defined as absence of any FHS at 8 years of age and reported FHS to specific foods, any FHS, non-milk FHS and milk FHS respectively at 12 years of age. Remission of FHS was defined as reported symptoms of FHS to specific foods, any FHS, non-milk FHS and milk FHS respectively at 8 years of age and absence of the corresponding symptoms at 12 years of age.
Statistical methods
Data were analyzed using the IBM SPSS Software version 21 (New York,, USA). The Chi-square test was used for comparison of proportions. Adjusted risk estimates expressed as odds ratio (OR) and confidence intervals were determined by logistic regression analysis for variables significantly associated to incidence or remission of FHS in the bivariate analyses. The independent variables included in the risk factor analyses were collected at age 8. The confidence interval was set to 95% (95% CI) and a p-value < 0.05 was considered statistically significant.
Results
Prevalence of FHS
The prevalence of any FHS increased from 21.4% at 8 years to 25.9% at 12 years of age (p < 0.001). Changes in the prevalence of perceived FHS to each specific food are presented in Figure 1. The greatest increase was seen for cow´s milk, with a raise from 9% to 13% (p < 0.001). The prevalence of perceived FHS to hen´s egg, fish, wheat, soy, peanuts and almonds remained statistically unchanged with age.
Figure 1.
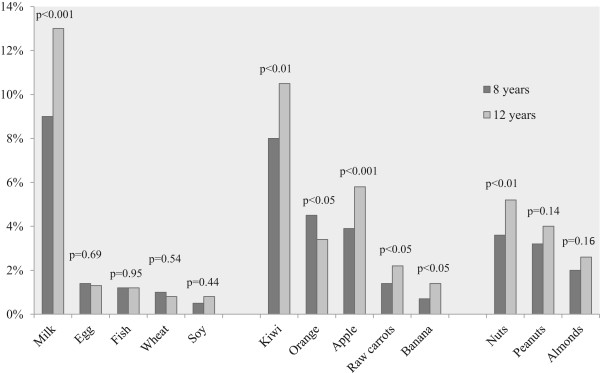
Prevalence (%) of reported food hypersensitivity to specific foods at age 8 and 12 years.
Incidence and remission
The incidence of any FHS from age 8 to 12 years was 14.7% and remission was 32.7% (Table 1). The specific food with the highest 4-year incidence was cow´s milk with 7.9% followed by kiwi with 4.6%, while the incidences were lower for other foods. The results were similar independent of whether children with no FHS or no FHS to the specific food at age 8 constituted the population at risk. Generally, remissions of FHS to all specific foods were high (27%-77%). Remission of milk FHS was 43.9%.
Table 1.
Cumulative incidence (%) and remission (%) of perceived food hypersensitivity (FHS) to different foods from age 8 to 12 years
| Incidence (*) | Incidence (**) | Remission (***) | ||||
|---|---|---|---|---|---|---|
| FHS | n | (%) | n | (%) | n | (%) |
| Milk | 147 | (7.9) | 190 | (8.8) | 94 | (43.9) |
| Egg | 8 | (0.4) | 11 | (0.5) | 15 | (44.1) |
| Fish | 4 | (0.2) | 10 | (0.4) | 12 | (38.7) |
| Wheat | 8 | (0.4) | 15 | (0.6) | 17 | (77.3) |
| Soy | 6 | (0.3) | 13 | (0.5) | 7 | (58.3) |
| Kiwi | 85 | (4.6) | 141 | (6.5) | 87 | (44.6) |
| Orange | 28 | (1.5) | 47 | (2.1) | 74 | (69.2) |
| Apple | 45 | (2.4) | 70 | (3.1) | 25 | (26.9) |
| Raw carrots | 9 | (0.5) | 37 | (1.6) | 17 | (51.5) |
| Banana | 6 | (0.3) | 26 | (1.1) | 8 | (50.0) |
| Nuts | 36 | (1.9) | 71 | (3.1) | 33 | (38.8) |
| Peanuts | 25 | (1.3) | 48 | (2.1) | 28 | (36.8) |
| Almonds | 16 | (0.9) | 40 | (1.7) | 25 | (54.3) |
| Any FHS | 274 | (14.7) | 274 | (14.7) | 167 | (32.7) |
*Children without any FHS at age 8 constituted the population at risk (n = 1868).
