Abstract
Greater sleep difficulty following a challenging event, or a vulnerability to stress-related sleep disturbance (i.e., sleep reactivity), is characteristic of insomnia. However, insomnia is rarely observed in isolation; rather it is frequently seen in combination with other problems, such as depression. Despite the link between depression and increased sensitivity to stress, relatively little is known about the role sleep reactivity has in explaining variability in depressive symptoms. Therefore, the current study examined whether sleep reactivity was associated with depressive symptoms, and whether this relationship was mediated by insomnia. We assessed sleep reactivity, insomnia, and depressive symptoms among 2250 young adults (1244 female; Mage = 23.1, SDage = 2.97) from the Colorado Longitudinal Twin Study and Community Twin Study. Results indicated that greater sleep reactivity was significantly associated with elevated depressive symptoms, and that this link was partially mediated by insomnia. This is one of the first studies to demonstrate an independent association between sleep reactivity and depressive symptomatology. These findings suggest that a greater sensitivity to stress-related sleep disturbance may also be a predisposing factor to depression, and highlight the need for a better understanding of sleep reactivity, as it may represent a more global vulnerability construct.
Keywords: sleep reactivity, insomnia, depressive symptoms, stress
Insomnia is a major source of daytime impairment (Riedel & Lichstein, 2000; Drake, Vargas, Roth, & Friedman, 2014) and is associated with a number of negative health consequences (Ohayon, 2002). While a number of factors increase vulnerability to insomnia (see Drake & Roth, 2006 for review), one factor that has gained increasing support is sleep reactivity (Drake, Richardson, Roehrs, Scofield, & Roth, 2004; Drake, Friedman, Wright, & Roth, 2011; Jarrin, Chen, Ivers, & Morin, 2013; Nakajima et al., 2014). Sleep reactivity is the degree to which a person experiences sleep disturbance in response to various challenges, and is commonly considered a predisposing factor to insomnia (Drake & Roth, 2006). While the consequences of greater sleep reactivity have almost exclusively been examined in the context of insomnia, sleep reactivity is actually a tendency for sleep-related disturbances in response to stress, and thus not inherently specific to insomnia. Yet, the impact of sleep reactivity on other related disorders, for example depression, is relatively unknown. This gap is important as identifying potential factors that increase vulnerability to depression is also a research priority (De Raedt & Koster, 2010). Therefore, in the current study we investigated whether sleep reactivity was also associated with variability in depressive symptomatology. Furthermore, we examined the extent to which a comorbid insomnia diagnosis explained this relationship.
Stress is associated with increased sleep disturbance, such as lower sleep efficiency (Hall et al., 2008), increased sleep fragmentation (Mezick et al., 2009), and greater self-reported insomnia symptoms (Bernert, Merrill, Braithwaite, Van Orden, & Joiner, 2007). Moreover, stress predicts the onset of insomnia (Gregory, Caspi, Moffitt, & Poulton, 2006). One recent study (Pillai, Roth, Mullins, & Drake, 2014) suggests that specific maladaptive coping behaviors (i.e., behavioral disengagement, distraction, and substance use) may explain this link between stress exposure and subsequent insomnia. Alternatively, stress-related sleep disturbance, or sleep reactivity, may explain the link between stress and insomnia. Previous studies have demonstrated a consistent link between sleep reactivity and insomnia (Jefferson et al., 2005; Drake et al., 2011; Nakajima et al., 2014; Jarrin et al., 2014). However, individuals vary in the degree to which they have a vulnerability to stress-related sleep disturbance, as sleep reactivity is not conceptualized in absolute terms. Furthermore, not all individuals who report high sleep reactivity go on to develop insomnia, given that the impact of sleep reactivity on insomnia also varies as a function of stress exposure (Drake, Pillai, & Roth, 2014). Taken together, these findings suggest that insomnia may be linked to a greater sensitivity to stress, and therefore individuals with insomnia or those vulnerable to developing insomnia are more susceptible to the consequences of stress exposure. For example, individuals with insomnia appraise negative life events as more stressful and use less effective coping strategies to deal with that stress compared to good sleepers (Morin, Rodrigue, & Ivers, 2003; Pillai et al., 2014). These maladaptive cognitive and behavioral responses to stress may in turn hinder a person’s ability to fall asleep during stressful periods.
