Unlike animals, plants have two alternating generations: a diploid sporophyte and a haploid gametophyte. Through meiosis, the sporophyte produces haploid spores, which then give rise to gametophytes, a multicellular haploid structure that produces gametes for sexual reproduction. All seed plants and some non–seed plants are heterosporous, producing spores of different sizes: large female spores and small male spores. Most ferns, on the other hand, are homosporous; they produce a single type of spore. After germination, each fern spore has the potential to develop into a male, female, or hermaphrodite gametophyte. The developmental decision that determines the sex of a particular gametophyte depends on an interplant communication system mediated by chemical signals, termed antheridiogens (1, 2). On page 469 of this issue, Tanaka et al. (3) show that an antheridiogen in the Japanese climbing fern (Lygodium japonicum) functions as more than a simple chemical signal. Covalent modification of the antheridiogen is required to trigger the fern's response to form male gametophytes. Temporal and spatial separation of the biosynthetic pathway between the early- and late-maturing gametophytes ensures the production of the antheridiogen, and the active male-inducing chemically modified form, at just the right time and place.
In a fern population, the early-maturing gametophytes (from spores that germinated first) produce and secrete into the aqueous environment antheridiogens, which promote differentiation of the neighboring younger gametophytes into males. These early-maturing antheridiogen-producing gametophytes will mainly develop the egg-forming female sex organ. This mechanism results in a small number of female gametophytes being surrounded by male gametophytes, promoting out-crossing and the maintenance of genetic diversity. Although this phenomenon was first discovered in 1950 (4), very little is known about how antheridiogen production is regulated and how this signal is perceived.
Tanaka et al. (3) show that an antheridiogen gibberellin (GA) A9 methyl ester (GA9-Me) is C3 hydroxylated and de-methylated to become an active GA (GA4). This antheridiogen is produced via the conserved gibberellin biosynthetic pathway in plants (3, 5, 6), although the methyl transferase and methyl esterase for the conversion between GA9 and GA9-Me have not yet been identified. Importantly, Tanaka et al. find that genes encoding several enzymes for the production of GA9 are more highly expressed in the early-maturing gametophyte than in the late-maturing young gametophytes. In contrast, the gene encoding the enzyme (GA 3-oxidase) for the conversion of GA9 to the active GA4 is preferentially expressed in the late-maturing young gametophytes, which are known to be highly sensitive to antheridiogens. This gene expression partitioning explains why only the early-maturing gametophytes produce the antheridiogen GA9-Me and only the late-maturing younger gametophytes can respond to this pheromone to become males (see the figure).
Figure. Sorting out sexual differentiation.
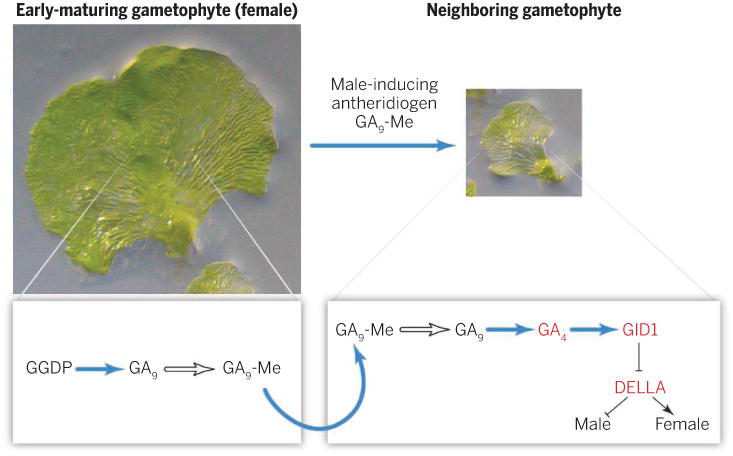
Interplant communication via antheridiogen between early- and late-maturing gametophytes is achieved by physical separation of the GA biosynthetic pathway in these individuals. The antheridiogen (GA9-Me) is produced and secreted by the early-maturing gametophyte (left). Once taken up by the neighboring late-maturing young gametophyte (right), GA9-Me is converted to the active GA4. GA4 activates its receptor GID1, which in turn promotes male sex organ formation by inhibiting the GA signaling repressor DELLA. The open arrows represent steps catalyzed by unidentified enzymes. GGDP, geranylgeranyl diphosphate.
