Abstract
Humans infected with West Nile virus (WNV) develop immunoglobulin M (IgM) antibodies soon after infection. The microtiter-based assays for WNV IgM antibody detection used by most state public health and reference laboratories utilize WNV antigen isolated from infected Vero cells or recombinant envelope protein produced in COS-1 cells. Recombinant antigen produced in COS-1 cells was used to develop a WNV IgM capture enzyme immunoassay (EIA). A supplementary EIA using WNV envelope protein expressed in Drosophila melanogaster S2 cells was also developed. Both assays detected WNV IgM in the sera of experimentally infected rhesus monkeys within approximately 10 days postinfection. Human sera previously tested for WNV IgM at a state public health laboratory (SPHL) were evaluated using both EIAs. Among the sera from 20 individuals with laboratory-confirmed WNV infection (i.e., IgM-positive cerebrospinal fluid [CSF]) that were categorized as equivocal for WNV IgM at the SPHL, 19 were IgM positive and one was negative by the new EIAs. Of the 19 IgM-positive patients, 15 were diagnosed with meningitis or encephalitis; the IgM-negative patient was not diagnosed with neurological disease. There was 100% agreement between the EIAs for the detection of WNV IgM. CSF samples from 21 individuals tested equivocal for WNV IgM at the SPHL; all 21 were positive in both bead assays, and 16 of these patients were diagnosed with neurological disease. These findings demonstrate that the new EIAs accurately identify WNV infection in individuals with confirmed WNV encephalitis and that they exhibit enhanced sensitivity over that of the microtiter assay format.
Some of the arboviruses responsible for significant human illness in the United States and Canada include eastern equine encephalitis (EEE) virus (family, Togaviridae), California group virus (CGV) (family, Bunyaviridae), and Powassan virus (family, Flaviviridae) (2). Among the flaviviruses, the Japanese encephalitis (JE) serogroup viruses are responsible for the majority of clinically important infections, including Murray Valley encephalitis, St. Louis encephalitis (SLE), and West Nile virus (WNV). SLE virus was the only mosquito-borne flavivirus causing significant morbidity and mortality in the United States until 1999, when WNV emerged as a new threat to humans and animals (4, 13). In 2002, WNV spread to 44 states, resulting in over 4,100 reported cases of West Nile meningitis or encephalitis or West Nile fever and 284 deaths (data available at http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount02.htm). In 2003, there were nearly 9,000 reported human cases of West Nile virus-associated disease and 218 deaths (data available at http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount03.htm). During the epidemic it has been essential that clinically relevant diagnostic tests be available to discriminate WNV infection from that of other JE serogroup viruses. Assays for detecting flavivirus infection have included indirect immunofluorescence, the plaque-reduction neutralization test (PRNT), the hemagglutination inhibition test, complement fixation, and direct virus isolation (1, 11). While providing needed specificity, these assays do not provide the sensitivity and ease of use afforded by enzyme-linked immunosorbent assays (ELISAs) for immunoglobulin M (IgM) detection (3, 11). Serodiagnostic assays for the detection of flavivirus IgM-class antibodies (IgM antibody capture ELISAs [MAC-ELISAs]) that utilize antigen produced in the brains of infected suckling mice (SMB) have been developed (11), and while they are relatively robust in their ability to discriminate between SLE virus and WNV infections, they still exhibit some cross-reactivity (11). Another MAC-ELISA that uses extracts from Vero cells experimentally infected with WNV as the antigen source demonstrated 96% agreement with PRNT and 97% agreement with MAC-ELISAs performed at public health testing laboratories for identification of individuals infected with WNV (14).
Recent work on the design of DNA vaccines to prevent infection with JE virus or WNV has led to the development of in vitro methods for the production of antigens capable of detecting IgM antibodies (6). The expression of a WNV premembrane-membrane-envelope (prME) recombinant protein in COS-1 cells provided a useful reagent for the design of a MAC-ELISA. This MAC-ELISA discriminated between individuals infected with SLE virus and those infected with WNV and is currently used at many state public health laboratories (SPHLs) (6). This assay demonstrates reasonable sensitivity and specificity but requires SPHLs to acquire reagents and standardize assay performance independently. We have used the WNV prME protein expressed in COS-1 or Drosophila melanogaster Schneider-2 (S2) cells as an antigen source for establishing enzyme immunoassays (EIAs) that use the quarter-inch-polystyrene-bead format. The assays demonstrate improved sensitivity relative to that of the microtiter assay currently in use at SPHLs.
MATERIALS AND METHODS
Expression of prME protein in COS-1 cells.
WNV recombinant prME protein was expressed by a stably transfected COS-1 cell line obtained from the Centers for Disease Control and Prevention (CDC), Fort Collins, Colo. (6). Cell growth and expansion were performed in 75-cm2 tissue culture flasks as previously described (6). The WNV antigen was harvested from the cell culture medium by overnight precipitation with 10% polyethylene glycol 8000 (PEG 8000) at 4°C. The precipitate was collected by centrifugation at 10,000 × g in a Beckman model JA-14 rotor at 4°C for 30 min and then suspended in 0.01 volume (relative to the amount of harvested medium) of TNE buffer (10 mM Tris [pH 7.5], 100 mM NaCl, 10 mM EDTA). Antigen was aliquoted and stored at −70°C.
Expression of prME protein in Drosophila S2 cells.