The onset of depression and depressive symptomatology have also been consistently linked to greater stress exposure (Hammen, 2005) and sensitivity to stress (Monroe & Harkness, 2005). Therefore, one common link between insomnia and depression may be this shared vulnerability to stress-related disturbances. For example, studies have consistently demonstrated an association between depression and an atypical endocrine stress response (Burke, Davis, Otte, & Mohr, 2005; Morris & Rao, 2014). Similarly, individuals with depression have a greater cognitive vulnerability to stress, or a tendency to make more negative attributions following a stressful life event (Haeffel & Grigorenko, 2007). In contrast, few studies have examined whether depression is directly associated with a vulnerability to stress-related sleep disturbance (i.e., sleep reactivity). Yet, it is possible that greater sleep reactivity is also a predisposing factor to depression. Sleep disturbances are commonly reported symptoms of depression that typically increases in response to stress, and thus a tendency to experience sleep problems following stress may also be characteristic of depression (Åkerstedt et al., 2002). This is one of the first studies to investigate the impact of sleep reactivity on depressive symptomatology.
Alternatively, it is possible that the hypothesized link between sleep reactivity and depressive symptomatology may be explained by their common link to insomnia. Insomnia is rarely seen in isolation. It is highly comorbid with various other health conditions (Ohayon, Caulet, & Lemoine, 1998), particularly depression (Buysse et al., 2008). In fact, insomnia is considered a major risk factor for the development of a major depressive episode (Riemann & Voderholzer, 2003; Baglioni et al., 2011). Therefore, sleep reactivity may instead have an indirect effect on depressive symptomatology, such that the impact of sleep reactivity on depression may be explained via its effect on insomnia (i.e., insomnia as a mediator). For example, those individuals with high levels of sleep reactivity who go on to develop insomnia may also be especially vulnerable to developing subsequent depression. This would suggest that a greater vulnerability to stress-related sleep disturbances may be a potential shared link between insomnia and depression and help explain the high comorbidity between these disorders. This is consistent with other research suggesting both insomnia and depression are associated with a greater sensitivity to atypical cognitive (Abramson et al., 1999; Morin et al., 2003, Fernandez-Mendoza et al., 2010; Haynes et al., 2012) and physiological (Basta, Chrousos, Vela-Bueno, & Vgontzas, 2007; Morris, Rao, & Garber, 2012) responses to stress. However, the indirect effect of sleep reactivity on depressive symptomatology has yet to be examined.
The primary aim of the current study was to examine whether there was a meaningful association between sleep reactivity and depressive symptomatology. Consistent with prior research suggesting that depression is associated with an increased sensitivity to stress (Monroe & Harkness, 2005), we hypothesized that greater sleep reactivity would be associated with greater depressive symptoms. Moreover, we also examined whether insomnia significantly mediated the relationship between sleep reactivity and depressive symptoms. To this end, we predicted that greater sleep reactivity would be associated with greater depressive symptoms via sleep reactivity’s effect on insomnia. This would suggest that participants with greater sleep reactivity would be more likely to report an insomnia diagnosis, and therefore more likely to also experience depressive symptoms. In summary, this study is important as it may provide further insight regarding the potential mechanisms that may contribute to a greater vulnerability to developing insomnia and depression, which may have important treatment and prevention implications for both disorders.
Methods
Participants
Participants from the ongoing Colorado Longitudinal Twin Study and Community Twin Study (Rhea, Gross, Haberstick, & Corley, 2013) were invited to complete an online survey about their sleep and associated health outcomes. The response rate for the study was 65%. The original sub-sample for this study consisted of 2330 individual twins (56% female; n = 1309). The current study is a secondary analysis of this sub-sample. This sub-sample was originally recruited to examine the genetic and environmental contributions to the link between sleep reactivity and insomnia (Drake et al., 2011). Forty-two individuals (71% female; n = 30) were missing an insomnia classification because there was not enough information (frequency, duration, or interference) to determine their status, and were therefore excluded from all subsequent analyses. An additional 38 participants who reported sleep disturbances due to being pregnant or breastfeeding, or due to waking to care for small children, were excluded from subsequent analyses. The final sample for this study included 2250 individual twins (55% female; n = 1244). The average age of our final sample was 23.1 years (SD = 2.97).
Procedure
The survey took approximately 30–45 minutes to complete and participants were compensated $20 for completing the survey. The survey included additional questions on sleep and health behaviors, however, the current analyses only examined items regarding insomnia symptoms, depressive symptoms, and sleep reactivity. All research protocols were reviewed and approved by the University of Colorado Institutional Review Board. Furthermore, the current study is in compliance with APA ethical standards in the treatment of human subjects.
