Abstract
Immunological diagnosis of Mycobacterium bovis infection of cattle is often confounded by cross-reactive responses resulting from exposure to other mycobacterial species, especially Mycobacterium avium. Early secretory antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10) are dominant gamma interferon (IFN-γ)-inducing antigens of tuberculous mycobacteria, and they are absent from many environmental nontuberculous mycobacteria. Because M. avium exposure is the primary confounding factor in the diagnosis of M. bovis-infected animals, in vitro responses to a recombinant ESAT-6:CFP-10 (rESAT-6:CFP-10) fusion protein by blood leukocytes from cattle naturally exposed to M. avium or experimentally challenged with Mycobacterium avium subsp. avium or Mycobacterium avium subsp. paratuberculosis were compared to responses by M. bovis-infected cattle. Responses to heterogeneous mycobacterial antigens (i.e., purified protein derivatives [PPDs] and whole-cell sonicates [WCSs]) were also evaluated. Tumor necrosis factor alpha (TNF-α), IFN-γ, and nitric oxide responses by M. bovis-infected cattle to rESAT-6:CFP-10 exceeded (P < 0.05) the corresponding responses by cattle naturally sensitized to M. avium. Experimental infection with M. bovis, M. avium, or M. avium subsp. paratuberculosis induced significant (P < 0.05) IFN-γ and nitric oxide production to WCS and PPD antigens, regardless of the mycobacterial species used for the preparation of the antigen. Responses to homologous crude antigens generally exceeded responses to heterologous antigens. Nitric oxide and IFN-γ responses to rESAT-6:CFP-10 by blood leukocytes from M. bovis-infected calves exceeded (P < 0.05) the corresponding responses of noninfected, M. avium-infected, and M. avium subsp. paratuberculosis-infected calves. Despite the reported potential for secretion of immunogenic ESAT-6 and CFP-10 proteins by M. avium and M. avium subsp. paratuberculosis, it appears that use of the rESAT-6:CFP-10 fusion protein will be useful for the detection of tuberculous cattle in herds with pre-existing sensitization to M. avium and/or M. avium subsp. paratuberculosis.
Mycobacterium bovis, a member of the Mycobacterium tuberculosis complex, has a wide host range compared to those of other species in this disease complex, is infectious to humans, and is the species most often isolated from tuberculous cattle. Control of M. bovis in cattle is particularly difficult due to the presence of wildlife reservoirs, such as white-tailed deer, European badgers, and brush-tailed possums. Recently, there has been an increase in the prevalence of M. bovis infection in the United States. Detection of tuberculous cattle in Michigan, California, Texas, and New Mexico has resulted in the loss of the tuberculosis (TB)-free designation for these states (or portions thereof) and subsequent economic losses from increased TB testing costs and hindrances of interstate shipment of livestock from these zones. The demand for improved diagnostic capabilities is again being realized and emphasized with the increased TB testing in the United States. Tests currently approved for the detection of bovine TB include measurement of delayed-type hypersensitivity responses (i.e., skin testing) to purified protein derivative (PPD) antigens and an in vitro assay for gamma interferon (IFN-γ) produced in response to PPD stimulation (i.e., Bovigam; Biocor Animal Health, Omaha, Nebr.). A major limitation of these tests is the cross-reactivity of M. bovis PPD (PPDb) with responses induced by exposure to related bacteria, especially Mycobacterium avium subsp. avium (referred to hereafter as M. avium) and Mycobacterium avium subsp. paratuberculosis.
Early secretory antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10) are IFN-γ-inducing antigens of tuberculous mycobacteria. ESAT-6 and CFP-10 are cosecreted proteins that naturally form a tight 1:1 complex upon export (14). Genes for these proteins are absent in many environmental, nontuberculous mycobacteria as well as in the TB vaccine strain, M. bovis BCG. Use of ESAT-6 and/or CFP-10 for the stimulating antigen in IFN-γ-based TB assays enhances the specificity over the specificity of the standard test using PPDb as the stimulating antigen (3, 4, 11, 12, 16). Unfortunately, esat-6 and cfp-10 are present in a subset of nontuberculous mycobacteria, most notably Mycobacterium kansasii, Mycobacterium marinum, Mycobacterium leprae, and Mycobacterium smegmatis (6, 7, 8; N. C. Gey van Pittius, R. M. Warren, and P. D. van Helden, Letter, Infect. Immun. 70:6509-6510, 2002). Of particular relevance for diagnostic issues concerning bovine TB in the United States, esat-6 and cfp-10 have been reported to be present in M. avium and M. avium subsp. paratuberculosis (8). A search of the complete genome of M. avium subsp. paratuberculosis strain K10 (genome accession no. NC_002944) and M. avium strain 104 (http://www.tigr.org), however, did not reveal any sequences with similarity to esat-6 or cfp-10. Considering this discrepancy, the objective of the present study was to determine whether a recombinant ESAT-6:CFP-10 (rESAT-6:CFP-10) fusion protein can be used to differentially detect M. bovis-infected cattle from cattle infected with M. avium or M. avium subsp. paratuberculosis.
MATERIALS AND METHODS
Animals, challenge procedures, and bacterial culture.
