Abstract
Localized aggressive periodontitis (LAgP) is a chronic inflammatory disease characterized by severe destruction of periodontal tissues surrounding the first molars and incisors. LAgP subjects produce large amounts of immunoglobulin G2 (IgG2) antibody against oral pathogens, and this response is inversely correlated with the severity of disease. We previously demonstrated that platelet-activating factor (PAF) is required for optimal IgG2 responses. The present investigation was designed to determine the mechanism of IgG2 induction by PAF. Exogenous PAF acetylhydrolase suppressed approximately 80% of pokeweed mitogen-stimulated IgG2 production, confirming that PAF is essential for optimal responses. PAF-activated leukocytes produced gamma interferon (IFN-γ), a Th1 cytokine that has been associated with IgG2 responses in previous studies. The monocyte-derived cytokines interleukin-12 (IL-12) and IL-18 are upstream of IFN-γ production, and IgG2 production was suppressed by neutralizing antibodies against these proteins. In addition, PAF induced monocyte-derived dendritic cells (DC) but not macrophages (MΦ) to secrete IL-12 and IL-18. This observation was interesting because monocyte differentiation in LAgP subjects is skewed to the DC phenotype. Although other investigators have implicated IFN-γ in IgG2 production, its precise role in this response is controversial. Our studies suggest that IFN-γ induces isotype switching to IgG2 but only in concert with the Th2 cytokine IL-4. Thus, it appears that the unique PAF metabolism of LAgP monocytes or DC promotes Th1 responses that are essential for optimal IgG2 antibody production. As IgG2 antibodies opsonize oral bacteria and promote their clearance and destruction, these alterations in PAF metabolism may be essential for limiting disease severity in LAgP patients.
Localized aggressive periodontitis (LAgP) is classified as an early-onset form of periodontitis due to its propensity to develop in adolescents around the time of puberty. It is characterized by severe destruction of supporting periodontal structures, primarily around the incisors and primary molars (2, 34). The disease clearly has a bacterial origin and several oral pathogens have been linked to the disease, most notably Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis (11, 58). However, as LAgP is an inflammatory disorder, the host response also contributes to its pathogenesis and in fact may dictate the pattern and severity of the disease.
Several immunological anomalies have been noted in LAgP subjects. For example, LAgP neutrophils exhibit reduced chemotaxis, oxidative burst, and calcium responses compared to neutrophils from periodontally healthy (nonperiodontitis [NP]) individuals (15, 25, 46). The monocytes of LAgP subjects exhibit exaggerated responses to bacterial lipopolysaccharides and produce large amounts of tumor necrosis factor and prostaglandin E2 (PGE2) (44). Immunoglobulin production in LAgP subjects is also somewhat abnormal, as LAgP patients have ∼35% more serum immunoglobulin G2 (IgG2) antibody than do race-matched NP subjects. The levels of this antibody reactive with A. actinomycetemcomitans and P. gingivalis are inversely correlated with the severity of the disease (9, 22, 33), suggesting that IgG2 antibodies are protective and promote the phagocytosis and clearance of oral pathogens. The IgG2 antibody response tends to be elicited against carbohydrate antigens, and the oral pathogens (A. actinomycetemcomitans in particular) express serotype-specific carbohydrates that can be opsonized (10, 11, 56, 57).
In recent years, we have gained several insights into the mechanisms that underlie the apparent increase in IgG2 antibody production in LAgP subjects. These studies have been conducted with in vitro cultures of peripheral blood leukocytes (PBL) stimulated with a polyclonal activator, pokeweed mitogen (PWM) (59). LAgP PBL stimulated in this system produce larger amounts of IgG2 antibody than do NP PBL, but IgG1 production is similar for NP and LAgP PBL. These observations are consistent with the levels of IgG1 and IgG2 antibodies in the circulation of LAgP and NP subjects. This culture system has been used to delineate the contributions of the major cell types in PBL (B cells, T cells, monocytes) to the IgG2 antibody response. For example, when LAgP monocytes are added to NP T and B cells, a dose-dependent induction of IgG2 is observed. This effect can be recreated with the cell-free supernatants of LAgP monocytes, suggesting that these cells secrete mediators that augment IgG2 production (27). In contrast, NP monocytes do not augment IgG2 antibody production. Together, these data suggest that LAgP monocytes secrete soluble mediators that augment the IgG2 antibody response.
Activated monocytes produce a variety of immunoregulatory cytokines and lipid mediators, and several of these appear to have roles in the IgG2 response. For example, PWM-stimulated IgG2 production is suppressed by neutralizing antibodies against interleukin-1-α (IL-1-α) or IL-1-β. Neither cytokine can compensate for the other, suggesting that IL-1-α and IL-1-β have nonredundant roles in the IgG2 response (26). The lipid mediator PGE2 selectively induces IgG2 and appears to be essential for optimal induction, as inhibitors of PGE2 synthesis block ∼80% of PWM-stimulated IgG2 production (27). Interestingly, the lipid mediator platelet-activating factor (PAF; 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) appears to have one of the most critical roles in the IgG2 response. PAF receptor antagonists suppress nearly 90% of IgG2 production, and a neutralizing anti-PAF antibody has a similar effect (27). As the biological activities of PAF are mediated through its interaction with a receptor that is expressed on the surfaces of a variety of cells including B cells, T cells, and monocytes (5, 8, 38, 40, 47), the effects of PAF on the IgG2 response may be mediated through the activation of any or all of these cell types. The present investigation was performed to delineate the mechanism by which PAF induces IgG2. Our studies indicate that PAF promotes Th1 responses through the activation of monocytes and lymphocytes but does not directly induce isotype switching to IgG2 by B cells. The PAF-induced Th1 response culminates in the production of gamma interferon (IFN-γ), the cytokine most closely associated with IgG2 production (30, 31). Given the elevated levels of PAF that are present in the cells and tissues of periodontitis patients (18, 21, 35, 37), this may account for the high levels of IgG2 that are present in their sera.
MATERIALS AND METHODS
Human subjects.
Human studies were performed in compliance with all relevant federal guidelines and the institutional policies of Virginia Commonwealth University, Richmond. Buffy coat preparations were obtained from Virginia Blood Services (Richmond, Va.) and were used within 24 h of blood draw. In the IgG2 experiments, subjects for study were obtained by the Clinical Research Center for Periodontal Disease, School of Dentistry, Virginia Commonwealth University. Patients with LAgP were 35 years old or less and had localized patterns of severe periodontal destruction limited to the first molar or incisor teeth and up to two additional teeth. The NP control subjects were age- (within 5 years) and race-matched and had no evidence of attachment loss, except for recession on the buccal surface of anterior teeth at no more than one site and no pockets greater than 3 mm. All subjects were nonsmokers.
PAFAH purification.