Measures
Sleep reactivity
The Ford Insomnia Response to Stress Test (FIRST; Drake et al., 2004) is a 9-item questionnaire (four-point Likert scale) assessing vulnerability to stress-related sleep disturbance (i.e., sleep reactivity). The FIRST has been widely used in previous research (Drake, Scofield, & Roth, 2008; Drake et al., 2011; Yang, Chou, & Hsiao, 2011; Lin, Jen, & Yang, 2014), and has demonstrated good psychometric properties. Furthermore, previous studies have reported a significant correlation between FIRST scores and polysomnographic measures of sleep, as well as latencies on the Multiple Sleep Latency Test (Drake et al., 2004). The FIRST also demonstrated good internal consistency (α = 0.86) in the current sample. The FIRST asked how likely (1 = “not likely”; 2 = “somewhat likely”; 3 = “moderately likely”; 4 = “very likely”) you would have difficulty sleeping under nine situations: before an important meeting the next day, after a stressful experience during the day, after a stressful experience in the evening, after getting bad news during the day, after watching a frightening movie or TV show, after having a bad day at work, after an argument, before having to speak in public, and before going on vacation the next day. Participants were asked to rate the likelihood even if they had not experienced the situation recently. The total score was equal to the sum of the nine items.
Insomnia diagnosis
Participants reported how often (e.g., never, sometimes, usually, or always) they experienced the following two sleep problems: difficulty falling asleep and difficulty staying asleep. Individuals who did not report “never” for both items were asked a series of follow-up questions, including how long they had the sleep problem(s) (years and months), how often they had the sleep problem(s) (average days per week), and to what extent they considered it to interfere with their daily functioning (e.g., daytime fatigue, ability to function at work/daily chores, concentration, memory, mood, etc.), with 0 = “not at all interfering”, 1 = “a little”, 2 = “somewhat”, 3 = “much”, and 4 = “very much interfering”. Participants were judged to meet Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria for insomnia if they had at least one problem “usually” or “always” for at least three months, three nights per week, and with at least “somewhat” interference. According to their responses to these items, approximately 13.6% (n = 305) of the entire sample met DSM-5 criteria for insomnia. This rate is relatively consistent with previously reported population estimates of insomnia (Ohayon, 2002; Roth et al, 2011), thus is likely to be representative of sleep problems in the general population.
Sleep disturbance
Additional insomnia symptoms were assessed in the current study and included weekday total sleep time (TST), sleep onset latency (SOL), latency back to sleep after a nocturnal awakening (back to sleep latency), number of nocturnal awakenings, and nocturnal insomnia symptom duration and frequency. Specifically, participants were asked to report, on average, their TST, sleep onset latency, and back to sleep latency, in hours and minutes. For example, to assess for TST, participants were asked “during the past month, thinking about your average weekday, how long did you actually sleep?” and for back to sleep latency, “during the past month, on average, how long does it take you to fall back asleep after waking up?” Participants were also asked to report the average number of times, during the past month, they woke up during the night. We assessed insomnia symptom duration by asking participants how long they have been experiencing nocturnal insomnia symptoms (i.e., difficulty falling asleep or staying asleep), in years and months. Insomnia symptom frequency was the average number of days per week they had experienced one or more of these symptoms. These additional sleep disturbance variables were included in the current analyses in order to control for their impact on individual differences in sleep reactivity and depressive symptoms.
Depressive symptoms
Participants completed the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977) to assess current self-reported depressive symptoms. The CES-D consists of 20 four-point Likert scale items assessing various symptoms commonly reported in depression. Total scores on the full 20-item scale range from 0 – 60. High internal consistency (α = 0.85) and test retest reliability (r = 0.61) have been previously reported for the CES-D (Radloff, 1977; Devins et al., 1988). In the current study, the “restless sleep” item was removed from the scale in order to reduce overlap between the CES-D and insomnia items. The revised 19-item CES-D demonstrated good internal consistency (α = 0.90).
Statistical Analyses
We used a series of hierarchical linear mixed models via SPSS MIXED to examine the association among insomnia, sleep reactivity, and depressive symptomatology. Previous studies have demonstrated that sleep reactivity (Drake et al., 2011), insomnia (Watson, Goldberg, Arguelles, & Buchwald, 2006), and depression (Sullivan et al., 2000) all have strong genetic components, therefore, family members cannot be considered independent observations. Given that twin data includes non-independent observations, we used random-effects models and entered family status as a random-effect in order to control for within-family similarities, and utilize the correct within-family covariate structure. First, we tested the direct effect of the independent variable (FIRST) on the dependent variable (CES-D). Next, we tested the direct effect of FIRST on the mediator (insomnia), and lastly CES-D was regressed on insomnia, while controlling for FIRST scores. Sobel’s test (Sobel, 1982) was used to calculate the indirect effect of sleep reactivity on depressive symptoms. The subsequent models included insomnia diagnosis, FIRST scores, and any relevant covariates (e.g. age, sex, TST, sleep latency, back to sleep latency, nocturnal awakening frequency, insomnia symptom duration, and insomnia symptom frequency) as fixed effects. Twin status was not included as a fixed effect, given that our research aims were not specific to the association with twin status. All independent variables were mean-centered and skewed variables were log-transformed.