Cattle were obtained from TB-free herds. The study consisted of two experiments. In the first experiment, groups consisted of Hereford cattle from a herd with a history of widespread exposure to M. avium (n = 8) and M. bovis-challenged crossbred beef cattle (n = 8). Calves in this portion of the study were 10 months of age at the time of sample collection. Sixty percent (30 of 50) of adult cows and 78% (32 of 41) of preweaned calves in the herd of origin of the M. avium-exposed calves had positive IFN-γ responses to M. avium PPD (i.e., IFN-γ response with M. avium PPD [PPDa] stimulation minus IFN-γ response with no stimulation equals ≥0.05 optical density unit) (Bovigam; Biocor Animal Health). Calves in the M. bovis-challenged group were tested and confirmed negative for M. bovis and M. avium exposure prior to challenge (Bovigam; Biocor Animal Health). The M. avium-exposed cattle were housed outdoors, whereas M. bovis-infected cattle were housed within a biosafety level 3 confinement facility. For M. bovis infection, the challenge inoculum, 2.4 × 106 CFU in 0.2 ml of phosphate-buffered saline (PBS) (0.15 M, pH 7.2), was instilled directly into the tonsillar crypts of anesthetized cattle as previously described for inoculation of white-tailed deer (10). The M. bovis strain used for the challenge inoculum (95-1315, U.S. Department of Agriculture, Animal Plant and Health Inspection Service designation) was originally isolated from a white-tailed deer in Michigan (15). Inoculum consisted of mid-log-phase M. bovis grown in Middlebrook 7H9 medium supplemented with 10% oleic acid-albumin-dextrose complex (Difco, Detroit, Mich.) plus 0.05% Tween 80 (Sigma Chemical Co., St. Louis, Mo.). Lesions typical of M. bovis infection were detected in M. bovis-inoculated animals, and infection was confirmed by isolation of M. bovis from tissues.
In the second experiment, groups consisted of nonchallenged calves (n = 4), M. avium-challenged calves (n = 4), M. avium subsp. paratuberculosis-challenged calves (n = 3), and M. bovis-challenged calves (n = 4). Calves (males, castrated at ∼3 months of age) were obtained from Holstein herds free of TB and M. avium subsp. paratuberculosis at 2 to 4 days of age and housed indoors in temperature-controlled rooms (18 to 24°C) (biosafety level 3 facility for M. bovis-infected calves). M. bovis (strain 95-1315) and M. avium (strain 3988; bovine isolate) for challenge inoculum were grown in Middlebrook 7H9 medium (National Animal Disease Center, Ames, Iowa) supplemented with 10% oleic acid-albumin-dextrose complex plus 0.05% Tween 80 (Sigma Chemical Co.). Medium for M. avium subsp. paratuberculosis (strain K10; cattle isolate) was additionally supplemented with 2 mg of mycobactin J (Allied Monitor Inc., Fayette, Mo.) per liter. Challenge inoculum (∼104 CFU of M. bovis, ∼1010 CFU of M. avium, or ∼108 CFU of M. avium subsp. paratuberculosis) was instilled directly into the tonsillar crypts of sedated calves as described previously (19). Calves in M. avium subsp. paratuberculosis- and M. avium-challenged groups received four weekly doses of inoculum for a total dose of ∼4 × 1010 CFU of M. avium or ∼4 × 108 CFU of M. avium subsp. paratuberculosis.
Cell culture and antigens.
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coat fractions of samples of peripheral blood collected in 2× acid-citrate-dextrose (5). Individual wells of 96-well round-bottom microtiter plates (Falcon; Becton-Dickinson, Lincoln Park, N.J.) were seeded with 2 × 105 PBMC in a total volume of 200 μl per well. The medium was RPMI 1640 medium (GIBCO, Grand Island, N.Y.) supplemented with 2 mM l-glutamine, 25 mM HEPES buffer, 100 U of penicillin per ml, 0.1 mg of streptomycin per ml, 1% nonessential amino acids (Sigma), 2% essential amino acids (Sigma), 1% sodium pyruvate (Sigma), 50 μM 2-mercaptoethanol (Sigma), and 10% (vol/vol) fetal bovine serum. Wells contained medium plus 5 μg of PPDb (CSL Ltd.) per ml, 5 μg of PPDa (CSL Ltd.) per ml, 20 μg of M. bovis strain 95-1315 whole-cell sonicate (WCS) per ml, 20 μg of M. avium strain 3988 WCS per ml, 20 μg of M. avium subsp. paratuberculosis strain K10 WCS per ml, and 10 μg of rESAT-6:CFP-10 per ml or medium alone (no stimulation). Antigens consisting of sonicates of the challenge strains were prepared as described previously (17). After incubation of PBMC cultures for 48 h at 37°C in 5% CO2, culture supernatants were harvested and stored at −80°C until thawed for analysis.
Cloning and expression of rESAT-6:CFP-10 fusion protein.