**Children without FHS to the specific food at age 8 constituted the populations at risk.
***Children with FHS to the specific food at age 8 constituted the populations at risk.
The pattern of symptoms among incident cases of milk FHS differed significantly from incident cases of non-milk FHS (Figure 2). Therefore, any FHS, non-milk FHS and milk FHS were analyzed separately in the risk-factor-analyses.
Figure 2.
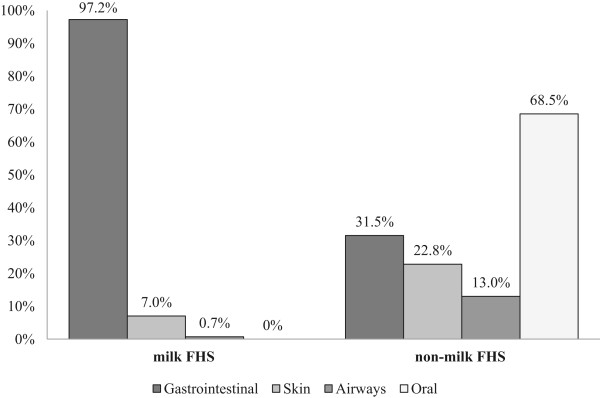
Patterns of symptoms elicited by the offending foods among incident cases of food hypersensitivity, milk FHS (n = 162) and non-milk FHS (n = 143) respectively.
Factors associated with incidence
Baseline characteristics in the cohort at 8 years of age are presented in Table 2. In bivariate analyses, the incidence of any FHS during 4 years was significantly associated with female sex, allergic heredity, current asthma, current rhinitis and allergic sensitization. In the multivariate analysis female sex (OR 1.8 CI 1.3-2.5), allergic heredity (OR 1.6 CI 1.2-2.1), current rhinitis (OR 3.4 CI 1.9-5.9) and allergic sensitization (OR 1.6 CI 1.1-2.3) remained statistically significant. The variables associated with the incidence of non-milk FHS were similar to those associated with the incidence of any FHS in both the bivariate and multivariate analyses. The incidence of milk FHS was significantly associated with female sex (OR 1.7, CI 1.2-2.4) and allergic heredity (OR 1.6, CI 1.1-2.4) (Table 3).
Table 2.
Baseline characteristics at 8 years of age (%) for all children participating in the study and according to sex and living area
| LuleÅ | PiteÅ | Kiruna | All | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 1242) | (n = 658) | (n = 478) | (n = 2378) | |||||||||
| Variables at 8 years | All | Girls | Boys | All | Girls | Boys | All | Girls | Boys | All | Girls | Boys |
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |
| Allergic heredity | 57.6 | 56.1 | 59.1 | 63.5 | 61.7 | 65.0 | 57.5 | 54.0 | 60.9 | 59.2 | 57.1 | 61.2 |
| Current asthma | 4.5 | 3.2 | 5.8 | 7.1 | 5.0 | 8.9 | 9.2 | 6.8 | 11.5 | 6.2 | 4.4 | 7.8 |
| Current rhinitis | 5.8 | 5.7 | 5.9 | 6.2 | 4.4 | 7.8 | 6.7 | 5.1 | 8.2 | 6.1 | 5.2 | 6.9 |
| Current eczema | 11.4 | 11.3 | 11.6 | 10.0 | 11.1 | 9.2 | 11.5 | 9.8 | 13.2 | 11.1 | 10.9 | 11.2 |
| Any positive SPT* | 29.8 | 26.9 | 32.8 | - | - | - | 29.3 | 26.4 | 32.2 | 29.7 | 26.8 | 32.6 |
*The children from LuleÅ and Kiruna were invited to Skin prick testing.
Table 3.
