Abstract
Intrauterine environments are related to fetal renal development and postnatal health. Influence of salty diets during pregnancy on renal functions and renin–angiotensin system (RAS) was determined in the ovine fetuses and offspring. Pregnant ewes were fed high-salt diet (HSD) or normal-salt diet (NSD) for 2 months during middle-to-late gestation. Fetal renal functions, plasma hormones, and mRNA and protein expressions of the key elements of renal RAS were measured in the fetuses and offspring. Fetal renal excretion of sodium was increased while urine volume decreased in the HSD group. Fetal blood urea nitrogen was increased, while kidney weight:body weight ratio decreased in the HSD group. The altered ratio was also observed in the offspring aged 15 and 90 days. Maternal and fetal plasma antidiuretic hormone was elevated without changes in plasma renin activity and Ang I levels, while plasma Ang II was decreased. The key elements of local renal RAS, including angiotensinogen, angiotensin converting enzyme (ACE), ACE2, AT1, and AT2 receptor expression in both mRNA and protein, except renin, were altered following maternal high salt intake. The results suggest that high intake of salt during pregnancy affected fetal renal development associated with an altered expression of the renal key elements of RAS, some alterations of fetal origins remained after birth as possible risks in developing renal or cardiovascular diseases.
Keywords: Fetus, offspring, renal function, high salt, rennin–angiotensin system
Introduction
Accumulating evidences have shown that the kidney is sensitive to environmental insults during critical developmental periods in animal models (Guyton et al. 1972, Hoy et al. 1999, Woods 2000, Tay et al. 2007, 2012). In humans, previous studies also demonstrated that the kidney may be affected in programming of renal and cardiovascular diseases (do Carmo Pinho et al. 2003, Bagby 2007). It is well known that high-salt diets (HSDs) are related to hypertension as well as renal injury in adults (Barker 1992, Boero et al. 2002, du Cailar et al. 2002, Logan 2006). There has been a fairly large body of research on the impacts of salt exposure in pregnancy (Coelho et al. 2006, Digby et al. 2010). During pregnancy, many conditions such as overheating, hemorrhage, diarrhea, and hyperemesis may result in sodium deficiency and a change in salt appetite, so pregnant women experience sodium deficiency and tend to prefer salty food (Brown & Toma 1986, Bowen 1992). Middle-to-late gestation period is critical for functional development of organs, including the kidney, and a number of studies demonstrated the importance of this period as a window for health and diseases in fetal origins. Thus, the present study focused on that pregnancy stage.
The renin–angiotensin system (RAS) is important in the control of body fluid homeostasis and renal development (Schunkert et al. 1991, Guron & Friberg 2000, De Wardener & MacGregor 2002). All key components of RAS (renin, angiotensinogen (AGT), angiotensin converting enzyme (ACE), and angiotensin II type-1 and -2 receptors (AT1R and AT2R)) are found in the kidney. Several lines of evidence have demonstrated an influence of salt loading on Ang II receptors in adults (Hettinger et al. 2002, de Resende & Mill 2007) and functional changes of RAS in adult rats after perinatal overloading of salt (Alves da Silva et al. 2003). Maternal HSDs may lead to alterations in uterine–placental perfusion and fetal growth, inducing sodium-dependent hypertension in rats (Barron et al. 2001, Sanders et al. 2005). Recent studies in our laboratory showed alterations in body fluid homeostasis and blood pressure in the offspring exposed to maternal HSDs or dehydration during pregnancy (Guan et al. 2009, Ding et al. 2010). However, limited information is available on the influence of HSDs on fetal local renal RAS, despite it being relatively clear that overconsumption of salty diets can significantly influence systemic RAS in the circulation (Thomson et al. 2006). Addressing such questions is important to understand fetal renal physiology and diseases of fetal origins. Therefore, fetal renal excretion, fetal and offspring hormonal responses (plasma renin activity (PRA), Ang I, Ang II, aldosterone (ALD), and antidiuretic hormone (ADH)), and the key elements of renal local RAS in both fetuses and offspring were determined in the present study to test the hypothesis that maternal high-salt intake during pregnancy may affect the development of fetal renal RAS, which may have long-term impacts on the local renal RAS in the offspring.