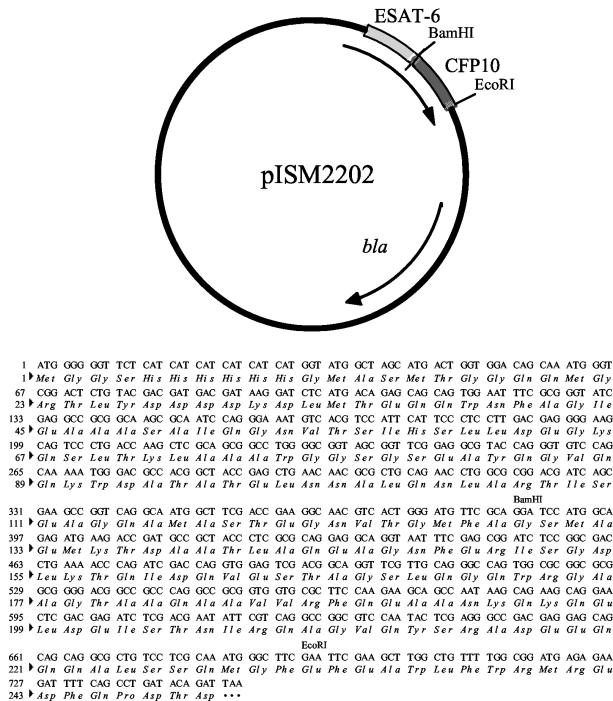
CFP10 was amplified from M. bovis genomic DNA with primers CFP10F (5′-AAGGATCCATGGCAGAGATGAAGACC) and CFP10R (5′-AAGAAGAATTCGAAGCCCATTTGCGAGGA) to incorporate BamHI and EcoRI restriction sites. The PCR products were ligated into pCR2.1 (Invitrogen, Carlsbad, Calif.) and transformed into Escherichia coli TOP10F′ chemically competent cells [mcrA Δ(mcrCB-hsdSMR-mrr) (φ80 lacZΔM15) Δ(lac)X74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL endA1 nupG F′(Tetr)]. Plasmid DNA was isolated using Qiaspin miniprep system (Qiagen, Valencia, Calif.) and screened by DNA sequencing. The appropriate plasmid was designated pISM2204. The expression vector pISM404 (pTrcHisB:ESAT-6) (9) and pISM2204 were digested with BamHI and EcoRI. The digested DNA was gel purified, ligated, and transformed into Escherichia coli DH5α cells [supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1]. Recombinant plasmids were sequenced to confirm the insertion, and the final plasmid was designated pISM2202 (Fig. 1).
FIG. 1.
Map of pISM2202 and sequence of ESAT-6:CFP10 fusion protein.
rESAT-6:CFP10 protein was purified from a 200-ml 2× yeast tryptone ampicillin (50 μg/ml) culture, induced to an optical density at 600 nm of 0.6 with 1 mM isopropyl thiogalactoside for 4 h at 37°C. Cells were lysed in 20 ml of lysis buffer (20 mM Tris [pH 8.0], 100 mM NaCl, 8 M urea) and then sonicated. Proteins were purified on 10 ml of Ni-nitrilotriacetic acid resin (Sigma) using the Biologic HR chromatography system (Bio-Rad, Hercules, Calif.). The resin was washed with 10 ml of water and then with 25 ml of lysis buffer, and the cell lysate was added. Nonspecific proteins were washed through the column with 60 ml of lysis buffer and then with 30 ml of lysis buffer containing 10 mM imidazole. The protein was eluted with 30 ml of lysis buffer containing 200 mM imidazole. Fractions were collected every 5 ml and analyzed by Western blotting against the six-histidine tag (6X His Tag; Clontech, Palo Alto, Calif.) (9). Fractions were then dialyzed overnight at 4°C in PBS and quantified by the Bradford assay.
IFN-γ assay.
Heparinized blood samples were dispensed in 1.5-ml aliquots into individual wells of a 24-well plate (Falcon 353047; Becton Dickinson and Company, Franklin Lakes, N.J.). Blood cultures were incubated for 48 h. Plasma was harvested and stored at −80°C. IFN-γ concentrations in stimulated plasma (i.e., whole-blood assay) or supernatants were determined using a commercial enzyme-linked immunosorbent assay (ELISA)-based kit (Bovigam; Biocor Animal Health). Absorbencies of standards and test samples were read at 450 nm using an ELISA plate reader (Molecular Devices, Menlo Park, Calif.). In addition, IFN-γ concentrations in supernatants (three pooled replicate samples) from PBMC cultures were analyzed. Concentrations (in nanograms per milliliter) in test samples were quantified by comparing absorbencies of test samples with absorbencies of standards within a linear curve fit. Duplicate samples for individual treatments were analyzed.
Griess reaction assay.
The amount of nitrite, the stable oxidation product of nitric oxide (NO), in culture supernatants is a correlate of the amount of NO produced by cells in culture. Nitrite was measured using the Griess reaction (13) performed in 96-well microtiter plates (Immunolon 2; Dynatech Laboratories, Inc., Chantilly, Va.). Culture supernatant (100 μl) was mixed with 100 μl of Griess reagent (0.5% sulfanilamide; Sigma) in 2.5% phosphoric acid (Mallinckrodt Chemicals, Inc., Paris, Ky.) and 0.05% N-(1-naphthyl) ethylenediamine dihydrochloride (Sigma). The mixture was incubated at 21°C for 10 min. Absorbencies of test and standard samples at 550 nm were measured using an automated ELISA plate reader. Samples were diluted in culture medium (RPMI 1640 medium with 2 mM l-glutamine and 10% fetal bovine serum [vol/vol]). Absorbencies of standards and test samples were converted to nanograms of nitrite per milliliter by comparing them to absorbencies of sodium nitrite (Fisher Chemicals, Fair Lawn, N.J.) standards within a linear curve fit. Assays were run on three sets of pooled triplicates for each treatment.