Plasma PAF acetylhydrolase (PAFAH) was partially purified using a previously described protocol (50). Briefly, 100 ml of 4% sodium phosphotungstate (pH 7.0) was added to 1 liter of citrated human plasma. Magnesium chloride was added to 45 mM and the resulting solution was centrifuged at 6,000 × g for 10 min. The resultant pellet was resuspended in 1 liter of 0.2% unbuffered sodium citrate, and the lipoproteins were reprecipitated by the addition of MgCl2 to 60 mM and 10 g of NaCl. The reprecipitation was repeated, and the final pellet (containing low-density lipoprotein and very-low-density lipoprotein) was resuspended in 1 liter of 10 mM potassium phosphate (pH 6.8) containing 0.1% Tween 20. The pellet was stirred overnight to solubilize lipoprotein-associated PAFAH, and aliquots were stored at −20°C until use. In some cases, the purified PAFAH was treated with 4-[aminoethyl]benzenesulfonyl fluoride (AEBSF) to inhibit catalytic activity. The protocol for AEBSF treatment was based on a previous report (36) and involved incubation of purified PAFAH with 0.1 mM AEBSF for 30 min at 37°C. After the incubation, the PAFAH was dialyzed against phosphate-buffered saline (PBS) at 4°C overnight and then aliquoted and stored as described above. To determine the extent of inhibition, PAFAH activity was quantified with an assay that has previously been described (1). The AEBSF-treated PAFAH samples contained less than 1% residual catalytic activity (data not shown).
Isolation of leukocytes from peripheral blood.
Heparinized or citrated human peripheral blood (30 ml) was layered over 15 ml of lymphocyte separation media (Cappel) and centrifuged at 400 × g for 30 min. Peripheral blood leukocytes (PBL) were collected from the interface and washed with RPMI media. To obtain adherent monocytes, the PBL were cultured on plastic plates for 1 h at 37°C in 1 ml of RPMI containing 10% heat-inactivated fetal calf serum. Extensive washing was performed to remove nonadherent cells. In all cases, cell viability was at least 90%.
Macrophage (MΦ) cell cultures.
Adherent monocytes were cultured for 7 days in RPMI containing 10% heat-inactivated human AB serum (Biowhittaker), 50 μg of gentamicin/ml (Invitrogen Life Technologies), 2 mM glutamine (Invitrogen Life Technologies), and 1,000 U of recombinant human MΦ colony-stimulating factor (rhM-CSF) (R&D Systems) to induce differentiation to the MΦ phenotype. Cell cultures were maintained at 37°C, 7.5% CO2, and 100% humidity.
Monocyte-derived dendritic cell (DC) cultures.
Adherent monocytes were cultured for 7 days in RPMI containing 10% heat-inactivated fetal calf serum, 50 μg of gentamicin/ml (Invitrogen Life Technologies), and 2 mM glutamine (Invitrogen Life Technologies) in the presence of 500 U of rhIL-4/ml and 800 U of recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF; R&D Systems)/ml. Cell cultures were maintained at 37°C, 7.5% CO2 and 100% humidity.
Naïve B-cell separation.
CD19+ B cells were positively selected from PBL by magnetic labeling using a CD19 Multisort kit (Miltenyi Biotec, Auburn, Calif.) according to the manufacturer's instructions. CD19 microbeads were then released from B cells with the release reagent provided with the Multisort kit. Then, purified B cells were subjected to a second positive selection to isolate naïve B cells. In the second round of magnetic labeling, B cells were labeled with biotin-conjugated mouse anti-human IgD antibodies (Southern Biotech) at 1 μg/106 cells at 4°C for 10 min. This was followed by incubation with streptavidin microbeads (Miltenyi Biotec) at 10 μl/107 cells for 15 min. IgD+ naïve B cells were then positively selected according to the manufacturer's instructions (Miltenyi Biotec). The purity of the naïve B cells was analyzed by flow cytometric analysis with the following fluorescent antibodies: fluorescein isothiocyanate (FITC)-labeled anti-human CD19, FITC-labeled anti-human IgM, FITC-labeled anti-human CD3, or FITC-labeled anti-human CD14 (Pharmingen). The data were collected with a FACScan instrument (Becton-Dickinson) using Cell Quest Pro software and analyzed with WinMDI 2.8 software. All naïve B-cell preparations exceeded 95% CD19+ IgD+ cells (data not shown).
Naïve B-cell culture for analysis of isotype switching.
CD40 ligand (CD40L)-transfected 293 cells (2.5 × 104; kindly provided by Lori Covey, Rutgers University) were added to wells in a 96-well plate and irradiated with 4,000 rads. Then, 5 × 104 naïve B cells/well were added, and the cells were cultured in RPMI media containing 10% heat-inactivated fetal calf serum, 50 μg of gentamicin/ml (Invitrogen), and 2 mM glutamine (Invitrogen) in a 96-well plate in a 300-μl final volume at 37°C in 7.5% CO2 and 100% humidity. The cells were treated with 50 nM PAF (Avanti Polar Lipids), PAF vehicle (dimethyl sulfoxide [DMSO]), or 500 U of rhIFN-γ (R&D Systems)/ml in the absence or presence of 100 U of rhIL-4 (R&D Systems)/ml for 5 days.
RT PCR.
On day 5, the cells were harvested in 0.5 ml of Trizol (Invitrogen), and RNA extraction was performed according to the manufacturer's instructions. Total RNA (0.5 to 1.0 μg) was used in cDNA synthesis with a Thermoscript reverse transcriptase (RT) PCR system (Invitrogen). The reaction was primed with oligo(dT)20, and 5 μl of the 20-μl RT reaction was used to amplify γ1 and γ2 germ line transcripts or mature transcripts in the PCR. To amplify γ1 and γ2 germ line transcripts (IH-CH), the following primers (described in reference 31) were used: sense γ1, 5′ ACGAGGAACATGACTGGATGC 3′; antisense γ1, 5′ TGAGTTTTGTCACAAGATTTGGG 3′; sense γ2, 5′ TCTCAGCCAGGACCAAGGAC 3′; antisense γ2, 5′ ACTCGACACAACATTTGCG 3′. The 5′ primers were complementary to sequences in the Iγ1 or Iγ2 region and the 3′ primers were complementary to sequences in the hinge region of Cγ1 or Cγ2. RT PCR was performed using the hot start method for 30 cycles, with each cycle consisting of 45 s at 94°C, 1 min at 60°C, and 90 s at 72°C (31). The γ1 and γ2 mature transcripts (VH-DJH-CH) were amplified with primers described in reference 12. The antisense γ1 primer was 5′ GTTTTGTCACAAGATTTGGGCTC 3′, the antisense γ2 primer was 5′ GTGGGCACTCGACACAACATTTGCG 3′, and the sense primer for both γ1 and γ2 was in framework region 3 and was 5′ GACACGGCTGTGTATTACTGTGCG 3′. The mature transcripts were amplified in RT PCRs for 30 cycles consisting of 1 min at 94°C, 1 min at 68°C, and 1 min at 72°C. PCR products were electrophoresed in 1.2% agarose gels containing ethidium bromide. The sizes of PCR products were identified using a 100-bp DNA ladder (Invitrogen). The size of the γ1 germ line transcript is 535 bp, the γ2 germ line transcript is 344 bp, and the γ1 and γ2 mature transcripts are both 416 bp. The amplified products were quantified with a Fluorochem 8800 imager (Alpha Innotech).
Cytokine assays.
MΦ, monocyte-derived DCs, and nonadherent PBL were cultured overnight in the presence or absence of PAF (Avanti Polar Lipids) in RPMI containing 5 mg of Fraction V bovine serum albumin (Sigma, St. Louis, Mo.)/ml. The preparation of PAF was determined to be essentially endotoxin free (<0.33 pg of endotoxin/ml) with a Kinetic-QCL LAL testing kit from Biowhittaker. Cell-free supernatants were harvested and screened for IL-12, IL-18, and IFN-γ with enzyme-linked immunosorbent assay (ELISA) kits from Biosource (IL-12) or R&D Systems (IL-18 and IFN-γ). The cell monolayers were solubilized with 0.5% Triton X-100 and harvested to quantify protein mass (Bio-Rad dye binding assay). Cytokine production was normalized to protein recovery. The data are presented as n-fold induction, which is the ratio of cytokine production by PAF-treated cells to production by resting cells.