Incidence (%) of Any Food Hypersensitivity (FHS), non-milk FHS and milk FHS in relation to risk factors and adjusted risk analyzed by multiple logistic regression analyses expressed as odds ratios (OR) with 95% confidence intervals (CI)
| Incidence of Any FHS | Incidence of non-milk FHS | Incidence of milk FHS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables at 8 years | Adjusted risk* | Adjusted risk* | Adjusted risk* | |||||||
| (%) | P-value | OR (95% CI) | (%) | P-value | OR (95% CI) | (%) | P-value | OR (95% CI) | ||
| Sex | Boys | 12.3 | 1.0 | 7.2 | 1.0 | 6.1 | 1.0 | |||
| Girls | 16.9 | 0.005 | 1.8 (1.3-2.5) | 10.4 | 0.012 | 1.8 (1.3-2.5) | 9.5 | 0.006 | 1.7 (1.2-2.4) | |
| Allergic heredity | No | 11.0 | 1.0 | 6.3 | 1.0 | 5.7 | 1.0 | |||
| Yes | 17.3 | <0.001 | 1.6 (1.2-2.1) | 10.6 | 0.001 | 1.6 (1.1-2.2) | 9.2 | 0.005 | 1.6 (1.1-2.4) | |
| Current asthma | No | 14.0 | 1.0 | 8.1 | 1.0 | 7.5 | ||||
| Yes | 23.9 | 0.010 | 1.2 (0.7-2.1) | 19.3 | <0.001 | 1.6 (0.8-2.9) | 10.2 | 0.353 | ||
| Current rhinitis | No | 13.5 | 1.0 | 7.8 | 1.0 | 7.6 | ||||
| Yes | 40.3 | <0.001 | 3.4 (1.9-5.9) | 32.8 | <0.001 | 4.0 (2.2-7.3) | 10.4 | 0.359 | ||
| Any positive SPT | No | 12.9 | 1.0 | 7.0 | 1.0 | 7.4 | ||||
| Yes | 22.1 | <0.001 | 1.6 (1.1-2.3) | 16.3 | <0.001 | 2.1 (1.4-3.2) | 8.5 | 0.535 | ||
| Living area | LuleÅ | 13.7 | 8.7 | 6.7 | ||||||
| PiteÅ | 13.1 | 7.6 | 7.9 | |||||||
| Kiruna | 18.2 | 0.067 | 10.3 | 0.362 | 9.7 | 0.154 | ||||
*Significant variables in the bivariate analyses were included in the multivariate model.
Factors associated with remission
In bivariate analyses, remission of any FHS was negatively associated to allergic heredity, current asthma, current rhinitis and allergic sensitization. In multivariate analysis, only the association with allergic sensitization (OR 0.5 CI0.3-0.9) remained statistically significant. The variables associated with the remission of non-milk FHS were similar to those associated with remission of any FHS in the bivariate analyses. In multivariate analysis, current asthma (OR 0.5 CI 0.2-0.9) was the only variable significantly associated with non-milk FHS. The only variable significantly associated with remission of milk FHS was living in Kiruna, showing a negative association, OR 0.2 (CI 0.1-0.6) (Table 4).
Table 4.
Remission (%) of Any Food Hypersensitivity (FHS), non-milk FHS and milk FHS in relation to risk factors and adjusted risk analyzed by multiple logistic regression analyses expressed as odds ratios (OR) with 95% confidence intervals (CI)
| Remission of any FHS | Remission of non-milk FHS | Remission of milk FHS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables at 8 years | Adjusted risk* | Adjusted risk* | Adjusted risk* | |||||||
| (%) | P-value | OR (95% CI) | (%) | P-value | OR (95% CI) | (%) | P-value | OR (95% CI) | ||
| Female sex | Boys | 32.7 | 31.8 | 48.2 | ||||||
| Girls | 32.8 | 0.994 | 36.4 | 0.348 | 43.4 | 0.488 | ||||
| Allergic heredity | No | 39.8 | 1.0 | 45.1 | 1.0 | 46.7 | ||||
| Yes | 30.2 | 0.042 | 0.8 (0.5-1.1) | 30.9 | 0.013 | 0.7 (0.4-1.1) | 44.8 | 0.806 | ||
| Current asthma | No | 34.6 | 1.0 | 37.2 | 1.0 | 46.2 | ||||
| Yes | 18.6 | 0.014 | 0.5 (0.3-1.0) | 17.9 | 0.005 | 0.5 (0.2-0.9) | 33.3 | 0.333 | ||
| Current rhinitis | No | 34.5 | 1.0 | 37.2 | 1.0 | 44.4 | ||||
| Yes | 23.1 | 0.048 | 0.8 (0.4-1.4) | 22.7 | 0.018 | 0.8 (0.4-1.4) | 55.6 | 0.362 | ||
| Any positive SPT | No | 37.9 | 1.0 | 45.0 | 1.0 | 42.7 | ||||
| Yes | 22.4 | 0.001 | 0.5 (0.3-0.9) | 22.9 | <0.001 | 0.6 (0.3-1.0) | 40.4 | 0.792 | ||
| Current eczema | No | 34.5 | 37.7 | 1.0 | 43.9 | |||||
| Yes | 27.2 | 0.128 | 25.5 | 0.024 | 0.8 (0.5-1.4) | 51.2 | 0.390 | |||
| Living area | LuleÅ | 33.9 | 36.5 | 46.7 | 1.0 | |||||
| PiteÅ | 35.9 | 33.7 | 56.0 | 1.6 (0.8-3.1) | ||||||
| Kiruna | 25.5 | 0.219 | 29.3 | 0.278 | 18.5 | 0.006 | 0.2 (0.1-0.5) | |||
*Significant variables in the bivariate analyses were included in the multivariate model.