TNF-α assay.
Tumor necrosis factor alpha (TNF-α) was measured using a capture ELISA (protocol and reagents provided by L. Babiuk, Veterinary Infectious Diseases Organization, Saskatoon, Saskatchewan, Canada). TNF-α assays were performed in Immunolon II microtiter plates (Dynatech Laboratories, Inc.). Reagents consisted of a capture antibody (mouse ascitic fluid anti-bovine-TNF-α, immunoglobulin G [IgG] fraction), detection antibody (rabbit anti-bovine-TNF-α, IgG fraction), recombinant bovine TNF, biotinylated goat anti-rabbit IgG (Zymed Laboratories, Inc., South San Francisco, Calif.), horseradish peroxidase-conjugated streptavidin-biotinylated complex (Amersham Corporation, Arlington Heights, Ill.), substrate (H2O2 at a concentration of 0.1% [vol/vol]), and dye (2,2′-azinodi-ethylbenzothiazoline-sulfonic acid). Internal standards consisted of recombinant bovine TNF-α diluted serially in PBS (pH 7.2, 0.01 M) supplemented with Tween 80 (0.1% [vol/vol]) and gelatin (0.5% [vol/vol]) (PBST-g). Positive and negative controls and test samples were also diluted serially in PBST-g. Capture antibody was diluted (1:1,000 [vol/vol]) in carbonate buffer (pH 9.6, 0.01 M), and detection antibody was diluted in PBST-g (1:1,500 [vol/vol]). Biotinylated goat anti-rabbit Ig was diluted 1:10,000, and horseradish peroxidase-conjugated streptavidin-biotinylated complex was diluted 1:2,000 in PBST without gelatin. Intervening washes were done with PBST without gelatin. Enzyme substrate and indicator dye were diluted in citrate buffer. Incubations were at room temperature with the exception of capture antibody in carbonate buffer, which was incubated at 4°C. Absorbencies of standards and test samples was read at 405 and 490 nm using an ELISA plate washer and reader (Dynatech MR7000). TNF-α concentrations (in nanograms per milliliter) in test samples were determined by comparing the absorbencies of test samples with the absorbencies of standards within a linear curve fit.
Delayed-type hypersensitivity responses (i.e., skin testing).
Nine months after challenge, calves were tested for in vivo responsiveness to mycobacterial antigens using a modified, comparative cervical skin test as described previously (18). Animals were injected with 100 μl (1 mg/ml) each of PPDb, PPDa, and M. avium subsp. paratuberculosis PPD number 0202 (National Veterinary Services Laboratory, Ames, Iowa) intradermally. Data are presented as skin thickness (in millimeters) 72 h after injection of PPD minus preinjection skin thickness.
Statistics.
Data were assessed for normality prior to statistical analysis. Arithmetic and log10-transformed data were analyzed as a split plot with repeated-measure analysis of variance using Statview software (version 5.0; SAS Institute, Inc., Cary, N.C.). The statistical model included effects of treatments (infection type and recall antigen), time (months relative to establishment of infection), and the interaction of treatment and time on IFN-γ, TNF-α, and NO production by whole blood or enriched PBMC cultures. Scheffe's test was applied when effects (P < 0.05) detected by the model were significant (P ≤ 0.05).
RESULTS
Responses to rESAT-6:CFP-10 fusion protein by cattle naturally sensitized to M. avium. Natural exposure of cattle to M. avium was determined by detection of recall IFN-γ responses to PPDa. Seven of eight calves exposed to M. avium had positive IFN-γ responses to PPDa (i.e., IFN-γ response with PPDa stimulation minus IFN-γ response with no stimulation equals ≥0.05 optical density unit). IFN-γ responses to PPDa may also be indicative of M. avium subsp. paratuberculosis infection; however, fecal samples from cattle exposed to M. avium and adult members of their herd were negative for M. avium subsp. paratuberculosis growth using standard M. avium subsp. paratuberculosis culture techniques (19). IFN-γ responses by PBMC from M. avium-exposed calves to PPDa (7.5 ng/ml ± 3.0 ng/ml) exceeded (P < 0.01) parallel responses to PPDb (−2.4 ng/ml ± 1.1 ng/ml) (Table 1). Responses of M. bovis-infected calves to PPDa exceeded (P < 0.05) those of M. avium-exposed calves, indicating vigorous responses induced by M. bovis infection and the cross-reactivity of PPDs. As expected, PPDb-induced IFN-γ production by M. bovis-infected calves (55.2 ng/ml ± 9.7 ng/ml) exceeded (P < 0.01) responses elicited by PPDa (17.3 ng/ml ± 3.0 ng/ml). IFN-γ responses of M. bovis-infected calves to rESAT-6:CFP-10 also exceeded (P < 0.01) those of calves exposed to M. avium. Most importantly, stimulation of PBMC from M. avium-exposed calves with rESAT-6:CFP-10 did not elicit a significant IFN-γ response (Table 1). TNF-α and NO responses to PPDa, PPDb, and rESAT-6:CFP-10 were qualitatively similar to IFN-γ responses (Tables 2 and 3).
TABLE 1.