Antibody production.
PBL (106 per sample) were cultured in RPMI 1640 (Mediatech, Herndon, Va.) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah), 2 mM glutamine, 100 U of penicillin/ml, and 100 mg of streptomycin sulfate (Sigma)/ml and cultured in 75-mm tubes (Falcon, Lincoln Park, N.J.) in a volume of 1 ml/tube at 37°C in a humidified atmosphere of 5% CO2. Nine microliters of PWM was added to the cell cultures to induce antibody production. In some experiments, the cells were cultured in the presence of neutralizing antibodies (R&D Systems) against IL-12 (100 μg/ml) or IL-18 (50 μg/ml) or an isotype-matched control antibody. The cultures were incubated for 7 days, and then supernatant fluids were collected and assayed for IgG2 as outlined below.
IgG2 assays.
IgG2 levels in supernatant fluids were determined by a sandwich ELISA as described previously (4). Briefly, Fc-specific goat anti-mouse antibody (10 μl/ml) (ICN, Aurora, Ohio) in pH 9.6 carbonate buffer was used to coat the microtiter plates (Dynex, Chantilly, Va.). Mouse anti-human IgG2 monoclonal antibody (1 μg/ml) (Sigma) was then added to the plates to serve as capture antibody. The plates were incubated overnight at 4°C and washed, and bovine serum albumin-PBS was applied to minimize nonspecific binding. A known standard for human IgG2 antibody and the culture supernatant fluids were applied to different wells in plates and incubated for 1 h at room temperature. Horseradish peroxidase-conjugated goat anti-human immunoglobulin (Sigma) was added and the plates were incubated for 1 h at room temperature. The plates were washed three times with PBS containing 0.05% Tween 20 between each step. The color was developed using O-phenylenediamine dihydrochloride (Sigma) as substrate. Optical density at 450 nm was measured using a microplate reader (Dynex). The IgG2 concentration in each test sample was calculated using the standard curve generated in each assay.
Statistical analyses.
All experiments were replicated with at least three different individuals or preparations of cells. Each experimental analysis was performed in triplicate, and the mean and standard deviations of each set of replicates were determined. For the neutralizing antibody experiments, statistical significance was determined through unpaired t tests, with a P of <0.05 as the cutoff for significance. In the cytokine experiments, the data were not normally distributed, so analyses were performed on log transforms. Responses within an experiment were modeled with a mixed-model repeated-measures analysis of variance. For the experiments with exogenous PAFAH, the percentage of control was analyzed using the difference of the logs of the two values in a repeated-measures mixed-model analysis (SAS version 8). The predicted values and confidence intervals were then back-transformed to the original scale for presentation.
RESULTS
Exogenous PAFAH selectively suppresses IgG2 production.
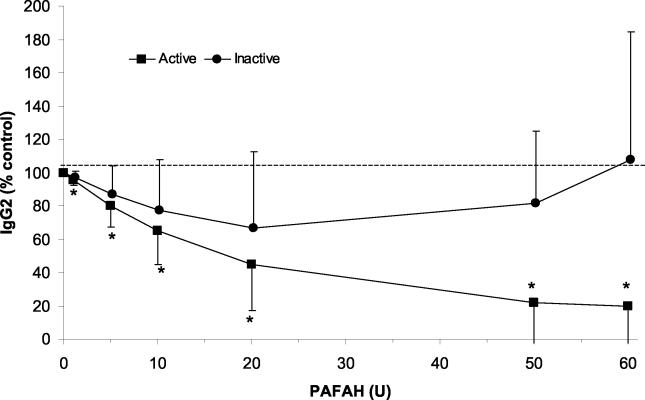
The monocytes of LAgP subjects contain lower levels of PAFAH (the phospholipase that catabolizes PAF) than do monocytes from periodontally healthy (NP) subjects (1). LAgP leukocytes produce large amounts of IgG2 when stimulated in vitro (59), and we hypothesized that this might be a reflection of the low levels of PAFAH that are produced by LAgP monocytes. To test this hypothesis, we determined the effect of exogenous PAFAH on PWM-stimulated IgG2 production. Various doses of partially purified PAFAH were added to PBL stimulated with 9 μl of PWM/ml. Cell-free supernatants were collected and IgG2 was quantified with an ELISA. As shown in Fig. 1, PAFAH suppressed IgG2 production in a dose-dependent manner. The exogenous enzyme suppressed IgG2 production by up to ∼80%. This degree of suppression is similar to that observed with a PAF receptor antagonist and an anti-PAF antibody (27). Catalytically inactive PAFAH (prepared by treating the enzyme with the serine-modifying agent AEBSF) had no effect on IgG2 production. Exogenous PAFAH did not suppress IgG1 production (data not shown), consistent with previous data showing the IgG2-specific effects of PAF (27).
FIG. 1.
Exogenous PAFAH suppresses IgG2 production. PBL were cultured for 7 days with 9 μl of PWM in the presence or absence of active or inactive PAFAH. Cell-free supernatants were collected and IgG2 antibody levels were quantified as described in Materials and Methods. The data are presented as percentages of control IgG2 production, where the control is PBL cultured with PWM in the absence of exogenous PAFAH. The data shown are compiled from four to six independent experiments with PBL from different individuals. Error bars indicate 95% confidence intervals. The dotted line indicates control response (100%). *, IgG2 production was lower in active PAFAH-treated samples (P < 0.05).
PAF induces Th1-associated cytokines that are essential for optimal IgG2 responses.