The WNV prME protein was obtained by PCR amplification from a recombinant plasmid containing the 5′ half of the WNV Eg101 genome. The cloned region encompassed the premembrane, membrane, and envelope regions (93, 75, and 501 amino acids, respectively). The signal sequence upstream of the premembrane was not included. PCR primers were designed for cloning of the PCR amplicon into the BglII and ApaI or PmeI sites of the Drosophila expression vector pMT/Bip/V5-HisA (Drosophila Expression System; Invitrogen, San Diego, Calif.). This vector encodes a signal for secretion of the expressed protein into the culture medium of transfected S2 cells. Cloning into the BglII-ApaI sites created a recombinant protein with a carboxy-terminal V5 epitope and six-His tags, while cloning into the BglII-Pme I sites produced a protein lacking the tags. The primers used to amplify the target region for cloning into the BglII-ApaI sites were preme-f (5′-GATCAGATCTGTTACCCTCTCTAACTTCCAA and preme-r (5′-GATCGGGCCCAGCGTGCACGTTCACGGAGAG-3′). The primer preme-r-pmeI (5′-GATCGTTTAAACTTATCAAGCGTGCACGTTCACGGAGAG-3′), when coupled with preme-f, was used for cloning into the BglII-PmeI sites.
Amplicons and vector DNA were digested with the requisite restriction enzymes and ligated overnight at 4°C. Escherichia coli XL1-Blue cells were transformed, and recombinants were identified by digestion of plasmid DNA with HindIII, which allowed the discrimination of recombinant plasmids with or without the C-terminal tags. Clones with the proper sequence were identified by sequence analysis of the entire insert region. The selection vector pCoHYGRO, a control vector expressing green fluorescent protein (pMTBiPGFPV5His), and the insertless parent vector (pMTBiPV5His-A) were also transformed into XL1-Blue cells. Large-scale plasmid preparations were made using LB medium and QIAGEN Maxi prep kits as described by the manufacturer. S2 cells were grown in Drosophila medium (Invitrogen) containing 10% heat-inactivated calf serum (complete medium). Cells were transfected via calcium phosphate precipitation of the expression plasmids and pCoHYGRO in a ratio of 19:1 (20 μg of total DNA). The insertless vector (pMTBiPV5His-A) and the control green fluorescent protein-expressing vector were transfected separately, along with pCoHYGRO, as controls. At day 3 posttransfection, the cells were washed with and replated into fresh complete medium. At day 5 posttransfection, the cells were washed and replated into complete medium containing 0.30 mg of hygromycin B/ml (i.e., selective medium). The selective medium was replaced at 4- to 5-day intervals for 25 days, at which time the cells were split 1:3. The cells were passaged at 1 million cells/ml into selective medium when the resistant-cell density reached 8 to 12 million cells/ml. S2 cells were maintained in 75-cm2 flasks in the presence of selective medium and scaled up into 200- to 600-ml spinner cultures for recombinant protein expression experiments. Recombinant protein expression in spinner cultures was induced by addition of CuSO4 to a final concentration of 333 μM. The cells were induced for 10 days at room temperature without changes of medium or inducer supplementation. Antigen secreted into the culture medium was harvested by PEG precipitation as described above. Expressed protein was detected by Western blotting using monoclonal antibodies directed against the V5 fusion tag or an anti-Kunjin virus antibody.
Immunochemical detection of recombinant antigen.
To monitor the expression of WNV recombinant envelope protein in either COS-1 or Drosophila S2 cells, an antigen capture assay was developed. The assay utilized as the capture antibody a flavivirus group-specific monoclonal IgG produced by the hybridoma cell line D1-4G2-4-15 (ATCC no. HB-112). Quarter-inch (6.4-mm) polystyrene beads were coated with this antibody at 1 μg/ml as previously described (7). Captured antigen was detected using the monoclonal antibody 6B6C-1 (16) conjugated with horseradish peroxidase (HRPO) (12). Cell culture medium or PEG-precipitated antigen was dissolved in TNE buffer and then diluted in PBS (pH 7.4) containing 0.05% Tween 20. After addition of the D1-coated bead and incubation for 2 h at room temperature with shaking, the beads were washed with distilled water. Conjugate (0.20 ml) diluted in PBS (pH 7.4) containing 0.5% Tween 20 and 5% nonfat dry milk was then added and incubated at room temperature for 60 min. The beads were washed with distilled water, and bound conjugate was detected by addition of 0.30 ml of 0.3% o-phenylenediamine in 0.1 M citrate buffer (pH 5.5), containing 0.02% H2O2. The reaction was quenched by the addition of 1 ml of 1 N H2SO4, and the optical density (OD) at 492 nm was determined. The OD is directly proportional to the amount of WNV antigen bound to the solid phase.
WNV IgM detection assays.
IgM antibody capture EIAs that employed a solid phase coated with antibody directed against human IgM were developed. Goat anti-human IgM was used to coat quarter-inch (6.4-mm) polystyrene beads as described above. The beads were incubated for 1 h at 37°C with 0.20 ml of serum diluted 1:336 in specimen diluent buffer (PBS [pH 7.0] containing 10% calf serum and 0.1% nonionic detergent). Cerebrospinal fluid (CSF) was diluted 1:5 in the same buffer. After incubation, the beads were washed with deionized water and then incubated for 2 h at 37°C with 0.10 ml of 6B6C-1-HRPO conjugate (described above) and 0.10 ml of WNV-specific recombinant envelope protein produced in COS-1 cells (COS-1 assay) or in Drosophila S2 cells (S2 assay). Recombinant antigen was added to the washed beads, followed immediately by the addition of the HRPO conjugate. Bound conjugate was detected as described above. A single initial test of human sera for WNV IgM was done; any sample for which the S/N ratio (the ratio of the signal obtained in the assay for the test sample [S] to the signal obtained for a negative sample [N]) was above or near the cutoff value was retested twice.
Volunteer donors.
The specificity of the WNV IgM assay utilizing the Drosophila S2 cell line-expressed antigen was determined by testing human sera from 140 volunteer blood donors obtained from the Gulf Coast Regional Blood Center, Houston, Tex.
Human specimens.