In seed plants, GA4 is an active hormone that plays a key role in regulating both vegetative and reproductive development (5, 6). However, GA9-Me is inactive. GA4 turns on the GA signaling pathway by activating its nuclear receptor GIBBERELLIN INSENSITIVE DWARF1 (GID1) to interact with and induce degradation of the DELLA master growth repressors (7, 8). Tanaka et al. find that GA4 in the Japanese climbing fern (a non–seed plant) activates the GA response pathway in a similar manner: GA4, but not GA9-Me, induces the GID1-DELLA interaction. Moreover, GID1 is preferentially expressed in the late-maturing young gametophyte, and treatment of such a gametophyte with a GID1 inhibitor blocks the male-inducing effect of GA9-Me, supporting the idea that the GAGID1-DELLA signaling module controls sex organ differentiation in this fern.
An outstanding critical question is how the fern is able to respond to a signal made endogenously at low levels and which is then further diluted upon secretion into the environment. Tanaka et al. measured the biochemical properties of the key enzyme GA 3-oxidase and the GA receptor GID1 and found that these proteins have up to 24- and 100-fold higher affinity, respectively, for substrate and ligand than their seed plant counterparts, facilitating the necessarily highly sensitive response to the scarce antheridiogen. Another key question the authors addressed is why ferns do not simply produce GA4 as an antheridiogen. Tanaka et al. show that GA9-Me is more efficiently taken up than GA4 by the young gametophytes, likely because the hydrophobic GA9-Me can easily diffuse through the cell membrane. However, how GA9-ME is transmitted through the aqueous environment is unknown; Tanaka et al. suggest that a protein transporter may facilitate this process.
Sex determination in plants can be controlled by genes located on the sex chromosomes or by chemical signals, such as hormones in seed plants, and pheromones (such as antheridiogens) in non–seed plants, such as ferns (2). However, the detailed molecular mechanisms of sex determination in plants are presently largely unknown. The identification of the conserved GA-GID1-DELLA signaling module as the central regulator in sex organ differentiation in the fern is important, especially because GA also controls sex determination in some seed plants, such as Zea mays (corn) and Cucumis sativa (cucumber). Future studies of the downstream signaling pathways from DELLA in different plant species will reveal whether the GA-regulated sex determining process is conserved more broadly across plants.
Acknowledgments
I thank K. Preyer and A. Silverstone for helpful discussions.
References and Notes
- 1.Yamane H. Int Rev Cytol. 1998;184:1. [Google Scholar]
- 2.Tanurdzic M, Banks JA. Plant Cell. 2004;16(suppl):S61. doi: 10.1105/tpc.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka J, et al. Science. 2014;346:469. doi: 10.1126/science.1259923. [DOI] [PubMed] [Google Scholar]
- 4.Döpp W. Ber Deut Bot Ges. 1950;63:139. [Google Scholar]
- 5.Hedden P, Thomas SG. Biochem J. 2012;444:11. doi: 10.1042/BJ20120245. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi S. Annu Rev Plant Biol. 2008;59:225. doi: 10.1146/annurev.arplant.59.032607.092804. [DOI] [PubMed] [Google Scholar]
- 7.Sun TP. Curr Biol. 2011;21:R338. doi: 10.1016/j.cub.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 8.Ueguchi-Tanaka M, Nakajima M, Motoyuki A, Matsuoka M. Annu Rev Plant Biol. 2007;58:183. doi: 10.1146/annurev.arplant.58.032806.103830. [DOI] [PubMed] [Google Scholar]