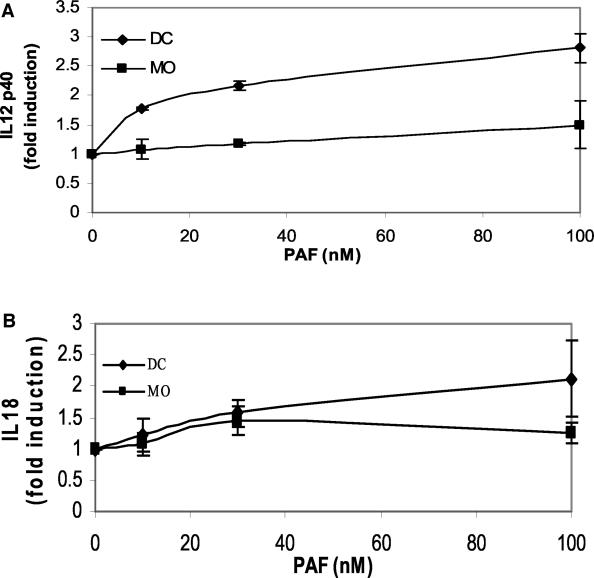
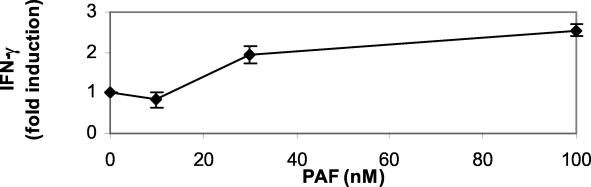
The data in Fig. 1 supported the hypothesis that PAF plays an essential role in the IgG2 response in LAgP subjects. We next sought to determine the mechanism of IgG2 induction by PAF and initially focused our attention on its effects on monocytes. Monocytes are the precursors of two important immunoregulatory cell types, DCs and MΦ, and are known to respond to PAF (1). It was recently demonstrated that LAgP monocytes exhibit a propensity to differentiate into DCs, compared to NP monocytes that primarily differentiate into MΦ (4). Enriched cultures of DCs and MΦ were prepared by culturing adherent NP monocytes in IL-4 plus GM-CSF or in M-CSF, respectively. After differentiation, the cells were treated with various doses of PAF. The cell-free supernatants were collected and screened for IL-12 and IL-18, two proinflammatory cytokines that are essential for optimal IgG2 antibody responses. PBL were cultured with PWM in the presence of neutralizing antibodies against IL-12 (100 μg/ml) and IL-18 (50 μg/ml) or an isotype-matched control antibody. Culture supernatants were harvested and screened for IgG2. An isotype-matched control antibody had no effect on IgG2 production. The data were compiled from three independent experiments with different individuals. Both anti-IL-12 and anti-IL-18 suppressed IgG2 production (P < 0.05, Student's t test). IgG2 production was 28% ± 5% and 23% ± 6% of control production in the anti-IL-12- and anti-IL-18-treated cultures, respectively. As shown in Fig. 2A, PAF induced DCs to secrete IL-12 while MΦ made no comparable response. A similar trend was observed for IL-18 (Fig. 2B), although the IL-18 response of PAF-treated DCs was not as robust as was the IL-12 response. The involvement of IL-12 and IL-18 in the IgG2 response is somewhat incongruous with the accepted paradigm of T-cell biology that segregates T-cell immunity into Th1 responses (associated with cell-mediated immunity and inflammation) and Th2 responses (associated with antibody responses) (51). However, other investigators have shown that IFN-γ, the prototypical Th1 cytokine, is essential for IgG2 antibody responses (30, 31). Thus, another potential role for PAF in the IgG2 response may be the induction of IFN-γ production by lymphocytes. To test this hypothesis, we treated nonadherent PBL with various doses of PAF, collected the cell-free supernatants, and screened for IFN-γ. These cultures contain primarily lymphocytes, although a limited number of monocytes may be present as well. In these lymphocyte-enriched cultures, PAF induced IFN-γ in a dose-dependent manner (Fig. 3). It is possible that this IFN-γ production is mediated through direct interactions between PAF and lymphocytes. Alternatively, residual monocytes in the cultures may be activated by PAF and produce IL-12 and IL-18 that then act on lymphocytes to induce IFN-γ. Together with the analysis of IL-12 and IL-18, these observations suggest that PAF promotes the production of IgG2 by inducing the Th1-associated cytokines that are essential for maximal responses.
FIG. 2.
DCs produce Th1-associated cytokines in response to PAF. DCs and MΦ (MO) were prepared from adherent monocytes as described in Materials and Methods. The cells were cultured overnight with various doses of PAF, and cell-free supernatants were harvested and screened for IL-12 (A) or IL-18 (B). Cytokine levels were normalized for protein recovery, and the data are presented as n-fold induction of cytokine over control (cells cultured in DMSO, the vehicle). The data shown are compiled from two to four independent experiments with monocytes from different individuals. PAF induced DCs to produce both IL-12 and IL-18 (P < 0.05) in a dose-dependent manner, while MΦ did not make this response.
FIG. 3.
Lymphocytes produce IFN-γ in response to PAF. Nonadherent PBL (primarily lymphocytes) were cultured overnight with various doses of PAF, and cell-free supernatants were harvested and screened for IFN-γ. Cytokine levels were normalized for protein recovery, and the data are presented as n-fold induction of cytokine over control (cells cultured in DMSO, the vehicle). The data shown are compiled from three independent experiments with lymphocytes from different individuals. PAF induced lymphocytes to produce IFN-γ in a dose-dependent manner (P < 0.05).
PAF does not induce isotype switching.
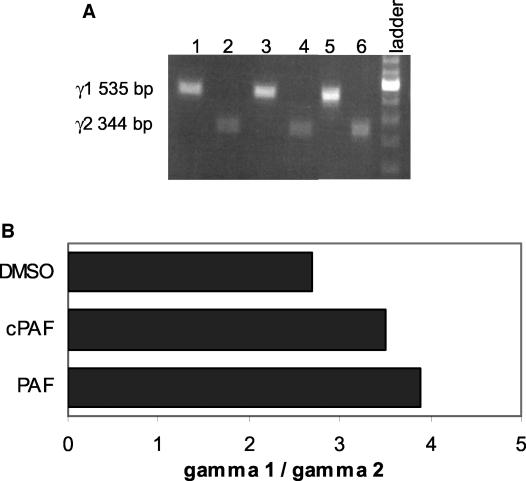
Other investigators have demonstrated that B cells express PAF receptors and can respond to this lipid mediator (5, 20). Therefore, we considered the possibility that PAF might act directly on B cells to induce isotype switching to IgG2. To address this hypothesis, naïve IgD+ B cells were stimulated with CD40L in the presence of various amounts of PAF, and RNA was isolated and screened for the presence of γ1 and γ2 germ line transcripts, essential prerequisites to isotype switching to IgG1 and IgG2, respectively. Raw data from a representative experiment are shown in Fig. 4A and a quantification of these data are shown in Fig. 4B. As expected, unstimulated naïve B cells expressed neither γ1 nor γ2 germ line transcripts (data not shown). However, stimulation with CD40L induced both γ1 and γ2 germ line transcripts. Similar ratios of γ1 to γ2 germ line transcripts were observed when CD40L-stimulated B cells were cultured with PAF, methylcarbamyl PAF (cPAF; nonhydrolyzable PAF analog), or vehicle control (DMSO). Other investigators have demonstrated that IL-4 synergizes with other cytokines to isotype switching (19, 23, 54). Therefore, we investigated the possibility that IL-4 might be required for PAF-induced isotype switching to IgG2. However, IL-4 coculture did not alter the ratio of γ1 to γ2 germ line transcripts in PAF- or cPAF-treated B cells (data not shown). These data suggest that PAF does not act directly on B cells to promote isotype switching but rather that the induction of IgG2 by PAF is mediated through its ability to activate accessory cells and induce the Th1 response and IFN-γ.
FIG. 4.
PAF does not induce γ2 germ line transcription in B cells. (A) Naïve B cells were cultured with CD40L-transfected 293 cells in the presence of vehicle (lanes 1 and 2), 100 nM PAF (lanes 3 and 4), or 100 nM cPAF (lanes 5 and 6). RNA was harvested and RT PCR performed to amplify γ1 (535 bp, lanes 1, 3, and 5) or γ2 (344 bp, lanes 2, 4, and 6) germ line transcripts. Data from a representative experiment are shown. The experiment was performed three times with similar results. (B) Ratio of intensity of γ1/γ2 germ line transcripts for this experiment. Band intensity was quantified with a Fluorochem 8000 imager.