The Michigan Department of Community Health (MDCH) provided panels of human sera and CSF which were unlinked to the source and identified only by code number and diagnosis at the time of sample collection. The samples were originally submitted to the MDCH during 2002 and 2003 for differential diagnosis of human arboviral infections by the MAC-ELISA procedures described by the CDC (10, 11) and standardized internally (H. Kapoor, S. D. Manning, P. Clark, F. P. Downes, P. Somsel, and K. Signs, presented at the 19th Annu. Clin. Virol. Symp. and Annu. Mtg. Pan Am. Soc. Clin. Virol., 2003). MAC-ELISAs for IgM specific to La Crosse encephalitis virus, SLE virus, EEE virus, and WNV were performed. Samples were considered WNV IgM positive if (i) the specimen tested positive in two consecutive runs and (ii) the S/N ratio obtained in the WNV MAC-ELISA was at least twice that obtained in the assay for SLE IgM. The S/N ratio ranges used for classifying serum specimens were as follows: a value of <2.0 indicated a negative result, a value of 2.0 to 5.0 indicated an equivocal result, and a value of >5.0 indicated a positive result. The S/N ratio ranges used for classifying CSF specimens were as follows: a value of <2.0 indicated a negative result, a value of 2.0 to 10.0 indicated an equivocal result, and a value of >10.0 indicated a positive result. The WNV IgM-positive and WNV IgM-equivocal human sera were from individuals with serologically confirmed WNV infections, i.e., with WNV IgM-positive CSF (per the CDC case definition). The human CSF panel consisted of specimens found to be WNV IgM positive at the MDCH following testing of undiluted samples. None of the patient sera used in this study were positive for CGV, SLE virus, or EEE virus IgM. The sera and CSF used in this study were not matched. A panel of 12 IgM-negative CSF samples was tested in both experimental EIAs, and the mean OD was used as the negative value to calculate S/N ratios and to establish a provisional cutoff value for the CSF assay.
WNV-infected animals.
Serial serum samples (taken at day 0 and at 1 to 14, 21, 28, 45, and 63 days postinfection) from experimentally infected rhesus monkeys (15) were tested for IgM antibodies using the S2 assay described above. S/N ratios were calculated by dividing the absorbance reading for each test sample by the absorbance value obtained for the day 0 (preinoculation) serum sample. S/N ratios greater than 4.20 were considered WNV IgM positive.
RESULTS
WNV antigen expression in COS-1 cells.
Recombinant WNV prME protein constitutively expressed in COS-1 cells as previously described (6, 8) was used as a source of antigen for the development of an EIA (referred to as the COS-1 assay). Antigen secreted into the cell culture medium was harvested by PEG precipitation, and yields were estimated by an antigen capture ELISA. The maximum ELISA titer for harvested cell culture medium was 1:80, and this titer was maintained in culture medium stored at 4°C for up to 83 days. Recombinant antigen of the expected size was detectable in the cell culture supernatant and PEG precipitates via sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Western blotting using the anti-Kunjin virus monoclonal antibodies (data not shown). Previous studies have shown the utility of recombinant WNV prME protein in the detection of WNV IgM antibodies, as well as its ability to discriminate between individuals infected with SLE virus (6).
Antigen expression in S2 cells.
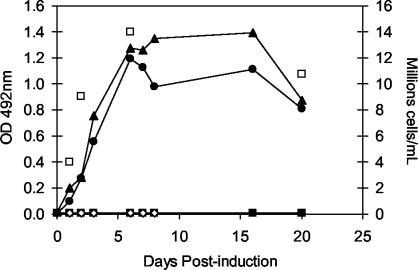
To provide an alternative source of WNV envelope protein for the development of a supplemental WNV IgM assay, the prME region of WNV isolate Eg101 was cloned into the Drosophila expression plasmid pMTBipV5His. This plasmid directs the expression of recombinant proteins in Drosophila S2 cells under the control of the inducible metallothionein promoter, with secretion of the protein into the culture medium (5, 9). The plasmid also allows for the production of proteins with carboxy-terminal epitopes (i.e., V5 and six His) that serve as tags for monitoring protein expression and/or isolation via immunochemical methods. We generated two constructs encoding the entire prME region of WNV Eg101: one that included sequences encoding the carboxy-terminal epitope tags and one that lacked these sequences (pMTBip-prME-V5His and pMTBip-prME, respectively). Preliminary studies using transiently transfected cells demonstrated the ability of both plasmids to direct the synthesis of immunoreactive WNV antigen. The amount of antigen secreted into the cell culture medium was estimated by the antigen capture ELISA (data not shown). Subsequently, stable cell lines were generated by cotransfection of an expression plasmid containing the hygromycin resistance gene (pCoHYGRO). Figure 1 shows a time course of WNV antigen production in S2 cells stably transfected with either of the two WNV expression plasmids, the insertless parent vector or the plasmid expressing green fluorescent protein. These results demonstrated that, for cells grown in flasks, cell density and antigen production reached a maximum at about 6 days postinduction. The large-scale production of antigen required growth in spinner cultures (200 to 600 ml) with cells seeded at 1 million cells per ml and induced immediately by the addition of 333 μM CuSO4. Induced cells were maintained in culture for 10 days at room temperature, at which time the cell culture medium was collected and the antigen was harvested by PEG precipitation. Typical yields from 250 ml of spinner culture medium produced sufficient antigen for approximately 2,000 assays. PEG-precipitated antigen resuspended in TNE buffer was found to be stable for more than 4 months when it was stored at 4°C (data not shown).
FIG. 1.