Comparison of IFN-γ responses by PBMC from M. bovis-infected cattle to responses by cattle naturally sensitized to M. avium
| Group | IFN-γ responsea to recall antigen:
|
||
|---|---|---|---|
| PPDa | PPDb | rESAT-6:CFP-10 | |
| M. bovis-infected (6 mo postinfection) | 17.3b (3.0) | 55.2c (9.7) | 29.1c (6.1) |
| M. avium-exposed (natural exposure) | 7.5 (3.0) | −2.4 (1.1) | −2.9 (0.7) |
Values are the mean IFN-γ responses (in nanograms per milliliter) (n = 8) of PBMC to antigen stimulation minus their response to medium alone after 48 h. Values in parentheses are the standard errors of the means.
Significantly different from the response by M. avium-exposed cattle (P < 0.05).
Significantly different from the response by M. avium-exposed cattle (P < 0.01).
TABLE 2.
Comparison of TNF-α responses by PBMC from M. bovis-infected cattle to responses by cattle naturally sensitized to M. avium
| Group | TNF-α responsea to recall antigen:
|
||
|---|---|---|---|
| PPDa | PPDb | rESAT-6:CFP-10 | |
| M. bovis-infected (6 mo postinfection) | 0.316 (0.084) | 1.370b (0.524) | 0.790b (0.285) |
| M. avium-exposed (natural exposure) | 0.164 (0.048) | 0.056 (0.052) | 0.038 (0.095) |
Values are the mean TNF-α responses (in nanograms per milliliter) (n = 8) of PBMC to antigen stimulation minus their response to medium alone after 48 h. Values in parentheses are the standard errors of the means.
Significantly different from the response by M. avium-exposed cattle (P < 0.05).
TABLE 3.
Comparison of NO responses by PBMC from M. bovis-infected cattle to responses by cattle naturally sensitized to M. avium
| Group | NO responsea to recall antigen:
|
||
|---|---|---|---|
| PPDa | PPDb | rESAT-6:CFP-10 | |
| M. bovis-infected (6 mo postinfection) | 196.1b (46.1) | 403.3b (86.4) | 415.7b (98.4) |
| M. avium-exposed (natural exposure) | 49.1 (9.1) | −4.5 (9.9) | −19.0 (10.1) |
Values are the mean NO responses (in nanograms per milliliter) (n = 8) of PBMC to antigen stimulation minus their response to medium alone after 72 h. Values in parentheses are the standard errors of the means.
Significantly different from the response by M. avium-exposed cattle (P < 0.01).
Sensitization upon intratonsillar challenge.
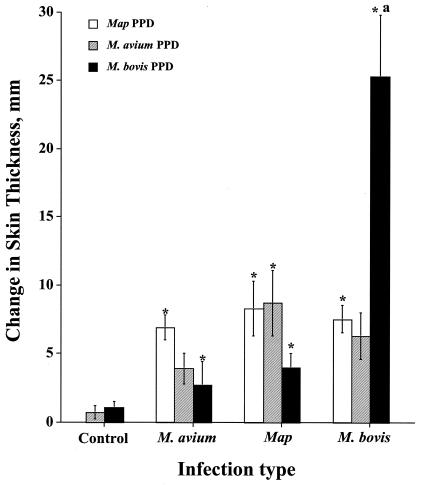
Because the dose and duration of natural exposure to M. avium are not amenable to determination or control, an experimental model of M. avium infection was developed by our group for comparison to responses induced by experimental M. bovis infection (19). At 9 months postchallenge, cutaneous delayed-type hypersensitivity responses to PPDs were detected (Fig. 2). With the exception of PPDb-induced responses by M. bovis-infected calves, responses to the various PPDs were similar, indicating cross-reactivity. Recall IFN-γ and NO responses by M. bovis-infected calves to M. bovis WCS exceeded (P < 0.05) concurrent responses to M. avium and M. avium subsp. paratuberculosis WCSs and responses induced by experimental M. avium and M. avium subsp. paratuberculosis infection to M. bovis WCS (Tables 4 and 5). Likewise, IFN-γ and NO responses by M. avium-infected calves to M. avium WCS exceeded (P < 0.05) concurrent responses to M. bovis WCS and M. avium subsp. paratuberculosis WCS and responses by noninfected calves to M. avium WCS (Tables 4 and 5). Responses of M. avium- and M. avium subsp. paratuberculosis-infected calves to M. avium and M. avium subsp. paratuberculosis WCSs did not differ, reflecting the many conserved antigens of these two closely related subspecies. Analysis of IFN-γ levels in PBMC supernatants and whole-blood cultures revealed similar effects of antigen stimulation and infection status (data not shown).
FIG. 2.
Delayed-type hypersensitivity responses elicited by PPDs upon experimental infection. PPDs from M. avium, M. avium subsp. paratuberculosis (Map), and M. bovis were used. Each value is the mean change (in milliliters) in skin thickness ± standard error of the mean (error bar) 72 h after injection of PPDs. Skin tests were performed approximately 9 months postchallenge. Values that were statistically significantly different (P < 0.05) from the response by control cattle (asterisk) are indicated. The response to PPDb by M. bovis-infected cattle that is statistically significantly different (P < 0.05) from concurrent responses to M. avium subsp. paratuberculosis PPD and PPDa is also indicated by the letter a.
TABLE 4.