Results
Descriptive Statistics
Table 1 presents correlations between all predictor variables included in the current analyses. In general, participants endorsed a relatively wide range of depressive symptoms (72.7% = minimal, 19.9% = mild, 7.4% = severe; range = 0–48). There were significant sex differences on all the target predictor variables. Specifically, females reported significantly greater FIRST scores, b = 3.19, t(1811) = 13.71, p < .001, and depressive symptoms, b = 2.21, t(1810) = 5.81, p < .001, compared to males. Similarly, females reported greater rates of insomnia compared to males, b = 0.06, t(1768) = 4.20, p < .001. Consequently, sex was included as a covariate in all subsequent models. Group means and standard deviations of all study variables are demonstrated in Table 2. Not surprisingly, participants with insomnia reported greater FIRST scores, depressive symptoms, and sleep disturbance (e.g., sleep onset latency, nocturnal awakenings, insomnia frequency).
Table 1.
Pearson’s correlations between sex, age, and all sleep variables. FIRST = Ford Insomnia Response to Stress Test; TST = Total Sleep Time; SOL = Sleep Onset Latency; BSL = Back to Sleep Latency.
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | |
|---|---|---|---|---|---|---|---|---|---|---|
| (1) Sex | -- | |||||||||
| (2) Age | .081** | -- | ||||||||
| (3) FIRST | −.287** | −.024 | -- | |||||||
| (4) DSM-5 Insomnia | −.087** | .023 | .321** | -- | ||||||
| (5) Symptom Duration | .069** | .193** | .139** | .147** | -- | |||||
| (6) Symptom Frequency | −.113** | .035 | .377** | .547** | .243** | -- | ||||
| (7) TST | .006 | .018 | −.058** | −.075** | −.023 | −.058* | -- | |||
| (8) SOL | −.044* | .043* | .231** | .326** | .106** | .382** | −.041 | -- | ||
| (9) Awakenings | −.033 | .032 | .154** | .179** | .094** | .251** | .002 | .159** | -- | |
| (10) BSL | −.080** | .088** | .158** | .207** | .045* | .235** | −.036 | .392** | .170** | -- |
p < .05;
p < .01.
Table 2.
Group means and standard deviations for all independent and dependent variables. Participants were judged to meet DSM-5 criteria for insomnia if they had at least one problem “usually” or “always” for at least three months, three nights per week, and with at least “somewhat” interference.
| Insomnia Group (n = 305) | No Insomnia Group (n = 1945) | p-value | |
|---|---|---|---|
| Sex, n (%) | < .001 | ||
| Male | 103 (33.8) | 903 (46.4) | |
| Female | 202 (66.2) | 1042 (53.6) | |
| Age, mean (SD) | 23.27 (2.92) | 23.08 (2.98) | 0.43 |
| Sleep Disturbance, mean (SD) | |||
| weekday TST (min) | 366.73 (92.47) | 452.28 (417.60) | < .001 |
| sleep latency (min) | 77.31 (56.60) | 33.65 (40.96) | < .001 |
| nocturnal awakenings | 3.44 (5.39) | 1.67 (2.87) | < .001 |
| back to sleep latency (min) | 32.70 (42.86) | 14.49 (26.81) | < .001 |
| symptom duration (months) | 53.80 (57.10) | 32.37 (50.70) | < .001 |
| symptom frequency (days/week) | 4.91 (1.32) | 2.10 (1.64) | < .001 |
| FIRST, mean (SD) | 23.86 (4.99) | 18.71 (5.23) | < .001 |
| CES-D, mean (SD) | 18.75 (10.25) | 9.82 (7.69) | < .001 |
To determine which covariates should be included in our primary analyses, we conducted preliminary, adjusted random-effects models to examine the independent effect of each covariate on depressive symptoms. All covariates (age, insomnia symptom duration, insomnia symptom frequency, TST, nocturnal awakenings, back to sleep latency, and SOL) were included as fixed effects in the same regression model. Notably, insomnia symptom frequency, SOL, back to sleep latency, and TST were significantly associated with depressive symptoms. Specifically, greater symptom frequency, b = 1.25, t(1786) = 9.89, p < .001, SOL, b = 2.83, t(1780) = 4.77, p < .001, back to sleep latency, b = 1.56, t(1780) = 3.69, p < .001, and lower TST, b = −4.03, t(1787) = −2.52, p = .01, were associated with greater depressive symptoms among the entire sample. All other covariates were unrelated to depressive symptoms, and therefore, only insomnia symptom frequency, SOL, back to sleep latency, and TST were included as sleep covariates in our final models.