Several other risk factors were evaluated. None of the following variables were associated with neither incidence nor remission of any of the FHS-groups; having older siblings, living in house versus apartment, furred animals at home during the first 2 years of life, mother or father smoked, mother smoked during pregnancy and breastfeeding < 3 months.
Correlation of Specific IgE and symptoms
The prevalence of a positive food-screening Immune-CAP was 17.0%. The prevalence of specific IgE to the foods included in the screening test; cow´s milk, hen´s egg, cod, soy, wheat and peanut, is presented in Table 5. In general, the agreement between the presence of symptoms and a positive food-specific IgE was poor. Only four out of 97 individuals reporting milk FHS were sensitized to milk and 40 out of 44 children with a positive specific IgE to cow´s milk did not report any milk-induced symptoms. In this subset of children, no one reported any symptoms to soy, though 16 were sensitized, and the 30 children with a positive IgE to wheat had no correlating symptoms. Of children reporting symptoms to peanut, 37% were peanut-sensitized and 38% of the children with a positive IgE-test for peanut described symptoms.
Table 5.
Prevalence of specific IgE and reported symptoms to cow´s milk, hen´s egg, cod, soy, wheat or peanut and number of children with or without specific IgE > 0.35 kU/L and with or without reported FHS to the specific foods*
| Prevalence of spec IgE > 0.35 kU/L | Prevalence of reported FHS | Spec IgE > 0.35 kU/L and reported FHS | Spec IgE > 0.35 kU/L and no reported FHS | Specific IgE < 0.35 kU/L and reported FHS | Specific IgE < 0.35 kU/L and no reported FHS | |
|---|---|---|---|---|---|---|
| n=652 | n=652 | n | n | n | n | |
| Cow´s milk | 6.7% | 14.9% | 4 | 40 | 93 | 515 |
| Hen´s egg | 4.3% | 2.2% | 7 | 21 | 7 | 617 |
| Cod | 1.2% | 1.8% | 5 | 3 | 7 | 637 |
| Soy | 2.5% | 0% | 0 | 16 | 0 | 636 |
| Wheat | 4.6% | 0.8% | 0 | 30 | 5 | 617 |
| Peanut | 4.4% | 5.7% | 11 | 18 | 19 | 604 |
*Calculations based on the 652 children participating in measurement of specific IgE at age 12 years.
Discussion
To our knowledge this is the first study to report data regarding incidence and remission of reported food hypersensitivity in a large population-based cohort of schoolchildren. We found an increase in the prevalence of perceived FHS from 21% at age 8 to 26% at age 12, with both high incidence and high remission. This pattern was particularly evident for FHS to cow´s milk. The high prevalence of any reported FHS was consistent with our hypothesis, while our predicted outcomes were contradicted by the high incidence of FHS to milk. Female sex, allergic heredity, current rhinitis and allergic sensitization were associated with the incidence of perceived FHS and allergic sensitization was negatively associated with remission. Incident cases of FHS to milk and non-milk foods were associated differently with symptoms and risk factors.
Female sex was a significant risk factor for the incidence of perceived FHS. Previous studies have shown a higher female prevalence of FHS among adolescents and adults [8, 17]. For food allergy, there is an increased risk among boys during childhood and a higher prevalence of food allergy among adult women [13, 19]. Endocrine influences have been suggested to contribute to this switch during adolescence [19]. In our cohort, reported FHS was more common among girls already at 8 years of age, mainly due to a higher female prevalence of FHS to cow´s milk [26]. This gender difference is in line with a recent Canadian study, where more women than men reported symptoms of lactose intolerance [27]. Also, symptoms of lactose malabsorption are associated with other diseases [10], of which irritable bowel syndrome is common even among younger people and has a female predominance [28]. However, since true lactose intolerance is reported to be rare among Swedish children [29] it is not likely to be a major contributor to the high prevalence of FHS in our study.