IFN-γ responses to crude mycobacterial antigens upon experimental infection
| Infection status | IFN-γ responsea to recall antigen (WCS):
|
||
|---|---|---|---|
| M. bovis strain 1315 | M. avium strain 3988 | M. avium subsp. paratuberculosis strain K10 | |
| Noninfected | −0.7 (0.4) A | −1.0 (0.4) A | −0.8 (0.4) A |
| M. bovis-infected | 60.0b (2.5) C | 15.2 (0.9) B | 9.2 (0.7) B |
| M. avium-infected | 13.1 (2.4) B | 29.6b (2.9) C | 16.8 (2.5) C |
| M. avium subsp. paratuberculosis-infected | 17.0 (5.2) B | 22.7 (7.5) BC | 30.9 (11.6) BC |
IFN-γ responses (whole-blood assay) were unaffected (P > 0.05) by the length of postinfection period. For this reason, values are the mean IFN-γ responses (in nanograms per milliliter) (i.e., response to 20 μg of antigen per ml) minus their response to medium alone after 48 h) during the period spanning 4 to 7 months postinfection. Treatment groups consisted of four animals except for the M. avium subsp. paratuberculosis-infected group, which had three animals. Values in parentheses are the standard errors of the means.
Letters A to C indicate that the treatment means for a specific type of stimulation (i.e., vertical comparisons) differ (P < 0.05). The same letter indicates that the IFN-γ responses were not significantly different (P < 0.05).
Significantly different from the responses by the other cattle infected with M. bovis or M. avium (i.e., horizontal comparisons) (P < 0.05).
TABLE 5.
Nitric oxide responses to crude mycobacterial antigens upon experimental infection
| Infection status | NO responsea to recall antigen (WCS):
|
||
|---|---|---|---|
| M. bovis strain 1315 | M. avium strain 3988 | M. avium subsp. paratuberculosis strain K10 | |
| Noninfected | −106 (53) A | −49 (51) A | −63 (43) A |
| M. bovis-infected | 451b (46) C | 184 (21) B | 97 (35) AB |
| M. avium-infected | 79 (17) B | 185b (20) B | 55 (14) AB |
| M. avium subsp. paratuberculosis-infected | 87 (52) AB | 213 (61) B | 278 (117) B |
NO responses by PBMC were unaffected (P > 0.05) by the length of postinfection period. For this reason, values are the mean NO responses (in nanograms per milliliter) (i.e., response to 20 μg of antigen per ml minus their response to medium alone after 72 h) during the period spanning 5 to 7 months postinfection. Treatment groups consisted of four animals except for the M. avium subsp. paratuberculosis-infected group, which had three animals. Values in parentheses are the standard errors of the means.
Letters A to C indicate that the treatment means for a specific type of stimulation (i.e., vertical comparisons) differ (P < 0.05). The same letter indicates that the NO responses were not significantly different (P < 0.05).
Significantly different from the responses by the other cattle infected with M. bovis or M. avium (i.e., horizontal comparisons) (P < 0.05).
Differential diagnosis using rESAT-6:CFP-10 fusion protein.
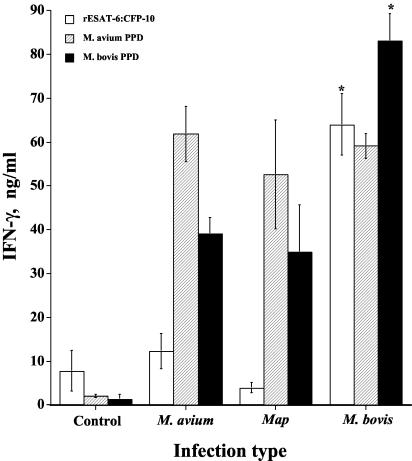
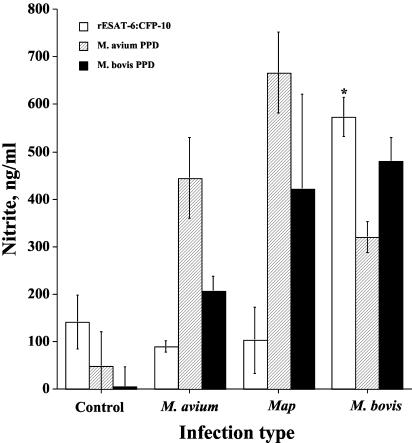
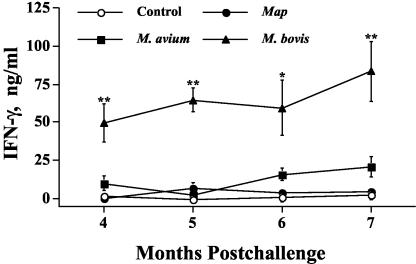
Differential IFN-γ responses to PPDa and PPDb are currently used for M. bovis diagnosis (Bovigam; Biocor Animal Health). For this reason, responses to rESAT-6:CFP-10 were compared with responses to PPDa and PPDb (Fig. 3 and 4). IFN-γ responses of M. bovis-infected calves to PPDb exceeded (P < 0.05) those of noninfected and M. avium- and M. avium subsp. paratuberculosis-infected calves (Fig. 3). IFN-γ and NO responses to PPDb, however, were induced by M. avium and M. avium subsp. paratuberculosis infection. Responses of these calves to PPDb exceeded (P < 0.05) those of noninfected calves (Fig. 3 and 4). NO responses of M. bovis-infected calves to PPDb exceeded (P < 0.05) those of noninfected and M. avium-infected calves (Fig. 4). IFN-γ and NO responses of M. bovis-, M. avium-, and M. avium subsp. paratuberculosis-infected calves to PPDa were comparable and exceeded (P < 0.05) those of noninfected calves. IFN-γ and NO responses of M. bovis-infected calves to rESAT-6:CFP-10 exceeded those of noninfected and M. avium- and M. avium subsp. paratuberculosis-infected calves (Fig. 3 and 4). IFN-γ and NO responses by M. avium- and M. avium subsp. paratuberculosis-infected calves to rESAT-6:CFP-10, in contrast to stimulation with PPDb, did not exceed (P < 0.05) those of noninfected calves. IFN-γ responses of M. bovis-infected calves to rESAT-6:CFP-10 were not different (P > 0.05) at 4, 5, 6, and 7 months postinfection (Fig. 5).