Impact of Sleep Reactivity on Depressive Symptoms and Insomnia
We first examined whether sleep reactivity predicted individual differences in depressive symptoms and insomnia via separate main-effects models. These models included FIRST scores, sex, and all sleep covariates as fixed effects. Our data indicated that greater sleep reactivity was independently associated with greater depressive symptoms, b = 0.31, t(1837) = 7.90, p < .001, after controlling for sex and other relevant sleep covariates. Similarly, sleep reactivity was associated with insomnia, such that, the insomnia group reported significantly greater FIRST scores compared to the no-insomnia group, b = 0.01, t(1820) = 5.07, p < .001. Please refer to Table 3 for all model estimates.
Table 3.
FIRST, sex, and all relevant sleep parameters predicting depressive symptoms. Adjusted model included insomnia group status as an additional fixed effect.
| Estimates of Fixed Effects | |||
|---|---|---|---|
|
| |||
| β | SE | t-value | |
| Unadjusted Model | |||
| Sex | 0.37 | 0.40 | 0.94 |
| FIRST | 0.31 | 0.04 | 7.90*** |
| Symptom Frequency | 1.06 | 0.12 | 9.17*** |
| TST | −3.35 | 1.57 | −2.13* |
| SOL | 2.07 | 0.58 | 3.55*** |
| Back to Sleep Latency | 1.55 | 0.37 | 4.17*** |
| Adjusted Model | |||
| Sex | 0.36 | 0.39 | 0.90 |
| Insomnia | −3.36 | 0.59 | 5.67*** |
| FIRST | 0.28 | 0.04 | 7.26*** |
| Symptom Frequency | 0.79 | 0.12 | 6.33*** |
| TST | −2.39 | 1.57 | −1.52 |
| SOL | 1.71 | 0.58 | 2.93** |
| Back to Sleep Latency | 1.53 | 0.37 | 4.15*** |
p < .05;
p < .01,
p < .001.
Mediation Analysis
In order to examine whether insomnia significantly mediated the relationship between sleep reactivity and depressive symptoms, we first tested the impact of insomnia on depressive symptoms while controlling for the effect of sleep reactivity. Our adjusted model revealed that greater sleep reactivity, b = 0.28, t(1836) = 7.26, p < .001, and insomnia, b = 3.36, t(1816) = 5.67, p < .001, both predicted greater depressive symptoms (Figure 1). Furthermore, results from our mediation analysis suggested that insomnia also partially mediated the effect of sleep reactivity on depression, Sobel’s test = 3.78, p < .001 (Figure 2).
Figure 1.
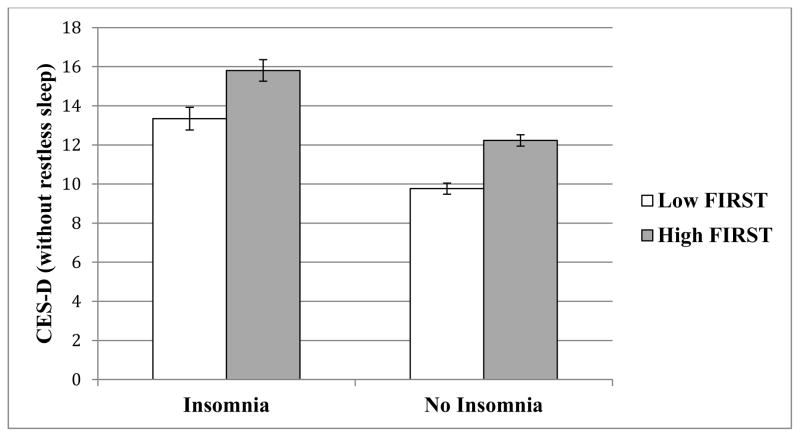
Mean depressive symptoms for insomnia and no insomnia groups represented as a function of FIRST scores. High (Mean = 24.1; SD = 3.4) and Low FIRST (Mean = 15.1; SD = 2.8) determined via median split for graphical representation only. FIRST scores range from 9 – 36. All reported group differences are significant at p < .001.
Figure 2.
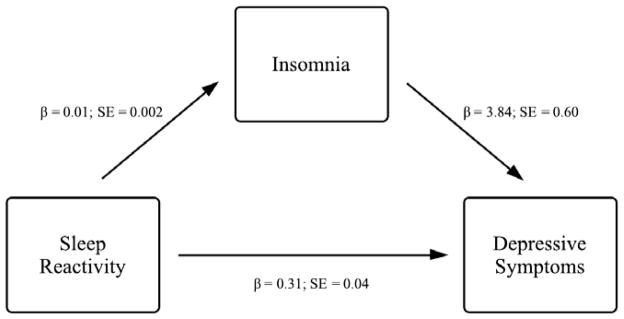
Insomnia as a mediator between sleep reactivity and depressive symptoms. Unstandardized beta estimates and standard errors for each step in the model are provided. All reported effects are significant at p < .001.