Expression time course of WNV prME protein in a stably transfected Drosophila S2 cell line. Cells were transfected with the insertless parent vector pMT/BiP/V5HisA (filled squares), a vector expressing green fluorescent protein (open diamonds), pMT/Bip/WNVpreME/V5His (filled circles), or pMT/Bip/WNVpreME (filled triangles) along with the selection vector pCoHYGRO and placed under selection for 19 days. One-sixth of the cells were transferred to a 25-cm2 flask and grown in selective medium at 28°C containing an inducer (333 μM CuSO4). Cell culture medium (0.5 ml) was harvested at various times after the addition of the inducer, and the presence of WNV antigen was detected by an antigen capture EIA following a 1:4 dilution into sample buffer. The total cell density was measured at 1, 2, 6, and 20 days postinduction (open squares). Only those cells transfected with WNV expression constructs produced detectable antigen in the culture medium.
WNV IgM bead EIAs.
Development of the WNV IgM antibody capture assays required the appropriate selection of sample diluent and conjugate diluent buffers, incubation times, and temperature. It was found that diluent buffers containing Triton X-100 eliminated the detection of WNV IgM from positive-control samples but that Tween 20 did not. Suitable dilutions of PEG-precipitated antigen and conjugate used in the final assay were those that returned S/N ratios greater than ∼5.0 for the lowest possible dilution of WNV-positive control serum (data not shown). Preliminary experiments used overnight incubation to capture IgM onto the solid phase; however, it was found that incubation with a sample for as little as 1 h at 37°C provided equivalent sensitivity. The final assay parameters were verified by repeated testing by multiple technicians of a proficiency panel of sera confirmed by PRNT to be WNV IgM positive (data not shown).
Assay specificity.
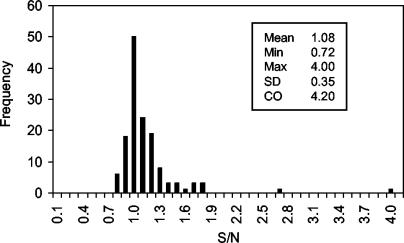
A provisional cutoff value was determined for the EIA utilizing the Drosophila S2 cell-expressed WNV prME antigen (S2 assay) by testing a panel of 140 serum samples from human volunteer donors. The cutoff value was set at 9 standard deviations above the mean S/N ratio for a normal population (Fig. 2). This value (4.20) minimized the number of repeatably reactive samples among the volunteer blood donors (presumed negative for WNV infection) and maximized the number of positive samples from each monkey seroconversion panel (see below) or presumed WNV IgM-positive human population. Among the 140 volunteer donor sera tested, none exhibited S/N ratios above the provisional cutoff of 4.20, resulting in a specificity for this population of 100%.
FIG. 2.
Frequency distribution of S/N ratios obtained by testing sera from 140 volunteer donors with the WNV IgM assay utilizing the Drosophila-expressed prME antigen. The cutoff value (CO) of 4.20 is 9 standard deviations (SD) above the population S/N ratio mean.
The specificity of the EIAs for human CSF was determined by testing a panel of 12 WNV IgM-negative CSF specimens (reactivity was determined by neat testing at the MDCH). The mean absorbance value obtained for each EIA was utilized as the denominator to calculate S/N ratios and to provide a basis for the establishment of provisional cutoff values. Assuming performance similar to that observed for the testing of human serum, a cutoff value for the CSF assay would be near an S/N ratio of 4.0 to 5.0. A provisional cutoff value of 4.0 is equivalent to 17 and 25 standard deviations above the 12-member IgM-negative CSF panel's S/N ratio mean for the S2 and COS-1 EIAs, respectively. We therefore set a cutoff S/N ratio of 4.0 for both EIAs for CSF testing.
IgM detection in experimentally infected monkeys.
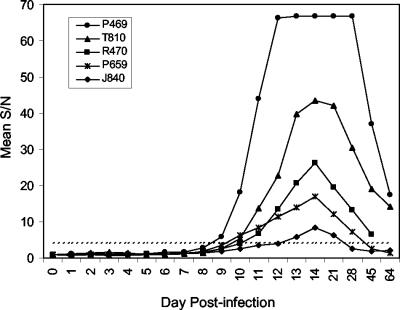
The natural history of WNV infection following experimental subdermal inoculation of rhesus monkeys was recently investigated by Ratterree and colleagues (15). This study included direct culture of virus from serum, detection of WNV RNA via reverse transcription-PCR, and examination of the immune response by complement fixation, hemagglutination inhibition testing, and PRNT. Serial blood samples taken at regular intervals after infection were previously shown to contain WNV-specific IgM by the COS-1 IgM assay (15). The results of IgM detection by the S2 assay are shown in Fig. 3. For three of the animals, concurrent IgM detection was observed for the two IgM assays (Table 1). However, for two animals, the serodiagnostic window was shortened when sera were tested using the S2 assay (Table 1). The S/N ratio for animal J840 at day 12 was 4.1, very close to the 4.20 S/N ratio cutoff for the S2 assay; the S/N ratio for this sample was 8.40 in the COS-1 assay. For the other three discrepant bleed dates, S/N ratios for the S2 assay were 2.6 to 3.5, whereas values ranged from 6.5 to 9.3 for the COS-1 assay (S/N ratio cutoff, 5.0). The overall agreement between the two assays was 90% (35 of 39 samples determined by the COS-1 assay to be positive were also positive by the S2 assay).
FIG. 3.
Detection of WNV IgM in sera of experimentally infected rhesus monkeys by using the Drosophila-expressed WNV prME antigen. Individual serum samples were diluted 1:336 and tested in the WNV EIA. Each diluted sample was assayed twice on consecutive days, and mean absorbance values were calculated. The dashed line represents the provisional cutoff value of 4.20 determined from testing of human volunteer donors (Fig. 2).
TABLE 1.