In the risk-factor-analyses, the incidence of any FHS and non-milk FHS during 4 years was significantly associated with current rhinitis and allergic sensitization. Furthermore, allergic sensitization was negatively associated with remission of any FHS. This is consistent with previous studies showing that atopy is a risk factor for incident and persistent FHS [3, 13, 14, 30]. Birch-pollen allergy and cross-reactions to birch-like proteins in vegetables are common in the Swedish population [31]. Others have shown that allergic reactions to fruits, nuts and peanuts constitute a large proportion of incident cases of FHS in Swedish schoolchildren [3]. This could explain the symptom-pattern among the non-milk FHS-cases, with a high percentage of symptoms of oral allergy syndrome (OAS) and rhinitis being the strongest risk factor associated with incidence of non-milk FHS.
The incidence and remission of milk FHS were differently associated with risk factors compared to incidence and remission of non-milk FHS and any FHS. Among incident cases of milk FHS, allergic heredity was the only atopy-related associated variable. It is difficult to find an explanation for this association. Since allergic heredity is a reported variable this could include reactions of non-IgE mediated origin. Also, parents with allergy might be more prone to suspect foods to cause symptoms in their children. The only variable associated with remission of milk FHS was living in the northernmost town, Kiruna, as compared to residence in other towns. This negative association could be a coincidence. However, the population in this area of Sweden includes inhabitants of Saami origin. The prevalence of lactose intolerance in Swedish Saami is unknown but among the Nenets, Samoyed people living in north-west Russia, the prevalence of the lactase down-regulating gene is high [32].
While food allergies are most common during childhood [6, 33, 34], the prevalence of reported FHS appears to remain high in adolescents and adults [4, 15–17]. In our study, there was a significant increase in the prevalence of perceived FHS from 8 to 12 years of age and this increase was mainly explained by a higher prevalence of reported FHS to milk among the 12-year olds. This finding could partly be explained by the onset of lactose intolerance [10]. Another feasible contributor could be an increased awareness in our society regarding foods, especially dairy products, as suspected triggers of various symptoms and diseases [10, 27, 28].
The high prevalence of a positive food-screening Immune-CAP supports the high prevalence of reported symptoms in our study population. However, the agreement between a positive IgE-test and reported symptoms was poor for all the tested foods. This was particularly evident for cow´s milk. A possible explanation for this finding could be parents reporting symptoms suspected to be induced by food in their children that either were not IgE-mediated or not actually caused by the suspected food. Also, IgE-tests can be false positive due to sensitization without symptoms, tolerance-development or cross-reactions [12, 31, 35]. Symptomatic FHS to soy and wheat are quite rare and these foods, and also peanut, have a high degree of cross-reactivity to birch- and timothy-pollens which could explain our findings. A Swedish study reported 7% of 8-year-olds to be sensitized to peanuts. The majority was sensitized to the birch-pollen-analog only and of these, only 17% experienced symptoms [31]. This could explain why only 38% of peanut-sensitized children experienced symptoms in the present study. However, since peanut allergy is mainly IgE-mediated [36], it is noteworthy that only 37% of children with reported FHS to peanut had a positive specific IgE.
A major strength of our study is the longitudinal and population-based design and the high participation-rate, supporting the representativeness of the population. Further, the children who contributed serum were very similar to the entire cohort with respect to the prevalence of allergic sensitization based on SPT, and indices of asthma and rhino conjunctivitis (data not shown). Furthermore, the prevalence of FHS in our study, although high, is consistent with previous findings [3, 6]. Studies investigating incidence and remission of FHS are rare and mostly performed on younger children or limited to one specific food [33, 34], which makes comparisons with our results difficult.
A possible study-limitation is parental reporting of symptoms, although a study from a previous pediatric OLIN-cohort showed a good agreement between reported symptoms of allergic diseases in teenagers and their parents [37]. Another study-limitation is that our results are mainly based upon reported data and since over-reporting of FHS is common [3, 6, 33], confirming studies using more objective diagnostic methods than specific IgE are needed. Nevertheless, previous studies show that people adapt their food-intakes according to perceived FHS, risking deficient intake of essential nutrients [9, 10]. Consequently, the high prevalence of FHS reflects the perceived reality to which children adapt their life and food-intakes.