FIG. 3.
IFN-γ responses upon experimental infection. IFN-γ responses (whole-blood assay) were unaffected (P > 0.05) by length of postinfection period. For this reason, each value represents the mean IFN-γ response (in nanograms per milliliter) (i.e., the response to antigen minus the response to medium alone after 48 h) during the period spanning 4 to 7 months postinfection ± standard error of the mean (error bar) (n = 4). The responses by M. bovis-infected animals that differ significantly (P < 0.05) from the responses by control animals and by M. avium- and M. avium subsp. paratuberculosis (Map)-infected animals for the particular antigen stimulation are indicated (asterisk).
FIG. 4.
Nitric oxide responses upon experimental challenge. NO responses by PBMC were unaffected (P > 0.05) by length of postinfection period. For this reason, each value represents the mean NO response (in nanograms per milliliter) (i.e., the response to antigen minus the response to medium alone after 72 h) ± standard error of the mean (error bar) during the period spanning 5 to 7 months postinfection (n = 3). The response by M. bovis-infected animals that differs significantly (P < 0.05) from the responses by control animals and by M. avium- and M. avium subsp. paratuberculosis (Map)-infected animals for the particular antigen stimulation is indicated (asterisk).
FIG. 5.
Longitudinal IFN-γ response to rESAT-6:CFP-10 fusion protein. Values indicate the mean responses to rESAT-6:CFP-10 stimulation (48 h) (in nanograms per milliliter) minus the response to medium alone ± standard error of the mean (error bars). Responses by M. bovis-infected animals that were significantly different (P < 0.05) from the responses by control animals and by M. avium- and M. avium subsp. paratuberculosis (Map)-infected animals are indicated (**). The response by M. bovis-infected animals that was significantly different from the responses by control animals (P < 0.05), M. avium-infected animals (P = 0.08), and M. avium subsp. paratuberculosis-infected animals (P < 0.05) is indicated (*).
DISCUSSION
Searches of publicly available bacterial genome sequences (partial or complete) have indicated that the ESAT-6 gene cluster (including esat-6, cfp-10, and flanking secretory apparatus genes) is of ancient origin, present in a subset of mycobacteria, and highly conserved (8). Proteins encoded by this gene cluster evoke potent T-cell responses that have been utilized in TB diagnostic tests (2-4, 11, 12, 16). In particular, use of these antigens enhances the specificity of IFN-γ-based tests over standard PPDs as the eliciting agent. However, humans infected with M. leprae, M. marinum, or M. kansasii exhibit recall IFN-γ responses to ESAT-6 and/or CFP-10, indicating that T-cell responses to these two proteins are not invariably specific for infection with M. tuberculosis complex mycobacteria (1, 6, 7; Gey van Pittius et al., letter). The reported presence of esat-6 and cfp-10 in M. avium and M. avium subsp. paratuberculosis is of particular concern for TB diagnosis in cattle (8). Recent studies have indicated that cattle with responses to PPDa resulting from natural exposure do not exhibit recall IFN-γ responses to ESAT-6 and CFP-10 antigens (11, 16). The dose and agents inducing the response to PPDa, however, are impossible to characterize under conditions of natural exposure. Additionally, natural exposure may result in inhibitory interactions due to exposure to multiple species of mycobacteria and/or repeated doses of antigen that induce tolerance.
In the present study, responses to ESAT-6:CFP-10 were not induced upon experimental inoculation of calves with doses of M. avium or M. avium subsp. paratuberculosis that clearly sensitized these animals to the respective mycobacterium. The challenge inoculum was delivered to neonatal calves housed in environmentally controlled rooms thereby limiting confounding interactions due to exposure to other mycobacteria. M. bovis-infected calves exhibited responses to rESAT-6:CFP-10 equal to those induced by stimulation with PPDb. These findings indicate that rESAT-6:CFP-10 should prove useful for the specific diagnosis of bovine TB, even under conditions of high exposure to M. avium or M. avium subsp. paratuberculosis.
Acknowledgments
Funds for this research were provided, in part, by grants from the U.S. Department of Agriculture and Animal Plant and Health Inspection Service.
We thank Shelly Zimmerman, Jessica Pollock, Jody Mentele, Peter Lasley, Janis Hansen, Don McDorman, and Trudy Bosworth for excellent technical support.