Comparison of EIAs for the detection of WNV IgM antibodies in the serum of experimentally infected monkeysa
| Monkey | Day monkey was identified as WNV IgM positive by:
|
|||
|---|---|---|---|---|
| COS-1 cell-based assay
|
S2 cell-based assay
|
|||
| First | Last | First | Last | |
| J840 | 12 | 28 | 13b | 21 |
| R470 | 11 | 45 | 11 | 45 |
| T810 | 10c | 63 | 10 | 63 |
| P469 | 9 | 63 | 9 | 63 |
| P659 | 9 | 45 | 10 | 28 |
Serum samples were collected on days 0, 1 to 14, 21, 28, 45, and 63 postinfection. All animals were euthanized on day 63 postinfection except for monkey R470, which was euthanized on day 45.
For monkey J840, the S/N ratio as determined by the S2 assay for the sample obtained on day 12 was 4.10, and the cutoff S/N ratio was 4.20.
For monkey T810, the sample obtained on day 12 was WNV RNA positive as determined by reverse transcription-PCR (15).
IgM detection in human sera or CSF.
A panel of 37 human sera was obtained from the MDCH. These individuals were referred to this state health lab for differential diagnosis of human flaviviral infections using microtiter MAC-ELISAs. MDCH assays use rare reagents and performance guidelines provided by the CDC but standardized at the testing laboratory. None of the patient sera used in this study were IgM positive for CGV, SLE virus, or EEE virus. The panel of sera was tested in both COS-1 and S2 EIAs as described in Materials and Methods. The results are summarized in Table 2. Samples with WNV IgM S/N ratios of <2.0 at the MDCH (i.e., WNV IgM negative) exhibited S/N ratios well below the cutoff values established for the bead assays. None of the WNV IgM-negative samples were from patients diagnosed with meningitis or encephalitis. All eight of the samples with S/N ratios in the positive range (i.e., >5.0) at the MDCH were IgM positive by both bead assays, and five of these were from patients with clinically apparent disease. Of the 20 samples with S/N ratios between 2.0 and 5.0 (considered equivocal for WNV IgM but obtained from individuals with confirmed WNV infections based on WNV IgM-positive CSF samples) at the MDCH, 19 were positive in both bead assays and one (specimen 18) was negative (S/N ratios of 3.8 and 3.6 for the COS-1 and S2 assays, respectively). Among these 20 individuals, 11 were diagnosed with encephalitis, meningitis, or meningoencephalitis, and 4 exhibited symptoms requiring hospitalization; patient 18 did not require hospitalization. For this panel of human samples, the experimental bead assays exhibited 100% concordance.
TABLE 2.
IgM reactivity (expressed as S/N ratios) to COS-1 and S2 cell-expressed WNV antigens in a panel of 37 human serum specimensa
| Specimen | S/N ratio obtained by indicated assay:
|
Diagnosisd | ||
|---|---|---|---|---|
| COS-1b | S2b | MDCHc | ||
| 4 | 77.8 | 44.7 | 3.00 | E |
| 32 | 77.8 | 43.7 | 3.20 | M/E |
| 28 | 77.8 | 48.2 | 3.30 | M/E |
| 36 | 77.8 | 48.2 | 3.50 | E |
| 31 | 77.8 | 30.8 | 3.60 | E |
| 43 | 77.8 | 27.9 | 3.60 | H |
| 15 | 77.8 | 48.2 | 3.80 | H |
| 30 | 77.8 | 48.2 | 4.70 | |
| 35 | 77.8 | 48.2 | 5.20 | |
| 38 | 77.8 | 48.2 | 5.50 | |
| 11 | 77.8 | 48.2 | 10.00 | E |
| 26 | 77.8 | 35.0 | 10.00 | M |
| 5 | 77.8 | 47.8 | 10.50 | E |
| 16 | 77.8 | 48.2 | 12.50 | M/E |
| 24 | 77.8 | 48.2 | 22.00 | E |
| 27 | 73.6 | 34.9 | 3.10 | M/E |
| 19 | 68.0 | 43.2 | 2.20 | E |
| 10 | 60.7 | 22.9 | 3.00 | |
| 9 | 60.1 | 21.9 | 2.30 | M |
| 6 | 59.3 | 44.2 | 2.90 | |
| 14 | 58.7 | 24.8 | 108.00 | |
| 37 | 56.3 | 23.2 | 2.60 | H |
| 33 | 44.0 | 24.5 | 2.50 | E |
| 17 | 43.8 | 27.3 | 2.60 | |
| 22 | 40.1 | 16.1 | 2.10 | E |
| 23 | 38.4 | 7.5 | 2.20 | E |
| 21 | 13.2 | 8.3 | 2.90 | H |
| 18 | 3.8 | 3.6 | 2.95 | |
| 41 | 2.1 | 1.8 | <2.0 | |
| 25 | 1.8 | 1.4 | <2.0 | HR |
| 42 | 1.1 | 1.0 | <2.0 | |
| 3 | 1.0 | 0.9 | <2.0 | |
| 40 | 1.0 | 0.9 | <2.0 | |
| 39 | 0.9 | 1.1 | <2.0 | |
| 1 | 0.8 | 0.9 | <2.0 | |
| 2 | 0.8 | 0.9 | <2.0 | |
| 34 | 0.8 | 1.0 | <2.0 | |
Tabular data are arranged according to S/N ratios from the COS-1 assay, listed from highest to lowest.
The COS-1 and S2 assay S/N ratios represent the means of duplicate determinations. Cutoff S/N ratios for the COS-1 and S2 assays were 5.0 and 4.20, respectively.
For the MAC-ELISA performed at the MDCH, samples with S/N ratios of <2.0 are considered IgM negative, those with S/N ratios from 2.0 to 5.0 are equivocal, and those with S/N ratios of >5.00 are considered positive for WNV IgM. MDCH results were obtained using the WNV IgM assay, and reagents were provided by the CDC.