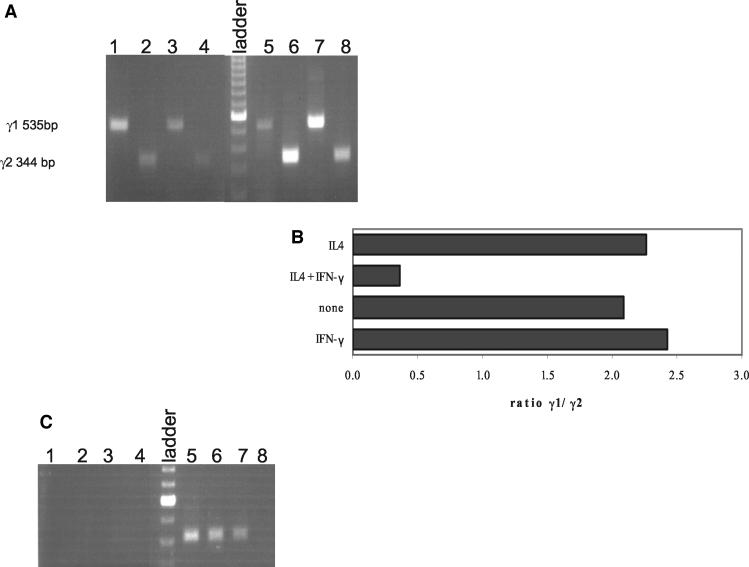
Although the necessity of IFN-γ for IgG2 production is well established, the mechanism is under dispute, with some studies suggesting a role in isotype switching while others suggest that IFN-γ affects later steps in the differentiation of IgG2-producing B cells (30, 31). Therefore, we performed experiments to investigate the role of IFN-γ in the IgG2 response. CD40L-stimulated naïve B cells were used to determine if IFN-γ induced γ2 germ line transcription. Similar to the experiment shown in Fig. 4, neither γ1 nor γ2 germ line transcripts were present in unstimulated naïve B cells (data not shown), but both were induced upon stimulation with CD40L (Fig. 5A and B) and were further induced in cells cultured with CD40L in the presence of IL-4 (compare lanes 3 and 4 to lanes 7 and 8). The ratio of γ1 to γ2 germ line transcripts was unchanged when CD40L-stimulated B cells were cultured with IFN-γ or IL-4 alone. However, both γ2 germ line (Fig. 5A and B) and mature (Fig. 5C) transcripts were induced when naïve B cells were treated with IL-4 and IFN-γ together. Thus, it appears that the role of PAF in the IgG2 response is in the induction of the Th1 cytokine IFN-γ that then synergizes with IL-4 to induce isotype switching to IgG2.
FIG. 5.
IL-4 and IFN-γ synergize to induce isotype switching by B cells. (A and B) Naïve B cells were cultured with CD40L-transfected 293 cells in the presence of 500 U of IFN-γ/ml (lanes 1 and 2), no addition (lanes 3 and 4), 500 U of IFN-γ/ml plus 100 U of IL-4/ml (lanes 5 and 6), or 100 U of IL-4/ml (lanes 7 and 8). RNA was harvested and RT PCR performed to amplify γ1 (535 bp, lanes 1, 3, 5, and 7) or γ2 (344 bp, lanes 2, 4, 6, and 8) germ line transcripts. Data from a representative experiment are shown. The experiment was performed three times with similar results. (A) Ethidium bromide image of agarose gel. (B) Ratio of intensity of γ1/γ2 germ line transcripts for this experiment. Band intensity was quantified with a Fluorochem 8000 imager. (C) RNA was harvested and RT PCR performed to amplify γ1 (410 bp, lanes 1, 3, 5, and 7) or γ2 (410 bp, lanes 2, 4, 6, and 8) mature transcripts from naïve B cells cultured with CD40L-transfected 293 cells and 500 U of IFN-γ/ml (lanes 1 and 2), no addition (lanes 3 and 4), 500 U of IFN-γ/ml plus 100 U of IL-4/ml (lanes 5 and 6), or 100 U of IL-4/ml (lanes 7 and 8). Data from a representative experiment are shown. The experiment was performed three times with similar results.
DISCUSSION
In previous studies, it was demonstrated that PAF is essential for optimal IgG2 antibody responses (27). The goal of this investigation was to delineate the mechanism by which PAF induces IgG2. Our data indicate that PAF activates monocytes to produce the Th1-associated cytokines IL-12 and IL-18 (Fig. 2) and potentially activates lymphocytes to produce IFN-γ (Fig. 3), a Th1 cytokine that other investigators have implicated in the induction of IgG2 (30, 31). We also show that IFN-γ induces B cells to undergo isotype switching to IgG2, but only in concert with the Th2 cytokine IL-4. Thus, both Th1 and Th2 cytokines are necessary for optimal IgG2 production. This requirement for the two classes of helper T-cell cytokines distinguishes IgG2 from other isotypes of antibody in the human system.
The commonly accepted paradigm dictates that antibody responses are induced by Th2 cytokines while Th1 cytokines are associated with inflammation and cell-mediated immunity (51). However, there is ample evidence in the human system for the dependence of IgG2 antibody on IFN-γ, the quintessential Th1 cytokine (30, 31). Similarly, in the murine system, exogenous IFN-γ induces IgG2a and IgG3, and these isotypes are suppressed when B cells are cultured in the presence of neutralizing antibodies against IFN-γ (6, 16, 32, 48). These observations are consistent with the recently reported requirement for the Th1-associated transcription factor T-bet in isotype switching to IgG2a and for the production of IgG2a, IgG2b, and IgG3 in the mouse system (39). Similarly, T cells from ICOS-deficient mice produce large amounts of IFN-γ, and the IgG3 response against T-independent antigens is elevated in these mice (52). Although T cells are required for optimal IgG2 responses in our PWM-stimulated system (59), it remains possible that T cells are not the only source of IFN-γ in our cell cultures. For example, activated natural killer (NK) cells produce large amounts of this cytokine (14). Thus, it is possible that the IL-12 produced by PAF-stimulated DCs activates NK cells that then produce IFN-γ and thereby induce IgG2.
The mechanism of the IFN-γ-mediated induction of human IgG2 remains somewhat controversial. By using preparations of human PBL, Kawano et al. (30) demonstrated that IFN-γ induced IgG2 secretion but that this did not occur if surface IgG2+ B cells were depleted from the PBL. This observation suggests that the effects of IFN-γ are limited to cells that have already committed to the IgG2-producing lineage and therefore occur after isotype switching has occurred. In contrast, Kitani and Strober (31) have shown that exogenous IFN-γ induces γ2 germ line transcription, an essential event that must take place before isotype switching to IgG2 can occur. Our observations are more consistent with those of Kitani and Strober, as IFN-γ enhanced the levels of γ2 germ line transcripts in our IgD+ naïve B cells while suppressing γ1 germ line transcription (Fig. 5A) and induced γ2 mature transcripts (Fig. 5C). The data in Fig. 5C suggest that IL-4 is essential for the generation of mature γ1 and γ2 transcripts. Although we cannot rule out the possibility that the mature γ1 and γ2 transcripts were present but not amplified in naïve B cells cultured with CD40L in the absence of IL-4, our results are consistent with other studies. For example, Ford et al. (19) demonstrated that Ramos B cells produced mature γ1 and γ2 transcripts only when cultured in the presence of both CD40L and IL-4. Cerutti et al. (12) reported similar observations for γ2 mature transcripts but observed a low level of γ1 mature transcripts in CL01 B cells cultured with CD40L alone. Thus, although it is possible that the apparent IL-4 dependence of γ1 mature transcription is an artifact, our results for γ2 mature transcripts are consistent with other published data suggesting that IL-4 is essential for this event.