Conclusion
In summary, in this large population of schoolchildren followed over four years, there was a high prevalence, a high cumulative incidence and a high remission of perceived FHS. This pattern was particularly evident for FHS to cow´s milk, while the incidences of FHS to other specific foods were lower. Generally, there was a poor agreement between food-induced symptoms and IgE-sensitization to the implicated foods. Perceived FHS to milk and non-milk foods differed in risk factor patterns for both incidence and remission. Since one fourth of the children in this study reported FHS and since children adapt their life and food- intake according to perceived FHS, further studies including valid diagnostic methods are needed to evaluate the association between reported symptoms to food and true FHS. This is particularly important for the large number of children reporting FHS to essential foods like cow´s milk.
Consent
Written informed consent was obtained from the patient’s guardian/parent/next of kin for the publication of this report and any accompanying images.
Electronic supplementary material
Additional file 1: The Olin Pediatric Questionnaire 2006. (DOC 200 KB)
Acknowledgements
We wish to acknowledge the participating children and their parents, the school personnel distributing the questionnaires and Sigrid Sundberg, Anders Bjerg and Britt-Marie Eklund for the collection of data. Professor Olle Hernell, professor Bo Lundbäck and Dr Stig Örjestad are acknowledged for valuable comments on the manuscript.
Funding source
Financial support was provided through; The Swedish Heart and Lung Foundation; The Swedish Asthma and Allergy Foundation; The Swedish research council; A regional agreement between UmeÅ University and Västerbotten County Council and Norrbotten County Councils (ALF); State Government funding for Health Care research (FoU); Norrbotten County Council, VISARE Norr. ThermoFisher Diagnostics, Uppsala, Sweden provided the analyses of specific IgE and ALK, Denmark is acknowledged for supporting the skin-prick-tests.
Abbreviations
- EAACI
European Academy of Allergology and Clinical Immunology
- FHS
Food hypersensitivity
- ISAAC
International Study of Asthma and Allergies in Childhood
- OAS
Oral allergy syndrome
- OLIN
The Obstructive Lung disease In Northern Sweden Studies
- OR
Odds ratio
- SPT
Skin prick test.
Footnotes
Competing interests
The authors declare that they have no competing interests. The sponsors, ThermoFisher Diagnostics provided the IgE testing, but had no involvement in the interpreting of data, writing the report or in the decision to submit the paper for publication.
Authors’ contributions
AW - contributed to the study design, analysis and interpretation of data, drafted the manuscript and approved the final manuscript as submitted. ÅS, LH, CEW and MSP – all contributed to the analysis and interpretation of data, reviewed and revised the manuscript and approved the final manuscript as submitted. ER - is responsible for study conception and design, has contributed to the analysis and interpretation of data, reviewed and revised the manuscript and approved the final manuscript as submitted. All authors read and approved the final manuscript.
Contributor Information
Anna Winberg, Email: anna.winberg@pediatri.umu.se.
Åsa Strinnholm, Email: asa.strinnholm@vll.umu.se.
Linnea Hedman, Email: linnea.hedman@nll.se.
Christina E West, Email: christina.west@pediatri.umu.se.
Matthew S Perzanowski, Email: mp2217@columbia.edu.
Eva Rönmark, Email: eva.ronmark@nll.se.