Clinical diagnosis abbreviations: E, encephalitis; M, meningitis; M/E, meningo-encephalitis; H, hospitalized; HR, hospital released.
A separate panel of 27 human CSF samples was tested in both COS-1 and S2 EIAs. Due to the limited volume of material available, the samples were diluted 1:5 prior to testing. A comparison of bead assay S/N ratios with those obtained at the MDCH, where fivefold-diluted samples were tested with the CDC microtiter assay format, is shown in Table 3. Bead assay S/N ratios greater than 4.0 were considered positive for WNV IgM (as described above). Of the 27 samples tested, 6 were identified as IgM negative (S/N ratio of <2.00) at the MDCH. Of these six, two were IgM positive in the bead assays (specimens 21 and 34), exhibiting S/N ratios of 4.8 to 13.8. The remaining four had S/N ratios of <4.0 in the bead assays and were thus considered IgM negative. Twenty-one specimens were identified as WNV IgM equivocal (S/N ratio of 2.00 to 10.0) at the MDCH; however, all 21 were IgM positive in the bead EIAs, with S/N ratios ranging from 15.1 to 74.1 and with all but one sample exhibiting S/N ratios greater than 20.0. As with the panel of human sera, the experimental bead assays exhibited 100% concordance for this panel of CSF specimens.
TABLE 3.
IgM reactivity (expressed as S/N ratios) to COS-1 and S2 cell-expressed WNV antigens in a panel of 27 human CSF specimensa
| Specimen | S/N ratio obtained by indicated assay:
|
Diagnosisd | ||
|---|---|---|---|---|
| COS-1b | S2b | MDCHc | ||
| 10 | 74.1 | 74.1 | 7.3 | E |
| 14 | 74.1 | 74.1 | 6.4 | M/E |
| 15 | 74.1 | 68.2 | 5.3 | |
| 17 | 74.1 | 74.1 | 5.7 | HR |
| 18 | 74.1 | 68.9 | 4.8 | M/E |
| 20 | 74.1 | 32.2 | 3.6 | E |
| 23 | 74.1 | 74.1 | 5.8 | |
| 24 | 74.1 | 57.9 | 3.5 | E |
| 25 | 74.1 | 65.9 | 6.4 | M |
| 26 | 74.1 | 74.1 | 5.8 | E |
| 28 | 74.1 | 74.1 | 5.9 | E |
| 30 | 74.1 | 74.1 | 6.4 | M/E |
| 31 | 74.1 | 74.1 | 5.3 | |
| 33 | 74.1 | 74.1 | 4.3 | E |
| 8 | 74.1 | 74.1 | 7.0 | |
| 9 | 74.1 | 74.1 | 5.6 | |
| 32 | 71.9 | 32.8 | 5.0 | H |
| 22 | 71.4 | 49.6 | 6.4 | E |
| 19 | 58.4 | 41.1 | 5.5 | E |
| 27 | 36.8 | 28.7 | 3.3 | M |
| 12 | 29.7 | 15.1 | 4.7 | E |
| 21 | 13.8 | 8.6 | 1.9 | M |
| 34 | 8.1 | 4.8 | 1.0 | |
| 16 | 3.5 | 2.5 | 1.4 | M |
| 11 | 1.7 | 2.4 | 1.7 | |
| 35 | 1.4 | 1.1 | 1.0 | E |
| 13 | 1.3 | 1.7 | 1.3 | E |
Reactivity for all three assays is reported as S/N ratios. Tabular data are arranged according to S/N ratios from the COS-1 assay, listed from highest to lowest. Samples were diluted 1:5 in assay buffer prior to testing.
COS-1 and S2 S/N ratios represent the means of duplicate determinations.
For results obtained at the MDCH, samples with S/N ratios of <2.0 are considered negative, those with S/N ratios from 2.0 to 10.0 are equivocal, and those with S/N ratios of >10.0 are positive for WNV IgM.
Clinical diagnosis abbreviations: see footnote d of Table 2.
DISCUSSION
Diagnostic testing of human serum, plasma, or CSF for IgM antibodies against JE group viruses (e.g., SLE virus, EEE virus, and WNV) has relied upon microtiter assays developed by the CDC (10, 11). These assays utilize viral antigens extracted from SMB or recombinant antigen produced in COS-1 cells. During the current WNV epidemic, a new assay for the detection of WNV IgM antibodies which used recombinant WNV envelope protein expressed and secreted by COS-1 cells was developed by the CDC (6). The recombinant-based assay was shown to have a sensitivity and specificity similar to those of the assay employing SMB-derived antigen. However, the dynamic range of the assay, like that of other assays utilizing SMB antigens, was limited. In addition, the assay required overnight incubation with antigen for maximal sensitivity. Our goal was to use the COS-1-expressed recombinant antigen to develop an ELISA for the detection of WNV IgM, utilizing quarter-inch-polystyrene-bead technology, that would exhibit improved ease of use and sensitivity compared to those of the microtiter assay. In addition, we sought to establish a stable Drosophila S2 cell line expressing the WNV prME antigen for large-scale production of material for use in a supplementary WNV IgM assay.
Our assay format is similar to that used by the CDC in that it comprises an IgM-class-antibody capture step, a WNV-specific antigen expressed stably in COS-1 or Drosophila S2 cells, and finally, a broadly group-reactive monoclonal antibody peroxidase conjugate. A comparison of assay performance using seroconversion panels from experimentally infected rhesus monkeys demonstrated 90% agreement between the COS-1 cell-based assay and the supplemental S2 assay utilizing Drosophila-expressed antigen. Among four monkey serum samples (from two animals) for which the S/N ratios were below the S2 assay cutoff value of 4.20, one exhibited an S/N ratio of 4.1, while the S/N ratios for the other three were also elevated (i.e., 2.5, 2.6, and 3.5). Though these were below the provisional cutoff established for this assay, there was a clear trend toward higher S/N ratios for serum samples obtained immediately preceding or following samples with S/N ratios clearly in the positive range (Fig. 3). The sensitivity of the S2 assay may be improved by the development of Drosophila S2 cell lines exhibiting higher levels of WNV prME protein expression. Decreasing the ratio of WNV antigen expression plasmid to pCoHYGRO (from 19:1, used to develop the existing cell lines, to 10:1 or even 1:1) should result in cell lines with higher copy numbers of the integrated WNV expression plasmid and thus potentially higher overall expression levels (9).