Our data suggest that the Th2 cytokine IL-4 is required for IFN-γ-mediated induction of γ2 isotype switching. Other investigators have demonstrated the induction of γ2 isotype switching by IL-4, and this is at least partially attributed to the effects of IL-4 as B-cell growth factor (12). As LAgP is an inflammatory disorder associated with elevated PAF and Th1 cytokines, one might question the availability of IL-4 in LAgP subjects. It has been reported that LAgP monocytes produce large amounts of PGE2, a proinflammatory lipid mediator that is also known to mediate bone resorption by activating osteoclasts (45). We have recently demonstrated that PGE2 acts on lymphocytes to elicit IFN-γ (53). Interestingly, PGE2 also has anti-inflammatory properties, as it both induces Th0 cells to adopt the Th2 phenotype and suppresses the differentiation of Th1 cells (24, 28, 29). Thus, we predict that activated LAgP monocytes secrete PGE2 that then drives Th2 cells to produce the IL-4 required to synergize with IFN-γ to induce isotype switching to IgG2. This hypothesis may appear inconsistent with the inflammatory Th1 nature of LAgP and with previous reports of a strong Th1 bias in LAgP (43, 53). However, we suggest that LAgP subjects produce both IFN-γ and IL-4, with the Th1 cytokine dominating the response, and that relatively low levels of IL-4 are sufficient to induce isotype switching.
PAF is a bioactive phospholipid that is primarily produced by activated monocytes and neutrophils (40). The biological activities of PAF are mediated through its interaction with a Gi-coupled receptor that is expressed on the surfaces of a variety of cells. Many of the biological activities of PAF are associated with the inflammatory response. For example, PAF induces the activation and aggregation of platelets, eicosanoid production by monocytes, the release of proinflammatory cytokines from monocytes and neutrophils, and the adhesion of activated leukocytes to the endothelium (13). LAgP is an inflammatory disease and several lines of evidence suggest that PAF has roles in its pathogenesis. The potential connection between PAF and periodontitis was recognized as far back as 1989, when it was reported that PAF levels were elevated in inflamed gingival tissues (37). Since that initial report, elevated PAF levels have been demonstrated in the saliva, gingival crevicular fluid, and gingival tissues of periodontitis patients (18, 35), including subjects with LAgP. It is likely that the high levels of PAF observed in LAgP and other periodontal diseases are at least partially due to increased synthesis, as activated inflammatory cells are the primary sources of PAF (40). However, PAF levels are also influenced by the rate of catabolism. PAF is catabolized by PAFAH, a secreted phospholipase A2 that is primarily produced by monocytes. When monocytes differentiate into MΦ, PAFAH expression increases nearly 100-fold (17). We have recently demonstrated that another form of differentiated monocyte, the monocyte-derived DC, expresses very low levels of PAFAH and secretes almost none of this activity. As a result, DCs secrete large amounts of PGE2 in response to PAF, while the response of MΦ is much more limited (1). Similar results were obtained in the present investigation, as PAF stimulated DCs but not MΦ to produce IL-12 and IL-18 (Fig. 2A and B). These observations are of particular interest because monocyte differentiation appears to be skewed to the DC phenotype in LAgP subjects (4). Indeed, PAFAH levels are lower in the adherent monocytes of LAgP subjects than in monocytes from race-matched NP controls (1). Thus, we predict that the skewing of monocyte differentiation in LAgP subjects results in low levels of the catabolic enzyme PAFAH and that this allows the bioactive lipid PAF to accumulate and thereby activate inflammatory cells.
A positive correlation has been observed between PAF levels and disease severity, which suggests that PAF may have a causative role in the disease process (21, 35). Indeed, periodontal therapies not only reduce the indices of disease but also result in reductions in salivary PAF (41). Furthermore, PAF activates monocytes and thereby elicits the production of PGE2 (3, 7, 55), another lipid mediator that then activates osteoclasts and thereby elicits bone resorption. Together, these data strongly suggest that there is a correlation between PAF and disease progression in LAgP. However, given its roles in the protective IgG2 antibody response, we contend that PAF should be viewed as a “doubled-edged sword.” IgG2 levels are elevated in LAgP subjects, and patients with the highest titers of IgG2 reactive with A. actinomycetemcomitans and P. gingivalis tend to have fewer sites with ≥2 mM attachment loss (9, 22). Thus, we suggest that the high levels of PAF that accumulate in LAgP subjects both contribute to disease progression and the production of protective IgG2 antibodies. Carbohydrate antigens tend to elicit the IgG2 response (42, 49). Both A. actinomycetemcomitans and P. gingivalis, the oral pathogens most closely associated with LAgP, are rich in carbohydrate antigens, and much of the IgG2 antibody response in LAgP is directed against these organisms (10, 11, 57). Thus, the adaptive immune system in LAgP subjects takes advantage of the chronic inflammation associated with the disease to generate a protective antibody response. This is largely dependent on the unique phenotype of the LAgP monocyte and its impact on PAF metabolism.
Acknowledgments
We thank Kimberley Lake for coordinating the acquisition of human subjects and Gail Smith for phlebotomy.
This work was supported by a grant from the National Institute of Dental and Craniofacial Research, DE-13102.