Discussion
In the current study, we examined the association between sleep reactivity and depressive symptoms among a sample of young adults with and without insomnia. Results showed that while insomnia partially mediated the association between sleep reactivity and depressive symptoms, the impact of sleep reactivity on depressive symptomatology was, in part, independent of insomnia. Therefore, these findings suggest that a vulnerability to stress-related sleep disturbances (i.e., sleep reactivity) may help explain some of the variability in depressive symptomatology, in addition to insomnia. These findings also have implications for identifying the common factors that increase vulnerability to both insomnia and depression.
Consistent with our hypothesis, greater sleep reactivity was significantly associated with elevated depressive symptoms in the current sample. Only one other study has examined the direct link between trait sleep reactivity and depressive symptomatology (Drake et al., 2014). However, that study used a DSM-IV diagnosis of insomnia and therefore, the present results extend to the more recent DSM-5 criteria which uses a more stringent duration criteria of a minimum of 3 months. Moreover, our results are also consistent with the conceptualization of depression as a disorder characterized by an increased sensitivity to stress and maladaptive responses to challenging life events (Monroe & Harkness, 2005). Specifically, the tendency for these types of maladaptive responses to stress (e.g., disturbed sleep) may be one mechanism that increases vulnerability to depression. For example, under stressful conditions, individuals with this vulnerability may be more likely to experience disturbed sleep. Greater sleep disturbances may subsequently lead to increased affective dysregulation, cognitive impairment, physiological hyperarousal, or other correlates of depression, given their demonstrated link to greater sleep difficulties (e.g. Fernandez-Mendoza et al., 2010; Williams, Cribbet, Rau, Gunn, & Czajkowski, 2013; Vargas & Lopez-Duran, 2014). Yet, given the methodological limitations of this study we are unable to answer these questions, and thus, future studies must further investigate the process by which sleep reactivity predisposes individuals to depression.
One other potential mechanism by which sleep reactivity leads to depression may be the shared link to insomnia. In fact, our data suggest that insomnia partially mediated the relationship between sleep reactivity and depressive symptoms. In particular, individuals with greater sleep reactivity were more likely to report having clinically significant insomnia symptoms, which in turn was associated with greater depressive symptoms. Prior research has consistently demonstrated a link between sleep reactivity and insomnia (Jefferson et al., 2005; Drake et al., 2011; Nakajima et al., 2014), as well a relationship between insomnia and depression (Riemann & Voderholzer, 2003; Baglioni et al., 2011). However, this is one of the first studies to suggest that the link between sleep reactivity and insomnia may be one pathway to depression. Yet, the partial mediation suggests the impact of sleep reactivity on depressive symptoms may not simply be a function of its impact on sleep. In contrast, these findings indicate that sleep reactivity is not specific to insomnia, and may increase vulnerability to other, related disorders, such as depression. Therefore, future studies should focus on gaining a better understanding of sleep reactivity as it may represent a more global vulnerability construct to stress-related disorders.
These findings should be considered in light of a number of study strengths and limitations. For example, the current study included a relatively large community-based sample of young adults. Whereas these findings may not necessarily generalize to older adults or children, the sample size does increase our confidence in the observed effects. Furthermore, the current study replicated previous findings shown in a relatively older sample (Drake et al., 2014). Our analyses also controlled for a number of potentially confounding variables (e.g., sex, sleep disturbance). By including these covariates in our models, this solidifies the independent effect of sleep reactivity on depressive symptoms. However, one potential confounding variable that was not examined in the current study is hypersomnia. While there is no study that has examined the association between sleep reactivity and hypersomnia, it is possible that sleep disturbance in response to stress is linked to hypersomnia as well, and thus may be an important area for future research. This was also the first study to examine the association between sleep reactivity and insomnia using DSM-5 criteria (American Psychiatric Association, 2013). Therefore, this study extends previous work (Drake et al., 2014) by replicating these finding using the latest diagnostic classifications. It is notable that while DSM-5 diagnostic criteria were used to determine insomnia group status, we did not establish diagnoses via standardized clinical interviews. The current study only included retrospective self-report measures of sleep disturbance (e.g., sleep onset latency, nocturnal awakenings, etc.) and depressive symptoms, which may be limited by reporter bias. Although clinical interviews and objective measurement of sleep could provide additional valuable information, insomnia is a symptom-based diagnosis that can be adequately tested via self-report questionnaires. Lastly, our findings are correlational, thus, causal inferences between sleep reactivity and depressive symptoms could not be determined. Additional studies examining the relationship between these variables prospectively are also needed in order to determine whether sleep reactivity predicts the future onset of a depressive episode.