The Drosophila expression system has advantages over the COS-1 mammalian cell expression system. First, Drosophila S2 cells grow very well at 28°C without the need of a CO2 incubator. Because the cells are semiadherent, they grow in standard plates or in shake or spinner flasks. COS-1 cells grow as adherent cells in a CO2 incubator at 37°C. Second, S2 cells grow well in serum-free Drosophila medium. We have transiently transfected S2 cells with the pMTBip-prME-V5His plasmid in the presence of serum and then expanded the culture in serum-free medium containing CuSO4 while monitoring the expression of WNV antigen. Expression levels were comparable to those observed for cells transfected and grown in the medium containing heat-inactivated 10% fetal calf serum (data not shown). Finally, based upon the similar immunoreactivities of WNV envelope protein expressed in COS-1 or Drosophila S2 cells, S2 cells must direct the expression of proteins in the proper conformation and glycosylation states. Hence, the Drosophila expression system provides a simple and potentially lower-cost alternative to COS-1 cell recombinant protein expression.
The comparison of the sensitivities of our COS-1 assay, the supplemental S2 cell-based assay, and the CDC assay format used at the MDCH was accomplished by testing a panel of sera from 37 individuals with suspected flaviviral infections (Table 3). For samples that tested positive (n = 8) or negative (n = 9) by the microtiter assay performed at the MDCH, the bead assays were in perfect agreement (Table 4). Among the eight IgM-positive patients, five were diagnosed with clinical disease, compared to none of the nine IgM-negative patients. In contrast, among the 20 patients with equivocal results in the microtiter assay, 19 were positive and 1 was negative in both experimental bead assays. Fifteen of these individuals were diagnosed with encephalitis, meningitis, or meningoencephalitis or exhibited clinical symptoms requiring hospitalization. All 15 of the patients with clinically apparent disease were unambiguously identified as WNV IgM positive by both EIAs described here (S/N ratio ranges, 7.5 to 48.2 for the S2 assay and 13.2 to 77.8 for the COS-1 cell-based assay). These results demonstrate the superior sensitivity of our bead assays for the detection of WNV IgM in human serum relative to that of the microtiter-based assay.
TABLE 4.
Agreement between assays for the detection of WNV IgMa
| MDCH result | No. of bead assay samples with indicated result
|
|||||
|---|---|---|---|---|---|---|
| Serum samples (n = 37)
|
CSF samples (n = 27)
|
|||||
| Positive | Negative | Total | Positive | Negative | Total | |
| Positive | 8 | 0 | 8 | 0 | 0 | 0 |
| Equivocal | 19 | 1 | 20 | 21 | 0 | 21 |
| Negative | 0 | 9 | 9 | 2 | 4 | 6 |
| Total | 27 | 10 | 37 | 23 | 4 | 27 |
COS-1 and S2 EIAs exhibited 100% agreement.
Because the detection of IgM antibody in CSF is diagnostic of recent WNV infection of the central nervous system, the ability of the bead assays to detect WNV IgM in human CSF was evaluated. Assuming a conservative negative-cutoff S/N ratio of 4.0 for either bead assay, four of six samples that were IgM negative (S/N ratio of <2.0) at the MDCH also tested negative in the bead assays, while two CSF samples that tested negative at the MDCH were positive in the bead EIAs (specimens 21 and 34). Among the 21 patients previously shown to be equivocal for WNV IgM at the MDCH, all 21 were IgM positive in the bead assays when their sera were tested at a 1:5 dilution (Table 4). All of the CSF specimens exhibited S/N ratios greater than 15.0, which is more than threefold higher than the provisional cutoff value of 4.0. Thus, the sensitivity of the COS-1 and S2 assays for the detection of WNV IgM in human CSF was superior to that of the microtiter assay format used at the MDCH when samples were tested at a 1:5 dilution.
Interestingly, four of the six IgM-negative CSF samples exhibited positive S/N ratios (22.5 to 185.5) when tested neat at the MDCH, while the remaining two had S/N ratios in the equivocal range (4.8 and 8.7) (data not shown). Three of these four WNV IgM-positive patients and one of the two IgM-equivocal patients were diagnosed with encephalitis or meningitis (Table 3). The apparent discrepancy between results obtained when CSF samples are tested neat versus those obtained when they are tested diluted in the microtiter assay may be due to the nonspecific binding of IgM to the solid phase. It is possible that false-positive results are obtained when CSF is not diluted in buffers containing nonspecific blocking agents (e.g., protein and surfactants). Different S/N ratios were also obtained for the 21 CSF samples with equivocal WNV IgM results after they were tested at a 1:5 dilution at the MDCH; that is, all 21 exhibited positive S/N ratios (12.0 to 181.0) when tested neat in the microtiter assay (data not shown). Adequate volumes of CSF samples were not available for testing undiluted in the experimental EIAs; however, given the high absorbance readings (>80% with ODs over 1.00) obtained for most of these samples at fivefold dilutions, it is not likely that testing neat samples would provide substantially increased sensitivity. Hence, while additional studies are needed to determine appropriate cutoff values, it is clear that our experimental bead assays are capable of detecting WNV IgM in human CSF samples from individuals with encephalitis or meningitis.