REFERENCES
- 1.Al-Darmaki, S., H. A. Schenkein, J. G. Tew, and S. E. Barbour. 2003. Differential expression of platelet-activating factor acetylhydrolase in macrophages and monocyte-derived dendritic cells. J. Immunol. 170:167-173. [DOI] [PubMed] [Google Scholar]
- 2.Baer, P. N. 1971. The case for periodontosis as a clinical entity. J. Periodontol. 42:516-520. [DOI] [PubMed] [Google Scholar]
- 3.Barbour, S. E., and E. A. Dennis. 1993. Antisense inhibition of group II phospholipase A2 expression blocks the production of prostaglandin E2 by P388D1 cells. J. Biol. Chem. 268:21875-21882. [PubMed] [Google Scholar]
- 4.Barbour, S. E., Y. Ishihara, M. Fakher, S. Al-Darmaki, T. H. Caven, C. P. Shelburne, A. M. Best, H. A. Schenkein, and J. G. Tew. 2002. Monocyte differentiation in localized juvenile periodontitis is skewed toward the dendritic cell phenotype. Infect. Immun. 70:2780-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastien, Y., B. J. Toledano, N. Mehio, L. Cameron, B. Lamoukhaid, P. Renzi, Q. Hamid, and B. D. Mazer. 1999. Detection of functional platelet-activating factor receptors on human tonsillar B lymphocytes. J. Immunol. 162:5498-5505. [PubMed] [Google Scholar]
- 6.Bossie, A., and E. S. Vitetta. 1991. IFN-gamma enhances secretion of IgG2a from IgG2a-committed LPS-stimulated murine B cells: implications for the role of IFN-gamma in class switching. Cell. Immunol. 135:95-104. [DOI] [PubMed] [Google Scholar]
- 7.Bulger, E. M., S. Arbabi, I. Garcia, and R. V. Maier. 2002. The macrophage response to endotoxin requires platelet activating factor. Shock 17:173-179. [DOI] [PubMed] [Google Scholar]
- 8.Calabresse, C., M. C. Nguer, O. Pellegrini, J. Benveniste, Y. Richard, and Y. Thomas. 1992. Induction of high-affinity paf receptor expression during T cell activation. Eur. J. Immunol. 22:1349-1355. [DOI] [PubMed] [Google Scholar]
- 9.Califano, J. V., J. C. Gunsolley, K. Nakashima, H. A. Schenkein, M. E. Wilson, and J. G. Tew. 1996. Influence of anti-Actinobacillus actinomycetemcomitans Y4 (serotype b) lipopolysaccharide on severity of generalized early-onset periodontitis. Infect. Immun. 64:3908-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Califano, J. V., H. A. Schenkein, and J. G. Tew. 1992. Immunodominant antigens of Actinobacillus actinomycetemcomitans serotype b in early-onset periodontitis patients. Oral Microbiol. Immunol. 7:65-70. [DOI] [PubMed] [Google Scholar]
- 11.Califano, J. V., R. E. Schifferle, J. C. Gunsolley, A. M. Best, H. A. Schenkein, and J. G. Tew. 1999. Antibody reactive with Porphyromonas gingivalis serotypes K1-6 in adult and generalized early-onset periodontitis. J. Periodontol. 70:730-735. [DOI] [PubMed] [Google Scholar]
- 12.Cerutti, A., H. Zan, A. Schaffer, L. Bergsagel, N. Harindranath, E. E. Max, and P. Casali. 1998. CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+IgD+ B cell line. J. Immunol. 160:2145-2157. [PMC free article] [PubMed] [Google Scholar]
- 13.Chu, E., L. C. Casey, J. E. Harris, and D. P. Braun. 1993. Suppression of the development of tumoricidal function in gamma interferon-treated human peripheral blood monocytes by lipopolysaccharide: the role of cyclooxygenase metabolites. J. Clin. Immunol. 13:49-57. [DOI] [PubMed] [Google Scholar]
- 14.Cooper, M. A., T. A. Fehniger, A. Fuchs, M. Colonna, and M. A. Caligiuri. 2004. NK cell and DC interactions. Trends Immunol. 25:47-52. [DOI] [PubMed] [Google Scholar]
- 15.Daniel, M. A., G. McDonald, S. Offenbacher, and T. E. Van Dyke. 1993. Defective chemotaxis and calcium response in localized juvenile periodontitis neutrophils. J. Periodontol. 64:617-621. [DOI] [PubMed] [Google Scholar]
- 16.Dobber, R., M. Tielemans, and L. Nagelkerken. 1995. The in vivo effects of neutralizing antibodies against IFN-gamma, IL-4, or IL-10 on the humoral immune response in young and aged mice. Cell. Immunol. 160:185-192. [DOI] [PubMed] [Google Scholar]
- 17.Elstad, M. R., D. M. Stafforini, T. M. McIntyre, S. M. Prescott, and G. A. Zimmerman. 1989. Platelet activating factor acetylhydrolase increases during macrophage differentiation. J. Biol. Chem. 264:8467-8470. [PubMed] [Google Scholar]
- 18.Emingil, G., S. Cinarcik, H. Baylas, and A. Huseyinov. 2001. Levels of platelet-activating factor in gingival crevicular fluid and gingival tissue in specific periodontal diseases. J. Periodontol. 72:1032-1037. [DOI] [PubMed] [Google Scholar]
- 19.Ford, G. S., C. H. Yin, B. Barnhart, K. Sztam, and L. R. Covey. 1998. CD40 ligand exerts differential effects on the expression of I gamma transcripts in subclones of an IgM+ human B cell lymphoma line. J. Immunol. 160:595-605. [PubMed] [Google Scholar]
- 20.Franklin, R. A., A. Tordai, B. Mazer, N. Terada, J. Lucas, and E. W. Gelfand. 1995. Platelet activating factor activates MAPK and increases in intracellular calcium via independent pathways in B lymphocytes. Biochem. Biophys. Res. Commun. 209:1111-1118. [DOI] [PubMed] [Google Scholar]
- 21.Garito, M. L., T. J. Prihoda, and L. M. McManus. 1995. Salivary PAF levels correlate with the severity of periodontal inflammation. J. Dent. Res. 74:1048-1056. [DOI] [PubMed] [Google Scholar]
- 22.Gunsolley, J. C., J. A. Burmeister, J. G. Tew, A. M. Best, and R. R. Ranney. 1987. Relationship of serum antibody to attachment level patterns in young adults with juvenile periodontitis or generalized severe periodontitis. J. Periodontol. 58:314-320. [DOI] [PubMed] [Google Scholar]
- 23.Hasbold, J., J. S. Hong, M. R. Kehry, and P. D. Hodgkin. 1999. Integrating signals from IFN-gamma and IL-4 by B cells: positive and negative effects on CD40 ligand-induced proliferation, survival, and division-linked isotype switching to IgG1, IgE, and IgG2a. J. Immunol. 163:4175-4181. [PubMed] [Google Scholar]
- 24.Hilkens, C. M., H. Vermeulen, R. J. van Neerven, F. G. Snijdewint, E. A. Wierenga, and M. L. Kapsenberg. 1995. Differential modulation of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur. J. Immunol. 25:59-63. [DOI] [PubMed] [Google Scholar]
- 25.Hurttia, H. M., L. M. Pelto, and L. Leino. 1997. Evidence of an association between functional abnormalities and defective diacylglycerol kinase activity in peripheral blood neutrophils from patients with localized juvenile periodontitis. J. Periodont. Res. 32:401-407. [DOI] [PubMed] [Google Scholar]
- 26.Ishihara, Y., J. B. Zhang, M. Fakher, A. M. Best, H. A. Schenkein, S. E. Barbour, and J. G. Tew. 2001. Non-redundant roles for interleukin-1 alpha and interleukin-1 beta in regulating human IgG2. J. Periodontol. 72:1332-1339. [DOI] [PubMed] [Google Scholar]
- 27.Ishihara, Y., J. B. Zhang, S. M. Quinn, H. A. Schenkein, A. M. Best, S. E. Barbour, and J. G. Tew. 2000. Regulation of immunoglobulin G2 production by prostaglandin E2 and platelet-activating factor. Infect. Immun. 68:1563-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapsenberg, M. L., C. M. Hilkens, T. C. van Der Pouw Kraan, E. A. Wierenga, and A. Snijders. 1996. The role of accessory cell products in the regulation of T cell cytokine production. Adv. Exp. Med. Biol. 409:305-308. [DOI] [PubMed] [Google Scholar]