References
- 1.Mills EN, Mackie AR, Burney P, Beyer K, Frewer L, Madsen C, Botjes E, Crevel RW, van Ree R. The prevalence, cost and basis of food allergy across Europe. Allergy. 2007;62(7):717–722. doi: 10.1111/j.1398-9995.2007.01425.x. [DOI] [PubMed] [Google Scholar]
- 2.Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TA, Ring J, Thien F, Van Cauwenberge P, Williams HC. Revised nomenclature for allergy for global use: report of the nomenclature review committee of the world allergy organization, October 2003. J Allergy Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 3.Östblom E, Lilja G, Pershagen G, van Hage M, Wickman M. Phenotypes of food hypersensitivity and development of allergic diseases during the first 8 years of life. Clin Exp All. 2008;38:1325–1332. doi: 10.1111/j.1365-2222.2008.03010.x. [DOI] [PubMed] [Google Scholar]
- 4.Nwaru BI, Hickstein L, Panesar SS, Muraro A, Werfel T, Cardona V, Dubois AE, Halken S, Hoffmann-Sommergruber K, Poulsen LK, Roberts G, Van Ree R, Vlieg-Boerstra BJ, Sheikh A. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014;69(1):62–75. doi: 10.1111/all.12305. [DOI] [PubMed] [Google Scholar]
- 5.Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69(8):992–1007. doi: 10.1111/all.12423. [DOI] [PubMed] [Google Scholar]
- 6.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Södergren E, Sigurdardottir ST, Lindner T, Goldhahn K, Dahlstrom J, McBride D, Madsen C. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Venter C, Pereira B, Grundy J, Clayton CB, Arshad SH, Dean T. Prevalence of sensitization reported and objectively assessed food hypersensitivity amongst six-year-old children: a population-based study. Pediatr Allergy Immunol. 2006;17(5):356–363. doi: 10.1111/j.1399-3038.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaya A, Erkocoglu M, Civelek E, Cakir B, Kocabas CN. Prevalence of confirmed IgE-mediated food allergy among adolescents in Turkey. Pediatr Allergy Immunol. 2013;24(5):456–462. doi: 10.1111/pai.12097. [DOI] [PubMed] [Google Scholar]
- 9.Meyer R, De Koker C, Dziubak R, Venter C, Dominguez-Ortega G, Cutts R, Yerlett N, Skrapak AK, Fox AT, Shah N. Malnutrition in children with food allergies in the UK. J Hum Nutr Diet. 2012;23(4):307–314. doi: 10.1111/jhn.12149. [DOI] [PubMed] [Google Scholar]
- 10.Wilt TJ, Shaukat A, Shamliyan T, Taylor BC, MacDonald R, Tacklind J, Rutks I, Schwarzenberg SJ, Kane RL, Levitt M. Lactose intolerance and health. Evid Rep Technol Assess. 2010;192:1–410. [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy. 2010;65(8):933–945. doi: 10.1111/j.1398-9995.2010.02342.x. [DOI] [PubMed] [Google Scholar]
- 12.Eller E, Kjaer HF, Host A, Andersen KE, Bindslev-Jensen C. Food allergy and food sensitization in early childhood: results from the DARC cohort. Allergy. 2009;64(7):1023–9. doi: 10.1111/j.1398-9995.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, Massing M, Cohn RD, Zeldin DC. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126:798–806. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lack G. Update on risk factors for food allergy. J Allergy Clin Immunol. 2012;129:1187–1197. doi: 10.1016/j.jaci.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 15.Sandin A, Annus T, Björksten B, Nilsson L, Riikjarv MA, van Hage-Hamsten M, BrÅbäck L. Prevalence of self-reported food allergy and IgE antibodies to food allergens in Swedish and Estonian schoolchildren. Eur J Clin Nutr. 2005;59(3):399–403. doi: 10.1038/sj.ejcn.1602087. [DOI] [PubMed] [Google Scholar]
- 16.Leung TF, Yung E, Wong YS, Lam CWK, Wong GWK. Parent-reported adverse food reactions in Hong Kong Chinese pre-schoolers: Epidemiology, clinical spectrum and risk factors. Pediatr Allergy Immunol. 2009;20(4):339–346. doi: 10.1111/j.1399-3038.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 17.Patelis A, Gunnbjornsdottir M, Borres MP, Burney P, Gislason T, Toren K, Forsberg B, Alving K, Malinovschi A, Janson C. Natural history of perceived food hypersensitivity and IgE sensitisation to food allergens in a cohort of adults. PLoS One. 2014;9(1):e85333. doi: 10.1371/journal.pone.0085333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soost S, Leynaert B, Almqvist C, Edenharter G, Zuberbier T, Worm M. Risk factors of adverse reactions to food in German adults. Clin Exp All. 2009;39(7):1036–1044. doi: 10.1111/j.1365-2222.2008.03184.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Mempel M, Schober W, Behrendt H, Ring J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy. 2008;63(11):1418–27. doi: 10.1111/j.1398-9995.