In conclusion, the polystyrene bead EIAs developed using WNV recombinant envelope protein expressed in COS-1 or Drosophila cells exhibit significantly enhanced sensitivity for the detection of IgM in human serum over the microtiter-based IgM assay currently in use at the MDCH and in many other SPHLs. Additionally, these assays provide reliable results when used to detect WNV IgM in human CSF.
Acknowledgments
We thank the following individuals for their contributions: Margo Brinton, Department of Biology, Georgia State University, Atlanta, Ga., for the WNV plasmids; Roy A. Hall, Department of Microbiology, University of Queensland, Queensland, Australia, for providing the anti-Kunjin virus monoclonal antibody; Jeff Chang and John Roehrig, Arbovirus Diseases Branch, CDC, Ft. Collins, Colo., for providing the 6B6C monoclonal antibody; and Akhtar Ali and Paul Coleman, Abbott Laboratories, for preparation of the 6B6C antibody HRPO conjugate used in the WNV antigen capture and IgM detection assays. The COS-1 cell line stably expressing WNV antigen was obtained under license from the CDC. Human sera and CSF specimens were obtained by the MDCH under institutional guidelines and were provided unlinked to the original source and identified only by code number and diagnosis.
REFERENCES
- 1.Beaty, B. J., C. H. Calisher, and R. E. Shope. 1995. Arboviruses. In E. H. Lennette, D. A. Lennette, and E. T. Lennette (ed.), Diagnostic procedures for viral, rickettsial and chlamydial infections, 7th ed. American Public Health Association, Washington, D.C.
- 2.Calisher, C. H. 1994. Medically important arboviruses of the United States and Canada. Clin. Microbiol. Rev. 7:89-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calisher, C. H., C. I. Pretzman, D. J. Muth, M. A. Parsons, and E. D. Peterson. 1986. Serodiagnosis of La Crosse virus infections in humans by detection of immunoglobulin M class antibodies. J. Clin. Microbiol. 23:667-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1999. Outbreak of West Nile-like viral encephalitis—New York. Morb. Mortal. Wkly. Rep. 48:845-849. [PubMed] [Google Scholar]
- 5.Culp, J. S., H. Johansen, B. Hellmig, J. Beck, T. J. Matthews, A. Delers, and M. Rosenberg. 1991. Regulated expression allows high level production and secretion of HIV-1 gp120 envelope glycoprotein in Drosophila Schneider cells. Biotechnology (New York) 9:173-177. [DOI] [PubMed] [Google Scholar]
- 6.Davis, B. S., G.-J. J. Chang, B. Cropp, J. T. Roehrig, D. A. Martin, C. J. Mitchell, R. Bowen, and M. L. Bunning. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75:4040-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez, R. A., G. J. Dawson, and I. K. Mushahwar. 1997. ELISA for detection of antibody to the E2 protein of GB virus C. J. Virol. Methods 69:1-6. [DOI] [PubMed] [Google Scholar]
- 8.Hunt, A. R., C. B. Cropp, and G. J. Chang. 2001. A recombinant particulate antigen of Japanese encephalitis virus produced in stably-transformed cells is an effective noninfectious antigen and subunit immunogen. J. Virol. Methods 97:133-149. [DOI] [PubMed] [Google Scholar]
- 9.Johansen, H., A. van der Straten, R. Sweet, E. Otto, G. Maroni, and M. Rosenberg. 1989. Regulated expression at high copy number allows production of a growth-inhibitory oncogene product in Drosophila Schneider cells. Genes Dev. 3:882-889. [DOI] [PubMed] [Google Scholar]
- 10.Martin, D. A., B. J. Biggerstaff, B. Allen, A. J. Johnson, R. S. Lanciotti, and J. T. Roehrig. 2002. Use of immunoglobulin M cross-reactions in differential diagnosis of human flaviviral encephalitis infections in the United States. Clin. Diagn. Lab. Immunol. 9:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin, D. A., D. A. Muth, T. Brown, A. J. Johnson, N. Karabatsos, and J. T. Roehrig. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J. Clin. Microbiol. 38:1823-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakane, P. K., and A. Kawaoi. 1974. Peroxidase-labeled antibody. A new method of conjugation. J. Histochem. Cytochem. 22:1084-1091. [DOI] [PubMed] [Google Scholar]
- 13.Nash, D., F. Mostashari, A. Fine, J. Miller, D. O'Leary, K. Murray, A. Huang, A. Rosenberg, A. Greenberg, M. Sherman, S. Wong, and M. Layton. 2001. The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 344:1807-1814. [DOI] [PubMed] [Google Scholar]
- 14.Prince, H. E., and W. R. Hogrefe. 2003. Detection of West Nile Virus (WNV)-specific immunoglobulin M in a reference laboratory setting during the 2002 WNV season in the United States. Clin. Diagn. Lab. Immunol. 10:764-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratterree, M. C., R. A. Gutierrez, A. P. Travassos Da Rosa, B. J. Dille, D. W. Beasley, R. P. Bohm, S. M. Desai, P. J. Didier, L. B. Birkenmeyer, G. J. Dawson, T. P. Leary, G. Schochetman, K. Phillipi-Falkenstein, J. Arroyo, A. D. Barrett, and R. B. Tesh. 2004. Experimental infection of rhesus monkeys with West Nile virus: level and duration of viremia and kinetics of the antibody response following infection. J. Infect. Dis. 189:669-676. [DOI] [PubMed] [Google Scholar]
- 16.Roehrig, J. T., J. H. Mathews, and D. W. Trent. 1983. Identification of epitopes on the E glycoprotein of Saint Louis encephalitis virus using monoclonal antibodies. Virology 128:118-126. [DOI] [PubMed] [Google Scholar]