- 29.Katamura, K., N. Shintaku, Y. Yamauchi, T. Fukui, Y. Ohshima, M. Mayumi, and K. Furusho. 1995. Prostaglandin E2 at priming of naive CD4+ T cells inhibits acquisition of ability to produce IFN-gamma and IL-2, but not IL-4 and IL-5. J. Immunol. 155:4604-4612. [PubMed] [Google Scholar]
- 30.Kawano, Y., T. Noma, and J. Yata. 1994. Regulation of human IgG subclass production by cytokines. IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J. Immunol. 153:4948-4958. [PubMed] [Google Scholar]
- 31.Kitani, A., and W. Strober. 1993. Regulation of C gamma subclass germ-line transcripts in human peripheral blood B cells. J. Immunol. 151:3478-3488. [PubMed] [Google Scholar]
- 32.Lin, M. S., and Y. W. Chen. 1993. B cell differentiation. II. Isotype potential of a single B cell. Cell. Immunol. 150:343-352. [DOI] [PubMed] [Google Scholar]
- 33.Lu, H., M. Wang, J. C. Gunsolley, H. A. Schenkein, and J. G. Tew. 1994. Serum immunoglobulin G subclass concentrations in peridontally healthy and diseased individuals. Infect. Immun. 62:1677-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manson, J. D., and T. Lehner. 1974. Clinical features of juvenile periodontitis (periodontosis). J. Periodontol. 45:636-640. [DOI] [PubMed] [Google Scholar]
- 35.McManus, L. M., and R. N. Pinckard. 2000. PAF, a putative mediator of oral inflammation. Crit. Rev. Oral Biol. Med. 11:240-258. [DOI] [PubMed] [Google Scholar]
- 36.Nakabo, Y., and M. J. Pabst. 1996. Lysis of leukemic cells by human macrophages: inhibition by 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF), a serine protease inhibitor. J. Leukoc. Biol. 60:328-336. [DOI] [PubMed] [Google Scholar]
- 37.Noguchi, K., I. Morita, and S. Murota. 1989. The detection of platelet-activating factor in inflamed human gingival tissue. Arch. Oral Biol. 34:37-41. [DOI] [PubMed] [Google Scholar]
- 38.Ouellet, S., E. Muller, and M. Rola-Pleszczynski. 1994. IFN-gamma up-regulates platelet-activating factor receptor gene expression in human monocytes. J. Immunol. 152:5092-5099. [PubMed] [Google Scholar]
- 39.Peng, S. L., S. J. Szabo, and L. H. Glimcher. 2002. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl. Acad. Sci. USA 99:5545-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prescott, S. M., G. A. Zimmerman, D. M. Stafforini, and T. M. McIntyre. 2000. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 69:419-445. [DOI] [PubMed] [Google Scholar]
- 41.Rasch, M. S., B. L. Mealey, T. J. Prihoda, D. S. Woodard, and L. M. McManus. 1995. The effect of initial periodontal therapy on salivary platelet-activating factor levels in chronic adult periodontitis. J. Periodontol. 66:613-623. [DOI] [PubMed] [Google Scholar]
- 42.Rijkers, G. T., L. A. Sanders, and B. J. Zegers. 1993. Anti-capsular polysaccharide antibody deficiency states. Immunodeficiency 5:1-21. [PubMed] [Google Scholar]
- 43.Salvi, G. E., C. E. Brown, K. Fujihashi, H. Kiyono, F. W. Smith, J. D. Beck, and S. Offenbacher. 1998. Inflammatory mediators of the terminal dentition in adult and early onset periodontitis. J. Periodont. Res. 33:212-225. [DOI] [PubMed] [Google Scholar]
- 44.Shapira, L., W. A. Soskolne, M. N. Sela, S. Offenbacher, and V. Barak. 1994. The secretion of PGE2, IL-1 beta, IL-6, and TNF alpha by adherent mononuclear cells from early onset periodontitis patients. J. Periodontol. 65:139-146. [DOI] [PubMed] [Google Scholar]
- 45.Shapira, L., W. A. Soskolne, and T. E. Van Dyke. 1996. Prostaglandin E2 secretion, cell maturation, and CD14 expression by monocyte-derived macrophages from localized juvenile periodontitis patients. J. Periodontol. 67:224-228. [DOI] [PubMed] [Google Scholar]
- 46.Sigusch, B., S. Eick, W. Pfister, G. Klinger, and E. Glockmann. 2001. Altered chemotactic behavior of crevicular PMNs in different forms of periodontitis. J. Clin. Periodontol. 28:162-167. [DOI] [PubMed] [Google Scholar]
- 47.Simon, H. U., P. W. Tsao, K. A. Siminovitch, G. B. Mills, and K. Blaser. 1994. Functional platelet-activating factor receptors are expressed by monocytes and granulocytes but not by resting or activated T and B lymphocytes from normal individuals or patients with asthma. J. Immunol. 153:364-377. [PubMed] [Google Scholar]
- 48.Snapper, C. M., T. M. McIntyre, R. Mandler, L. M. Pecanha, F. D. Finkelman, A. Lees, and J. J. Mond. 1992. Induction of IgG3 secretion by interferon gamma: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J. Exp. Med. 175:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soininen, A., I. Seppala, T. Nieminen, J. Eskola, and H. Kayhty. 1999. IgG subclass distribution of antibodies after vaccination of adults with pneumococcal conjugate vaccines. Vaccine 17:1889-1897. [DOI] [PubMed] [Google Scholar]
- 50.Stafforini, D. M., T. M. McIntyre, and S. M. Prescott. 1990. Platelet-activating factor acetylhydrolase from human plasma. Methods Enzymol. 187:344-357. [DOI] [PubMed] [Google Scholar]
- 51.Szabo, S. J., B. M. Sullivan, S. L. Peng, and L. H. Glimcher. 2003. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 21:713-758. [DOI] [PubMed] [Google Scholar]
- 52.Tafuri, A., A. Shahinian, F. Bladt, S. K. Yoshinaga, M. Jordana, A. Wakeham, L. M. Boucher, D. Bouchard, V. S. Chan, G. Duncan, B. Odermatt, A. Ho, A. Itie, T. Horan, J. S. Whoriskey, T. Pawson, J. M. Penninger, P. S. Ohashi, and T. W. Mak. 2001. ICOS is essential for effective T-helper-cell responses. Nature 409:105-109. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka, S., S. E. Barbour, A. M. Best, H. A. Schenkein, and J. G. Tew. 2003. Prostaglandin E2-mediated regulation of immunoglobulin G2 via interferon gamma. J. Periodontol. 74:771-779. [DOI] [PubMed] [Google Scholar]
- 54.Tangye, S. G., A. Ferguson, D. T. Avery, C. S. Ma, and P. D. Hodgkin. 2002. Isotype switching by human B cells is division-associated and regulated by cytokines. J. Immunol. 169:4298-4306. [DOI] [PubMed] [Google Scholar]
- 55.Thivierge, M., and M. Rola-Pleszczynski. 1995. Up-regulation of inducible cyclooxygenase gene expression by platelet-activating factor in activated rat alveolar macrophages. J. Immunol. 154:6593-6599. [PubMed] [Google Scholar]
- 56.Wilson, M. E., P. M. Bronson, and R. G. Hamilton. 1995. Immunoglobulin G2 antibodies promote neutrophil killing of Actinobacillus actinomycetemcomitans. Infect. Immun. 63:1070-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson, M. E., and R. E. Schifferle. 1991. Evidence that the serotype b antigenic determinant of Actinobacillus actinomycetemcomitans Y4 resides in the polysaccharide moiety of lipopolysaccharide. Infect. Immun. 59:1544-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zambon, J. J., V. I. Haraszthy, G. Hariharan, E. T. Lally, and D. R. Demuth. 1996. The microbiology of early-onset periodontitis: association of highly toxic Actinobacillus actinomycetemcomitans strains with localized juvenile periodontitis. J. Periodontol. 67(Suppl.):282-290. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, J. B., S. M. Quinn, M. Rausch, J. C. Gunsolley, H. A. Schenkein, and J. G. Tew. 1996. Hyper-immunoglobulin G2 production by B cells from patients with localized juvenile periodontitis and its regulation by monocytes. Infect. Immun. 64:2004-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]