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 20.Rönmark E, Bjerg A, Perzanowski M, Platts-Mills T, Lundbäck B. Major increase in allergic sensitization in schoolchildren from 1996 to 2006 in northern Sweden. J Allergy Clin Immunol. 2009;124:357–363. doi: 10.1016/j.jaci.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjerg A, Sandstrom T, Lundbäck B, Rönmark E. Time trends in asthma and wheeze in Swedish children 1996–2006: prevalence and risk factors by sex. Allergy. 2010;65(1):48–55. doi: 10.1111/j.1398-9995.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 22.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW, Strachan D, Weiland SK, Williams HC. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 23.Rönmark E, Bjerg A, Hedman L, Perzanowski M, Sundberg S, Lundbäck B. The Obstructive Lung Disease in Northern Sweden (OLIN) longitudinal paediatric study I–the first 10 years. Clin Respir J. 2008;2(Suppl 1):26–33. doi: 10.1111/j.1752-699X.2008.00081.x. [DOI] [PubMed] [Google Scholar]
- 24.Dreborg S. The skin prick test in the diagnosis of atopic allergy. J Am Acad Dermatol. 1989;21:820–821. doi: 10.1016/S0190-9622(89)70256-5. [DOI] [PubMed] [Google Scholar]
- 25.Rönmark E, Perzanowski M, Platts-Mills T, Lundbäck B. Four-year incidence of allergic sensitization among schoolchildren in a community where allergy to cat and dog dominates sensitization: report from the Obstructive Lung Disease in Northern Sweden Study Group. J Allergy Clin Immunol. 2003;112:747–754. doi: 10.1016/S0091-6749(03)01866-9. [DOI] [PubMed] [Google Scholar]
- 26.Strinnholm A, Winberg A, West C, Hedman L, Rönmark E. Acta Paediatr. 2014. Food hypersensitivity is common in Swedish schoolchildren, especially oral reactions to fruit and gastrointestinal reactions to milk. [DOI] [PubMed] [Google Scholar]
- 27.Barr SI. Perceived lactose intolerance in adult Canadians: a national survey. Appl Physiol Nutr Metab. 2013;38:830–835. doi: 10.1139/apnm-2012-0368. [DOI] [PubMed] [Google Scholar]
- 28.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 29.Vuorisalo T, Arjamaa O, Vasemagi A, Taavitsainen JP, Tourunen A, Saloniemi I. High lactose tolerance in North Europeans: a result of migration, not in situ milk consumption. Perspect Biol Med. 2012;55:163–174. doi: 10.1353/pbm.2012.0016. [DOI] [PubMed] [Google Scholar]
- 30.Ballardini N, Kull I, Lind T, Hallner E, Almqvist C, Östblom E, Melen E, Pershagen G, Lilja G, Bergström A, Wickman M. Development and comorbidity of eczema, asthma and rhinitis to age 12: data from the BAMSE birth cohort. Allergy. 2012;67(4):537–544. doi: 10.1111/j.1398-9995.2012.02786.x. [DOI] [PubMed] [Google Scholar]
- 31.Asarnoj A, Moverare R, Östblom E, Poorafshar M, Lilja G, Hedlin G, van Hage M, Ahlstedt S, Wickman M. IgE to peanut allergen components: relation to peanut symptoms and pollen sensitization in 8-year-olds. Allergy. 2010;65(9):1189–1195. [Google Scholar]
- 32.Khabarova Y, Grigoryeva V, Tuomisto S, Karhunen PJ, Mattila K, Isokoski M. High prevalence of lactase non-persistence among indigenous nomadic Nenets, north-west Russia. Int J Circumpolar Health. 2012;71:1–6. doi: 10.3402/ijch.v71i0.17898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Higgins B, Arshad SH, Dean T. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63(3):354–359. doi: 10.1111/j.1398-9995.2007.01570.x. [DOI] [PubMed] [Google Scholar]
- 34.Host A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow's milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol. 2002;13(Suppl 15):23–28. doi: 10.1034/j.1399-3038.13.s.15.7.x. [DOI] [PubMed] [Google Scholar]
- 35.Geroldinger-Simic M, Zelniker T, Aberer W, Ebner C, Egger C, Greiderer A, Prem N, Lidholm J, Ballmer-Weber BK, Vieths S, Bohle B. Birch pollen-related food allergy: clinical aspects and the role of allergen-specific IgE and IgG4 antibodies. J Allergy Clin Immunol. 2011;127:616–622. doi: 10.1016/j.jaci.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 36.Ho MH, Wong WH, Heine RG, Hosking CS, Hill DJ, Allen KJ. Early clinical predictors of remission of peanut allergy in children. J Allergy Clin Immunol. 2008;121:731–736. doi: 10.1016/j.jaci.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 37.Hedman L, Bjerg A, Perzanowski M, Rönmark E. Good agreement between parental and self-completed questionnaires about allergic diseases and environmental factors in teenagers. J Clin Epidemiol. 2010;63:783–789. doi: 10.1016/j.jclinepi.2009.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: The Olin Pediatric Questionnaire 2006. (DOC 200 KB)