REFERENCES
- 1.Arend, S. M., K. E. van Meijgaarden, K. de Boer, E. C. de Palou, D. van Soolingen, T. H. Ottenhoff, and J. T. van Dissel. 2002. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J. Infect. Dis. 186:1797-1807. [DOI] [PubMed] [Google Scholar]
- 2.Arend, S. M., T. H. Ottenhoff, P. Andersen, P., and J. T. van Dissel. 2001. Uncommon presentations of tuberculosis: the potential value of a novel diagnostic assay based on the Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:680-686. [PubMed] [Google Scholar]
- 3.Buddle, B. M., A. R. McCarthy, T. J. Ryan, J. M. Pollock, H. M. Vordermeier, R. G. Hewinson, P. Andersen, and G. W. de Lisle. 2003. Use of mycobacterial peptides and recombinant proteins for the diagnosis of bovine tuberculosis in skin test-positive cattle. Vet. Rec. 153:615-620. [DOI] [PubMed] [Google Scholar]
- 4.Buddle, B. M., T. J. Ryan, J. M. Pollock, P. Andersen, and G. W. de Lisle. 2001. Use of ESAT-6 in the interferon-gamma test for diagnosis of bovine tuberculosis following skin testing. Vet. Microbiol. 80:37-46. [DOI] [PubMed] [Google Scholar]
- 5.Burton, J. L., and M. E. Kehrli. 1996. Effects of dexamethasone on bovine circulating T lymphocyte populations. J. Leukocyte Biol. 59:90-99. [DOI] [PubMed] [Google Scholar]
- 6.Geluk, A., K. E. Van Meijgaarden, K. L. Franken, B. Wieles, S. M. Arend, W. R. Faber, B. Naafs, and T. H. Ottenhoff. 2004. Immunological cross-reactivity of the Mycobacterium leprae CFP-10 with its homologue in Mycobacterium tuberculosis. Scand. J. Immunol. 59:66-70. [DOI] [PubMed] [Google Scholar]
- 7.Geluk, A., K. E. van Meijgaarden, K. L. Franken, Y. W. Subronto, B. Wieles, S. M. Arend, E. P. Sampaio, T. de Boer, W. R. Faber, B. Naafs, and T. H. Ottenhoff. 2002. Identification and characterization of the ESAT-6 homologue of Mycobacterium leprae and T-cell cross-reactivity with Mycobacterium tuberculosis. Infect. Immun. 70:2544-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gey Van Pittius, N. C., J. Gamieldien, W. Hide, G. D. Brown, R. J. Siezen, and A. D. Beyers. 2001. The ESAT-6 gene cluster of Mycobacterium tuberculosis and other high G+C Gram-positive bacteria. Genome Biol. 2:0044-0048. http://genomebiology.com/2001/2/10/research/0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon, S. A., M. J. Wannemuehler, G. G. Mahairas, and F. C. Minion. 2002. Mycobacterial ESAT-6 protein enhances mouse IFN-γ responses to Mycoplasma hyopneumoniae P71 protein. J. Interferon Cytokine Res. 22:807-813. [DOI] [PubMed] [Google Scholar]
- 10.Palmer, M. V., D. L. Whipple, and S. C. Olsen. 1999. Development of a model of natural infection with Mycobacterium bovis in white-tailed deer. J. Wildl. Dis. 35:450-457. [DOI] [PubMed] [Google Scholar]
- 11.Pollock, J. M., and P. Andersen. 1997. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J. Infect. Dis. 175:1251-1254. [DOI] [PubMed] [Google Scholar]
- 12.Pollock, J. M., R. M. Girvin, K. A. Lightbody, R. A. Clements, S. D. Neill, B. M. Buddle, and P. Andersen. 2000. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet. Rec. 146:659-665. [DOI] [PubMed] [Google Scholar]
- 13.Rajaraman, V., B. J. Nonnecke, S. T. Franklin, D. C. Hammell, and R. L. Horst. 1998. Effect of vitamins A and E on nitric oxide production by blood mononuclear leukocytes from neonatal calves fed milk replacer. J. Dairy Sci. 81:3278-3285. [DOI] [PubMed] [Google Scholar]
- 14.Renshaw, P. S., P. Panagiotidou, A. Whelan, S. V. Gordon, R. G. Hewinson, R. A. Williamson, and M. D. Carr. 2002. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6:CFP-10 complex. Implications for pathogenesis and virulence. J. Biol. Chem. 277:21598-21603. [DOI] [PubMed] [Google Scholar]
- 15.Schmitt, S. M., S. D. Fitzgerald, T. M. Cooley, C. S. Bruning-Fann, L. Sullivan, D. Berry, T. Carlson, R. B. Minnis, J. B. Payeur, and J. Sikarskie. 1997. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis. 33:749-758. [DOI] [PubMed] [Google Scholar]
- 16.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waters, W. R., M. V. Palmer, D. L. Whipple, M. C. Carlson, and B. J. Nonnecke. 2003. Diagnostic implications of antigen-induced IFN-γ, nitric oxide, and TNF-α production by blood mononuclear cells from Mycobacterium bovis-infected cattle. Clin. Diagn. Lab. Immunol. 10:960-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waters, W. R., M. V. Palmer, S. C. Olsen, R. E. Sacco, and D. L. Whipple. 2003. Immune responses of elk to Mycobacterium bovis bacillus Calmette Guerin vaccination. Vaccine 21:1518-1526. [DOI] [PubMed] [Google Scholar]
- 19.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of a humoral and cellular immune response during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]