In summary, these results advance our current understanding of the factors that potentially increase vulnerability to depressive symptomatology. Specifically, we provide some of the first evidence for the link between sleep reactivity and depressive symptoms, which was, in part, mediated by insomnia. While these findings suggest that a greater sensitivity to stress-related sleep disturbances may be associated with variability in depressive symptomatology, additional research is needed to confirm the prospective nature of this relationship. While this link can be partly explained by sleep reactivity being a predisposing factor for insomnia, our study revealed that greater sleep reactivity is also independently associated with greater depressive symptoms. Taken together, we highlight the need for a more comprehensive understanding of sleep reactivity’s role in psychopathology more broadly, as it may have implications for the treatment and prevention of insomnia and depression.
Acknowledgments
We thank Sally Ann Rhea for project management and Andy Gross for questionnaire implementation. This study was supported by Henry Ford Hospital and NIH grants MH063207 and DA011015 (NPF).
Footnotes
Disclosure Statement:
Mr. Vargas reported no financial interests/conflicts in this manuscript.
Dr. Friedman reported no financial interests/conflicts in this manuscript.
Dr. Drake has served as consultant for Teva. He has received research support from Merck and Teva. He has served on speakers bureau for Jazz, Purdue, and Teva.
References
- Abramson LY, Alloy LB, Hogan ME, Whitehouse WG, Donovan P, Rose DT, Raniere D. Cognitive vulnerability to depression: Theory and evidence. Journal of Cognitive Psychotherapy. 1999;13(1):5–20. [Google Scholar]
- Åkerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, Kecklund G. Sleep disturbances, work stress and work hours: A cross-sectional study. Journal of Psychosomatic Research. 2002;53(3):741–748. doi: 10.1016/S0022-3999(02)00333-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Riemann D. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders. 2011;135(1):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. Chronic insomnia and the stress system. Sleep Medicine Clinics. 2007;2(2):279–291. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert Ra, Merrill Ka, Braithwaite SR, Van Orden Ka, Joiner TE. Family life stress and insomnia symptoms in a prospective evaluation of young adults. Journal of Family Psychology. 2007;21(1):58–66. doi: 10.1037/0893-3200.21.1.58. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rössler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Raedt R, Koster EHW. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective & Behavioral Neuroscience. 2010;10(1):50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- Devins GM, Orme CM, Costello CG, Binik YM, Frizzell B, Stam HJ, Pullin WM. Measuring depressive symptoms in illness populations: Psychometric properties of the Center for Epidemiologic Studies Depression (CES-D) Scale. Psychology & Health. 1988;2(2):139–156. doi: 10.1016/S0022-3999(99)00004-5. [DOI] [Google Scholar]
- Drake CL, Friedman NP, Wright KP, Roth T. Sleep Reactivity and Insomnia: Genetic and Environmental Influences. Sleep. 2011;34(9):1179–1188. doi: 10.5665/sleep.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CL, Roth T. Predisposition in the evolution of insomnia: evidence, potential mechanisms, and future directions. Sleep Medicine Clinics. 2006;1(3):333–349. doi: 10.1016/j.jsmc.2006.06.005. [DOI] [Google Scholar]
- Drake C, Pillai V, Roth T. Stress and sleep reactivity: A prospective investigation of the stress-diathesis model of insomnia. Sleep. 2014;37(8):1295–1304. doi: 10.5665/sleep.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27(2):285–292. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- Drake CL, Scofield H, Roth T. Vulnerability to insomnia: the role of familial aggregation. Sleep Medicine. 2008;9(3):297–302. doi: 10.1016/j.sleep.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C, Vargas I, Roth T, Friedman NP. Quantitative Measures of Nocturnal Insomnia Symptoms Predict Greater Deficits Across Multiple Daytime Impairment Domains. Behavioral Sleep Medicine. 2014;12(1):1–15. doi: 10.1080/15402002.2014.880345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Mendoza J, Vela-Bueno A, Vgontzas AN, Ramos-Platón MJ, Olavarrieta-Bernardino S, Bixler EO, De la Cruz-Troca JJ. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosomatic Medicine. 2010;72(4):397–403. doi: 10.1097/PSY.0b013e3181d75319. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Moffitt TE, Poulton R. Family conflict in childhood: A predictor of later insomnia. Sleep. 2006;29(8):1063. doi: 10.1093/sleep/29.8.1063. [DOI] [PubMed] [Google Scholar]
- Haeffel GJ, Grigorenko EL. Cognitive vulnerability to depression: Exploring risk and resilience. Child and Adolescent Psychiatric Clinics of North America. 2007;16(2):435–448. doi: 10.1016/j.chc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Hall M, Buysse DJ, Nofzinger EA, Reynolds CF, III, Thompson W, Mazumdar S, Monk TH. Financial strain is a significant correlate of sleep continuity disturbances in late-life. Biological Psychology. 2008;77(2):217–222. doi: 10.1016/j.biopsycho.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Haynes PL, Ancoli-Israel S, Walter CM, McQuaid JR. Preliminary Evidence for a Relationship Between Sleep Disturbance and Global Attributional Style in Depression. Cognitive Therapy and Research. 2012;36(2):140–148. doi: 10.1007/s10608-011-9416-5. [DOI] [Google Scholar]
- Jarrin D, Chen I, Ivers H, Morin C. Does vulnerability to stress-related insomnia predict future incident and persistent insomnia among good sleepers? Sleep Medicine. 2013;14(1):e159–e160. doi: 10.1016/j.sleep.2013.11.368. [DOI] [Google Scholar]
- Jefferson C, Roehrs T, Roth T, Drake C. Sleep reactivity to stress in insomniacs. Proceedings of the 1st Congress of the World Association of Sleep Medicine; 2005. pp. 79–82. [Google Scholar]
- Lin Y-H, Jen C-H, Yang C-M. Information Processing during Sleep and Stress-Related Sleep Vulnerability. Psychiatry and Clinical Neurosciences. 2014:1–9. doi: 10.1111/pcn.12206. [DOI] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall M, Kamarck TW, Buysse DJ, Owens JF, Reis SE. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology. 2009;34(9):1346–1354. doi: 10.1016/j.psyneuen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychological Review. 2005;112(2):417. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosomatic Medicine. 2003;65(2):259–267. doi: 10.1097/01.PSY.0000030391.09558.A3. [DOI] [PubMed] [Google Scholar]
- Morris MC, Rao U. Cortisol response to psychosocial stress during a depressive episode and remission. Stress. 2014;17(1):51–58. doi: 10.3109/10253890.2013.857398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Rao U, Garber J. Cortisol responses to psychosocial stress predict depression trajectories: Social-evaluative threat and prior depressive episodes as moderators. Journal of Affective Disorders. 2012;143(1):223–230. doi: 10.1016/j.jad.2012.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Okajima I, Sasai T, Kobayashi M, Furudate N, Drake CL, Inoue Y. Validation of the Japanese version of the Ford Insomnia Response to Stress Test and the association of sleep reactivity with trait anxiety and insomnia. Sleep Medicine. 2013;15:196–202. doi: 10.1016/j.sleep.2013.09.022. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Medicine Reviews. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Caulet M, Lemoine P. Comorbidity of mental and insomnia disorders in the general population. Comprehensive Psychiatry. 1998;39(4):185–197. doi: 10.1016/S0010-440X(98)90059-1. [DOI] [PubMed] [Google Scholar]
- Pillai V, Roth T, Mullins HM, Drake CL. Moderators and Mediators of the Relationship Between Stress and Insomnia: Stressor Chronicity, Cognitive Intrusion, and Coping. Sleep. 2014;37(7):1199–1208. doi: 10.5665/sleep.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Rhea SA, Gross AA, Haberstick BC, Corley RP. Colorado twin registry – An Update. Twin Research and Human Genetics. 2013;16(1):351–357. doi: 10.1017/thg.2012.93.Colorado. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel BW, Lichstein KL. Insomnia and daytime functioning. Sleep Medicine Reviews. 2000;4(3):277–298. doi: 10.1053/smrv.1999.0074. [DOI] [PubMed] [Google Scholar]
- Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? Journal of Affective Disorders. 2003;76(1):255–259. doi: 10.1016/S0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- Roth T, Coulouvrat C, Hajak G, Lakoma MD, Sampson NA, Shahly V, Kessler RC. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders. Biological Psychiatry. 2011;69(6):592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociological Methodology. Washington DC: American Sociological Association; 1982. pp. 290–312. [Google Scholar]
- Sullivan PF, Neale M, Kendler KS. Genetic Epidemiology of Major Depression: Review and Meta-Analysis. American Journal of Psychiatry. 2000;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Vargas I, Lopez-Duran N. Dissecting the impact of sleep and stress on the cortisol awakening response in young adults. Psychoneuroendocrinology. 2014;40:10–16. doi: 10.1016/j.psyneuen.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29(5):645–649. doi: 10.1093/sleep/29.5.645. [DOI] [PubMed] [Google Scholar]
- Williams PG, Cribbet MR, Rau HK, Gunn HE, Czajkowski LA. The effects of poor sleep on cognitive, affective, and physiological responses to a laboratory stressor. Annals of Behavioral Medicine. 2013;46(1):40–51. doi: 10.1007/s12160-013-9482-x. [DOI] [PubMed] [Google Scholar]
- Yang CM, Chou CPW, Hsiao FC. The association of dysfunctional beliefs about sleep with vulnerability to stress-related sleep disturbance in young adults. Behavioral Sleep Medicine. 2011;9(2):86–91. doi: 10.1080/15402002.2011.557990. [DOI] [PubMed] [Google Scholar]