Materials and methods
Animals and experimental groups
Time-mated pregnant ewes (term ~148±3 days) were fed with standard laboratory food (0.6% NaCl, normal-salt diet (NSD) group) or HSD (8% NaCl, HSD group) for 60 days during gestational days (GD) 70–130 (all nutrients in sheep food are standard and the same for both groups except for the salt percentages). After birth, all offspring were fed with standard food. The experimental groups included the following: i) prenatal groups: pregnant ewes fed with HSD (n=5) or NSD (n=5) and prepared for surgery at GD 125±3 (mixed sex for fetuses: singleton=4 (three males and one female) and twins=1 (one male and other female)). ii) Fifteen-day-old offspring: newborn lambs were kept with their mothers after birth. At the age of 15 days, male lambs from the HSD group (n=5 ewes: singleton offspring=3 (two males and one female) and twin offspring=2 (two males and two females)) or the NSD group (n=6 ewes: singleton offspring=4 (three males and one female) and twin offspring=2 (two males and two females)) were prepared for collecting blood samples and kidneys. iii) Male offspring aged 90 days from the HSD group (n=5 ewes: singleton offspring=4 (three males and one female) and twin offspring=1 (one male and one female)) or the NSD group (n=7 ewes: singleton offspring=5 (three males and two females) and twin offspring=2 (three males and one female)) were prepared for collecting blood samples and kidneys. All sheep were housed indoors and acclimated to a 12 h light:12 h darkness period. Water was provided ad libitum. All procedures were approved by the Institutional Animal Care Committee and followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgical preparation
Pregnant ewes were anesthetized at GD 125±3. Anesthesia was initiated by ketamine hydrochloride 20 mg/kg i.m. and maintained using 3% isoflurane and 1 l/min oxygen (Xu et al. 2001). Polyethylene catheters (ID=1.8 mm, OD=2.3 mm) were inserted into maternal femoral vein and artery and advanced into the inferior cava and abdominal aorta. The uterus was exposed by a midline abdominal incision. Polyethylene catheters (ID=1.0 mm, OD=1.8 mm) were inserted into fetal femoral vein and artery, and a small hysterotomy was performed to provide access to the fetal bladder. The fetal bladder was catheterized (ID=1.3 mm, OD=2.3 mm) via cystostomy, and the fetal urachus suture was ligated to eliminate urine flow to the allantoic cavity. The fetus was then returned into the uterus, and the uterus and maternal abdomen were closed in layers. The catheters were exteriorized through a small incision on the ewe’s flank and placed in a cloth pouch. Offspring were anesthetized and prepared for vascular catheterization at 90 days of age using catheters (ID=1.0 mm, OD=1.8 mm) as described above. Animals were allowed 4–5 days for postoperative recovery, while antibiotics were intravenously administered daily to the ewes (70 mg gentamicin and 1 g oxacillin) and to the fetuses (10 mg gentamicin and 20 mg oxacillin) for 3 postsurgical days. Gentamicin (30 mg) and oxacillin (0.5 g) were given to offspring intramuscularly.
After postsurgical recovery, maternal and fetal blood samples (4 ml) were collected via arterial catheters. Fetal urine was collected via the bladder catheter by gravity into glass tubes under conscious conditions. There was a 30-min pre-collection period so that the bladder could be drained of residual urine before collection. Urine samples were collected for 1 h each time, twice on the collection day in a setting of balance between the control and experimental groups. The mean urine volume of two collections was used in the analysis.
The animals were killed with an overdose of pentobarbital sodium (100 mg/kg) and phenytoin sodium (10 mg/kg). The fetuses around GD 130 and the offspring aged 15 and 90 days were killed for collecting the kidney tissues. The collected tissues were stored at −70 °C before assay.
Assay for blood and urine values
Plasma electrolytes were measured using a Nova analyzer (Nova Biochemical, Model pHOx Plus L, MA, USA). Urine was tested for volume, osmolality, and Na+, K+, and Cl− concentrations. Plasma and urinary osmolality was measured by an Advanced Digimatic osmometer (Advanced Instruments, Needlham Heights, MA, USA). Urinary Na+, K+, and Cl− concentrations were measured using automatic biochemical analyzer (Hitachi 7600-020).
Plasma hormonal and biochemical assays
All RIAs were performed by the Institute of Hua-Ying Biological Technology (Beijing, China; Ding et al. 2010). For measuring PRA, Ang I, and Ang II, blood samples were collected into chilled plastic tubes containing 50 μl EDTA–Na2 (0.32 M), 50 μl 8-hydroxyguinoline (0.34 M), and 25 μl dimercaptopropanol (0.32 M). For measuring ALD and ADH, blood samples were collected into chilled plastic tubes containing 30 μl 10% EDTA–Na2 and 40 μl trasylol. Blood samples were centrifuged at 2200 g at 4 °C for 10 min and then plasma was collected and stored at −20 °C until RIA. Assay was performed in duplicate. All primary antibodies used for RIA were raised from rabbits (Sigma). 125-I was from Amersham. Samples were incubated with the primary antibodies at 4 °C overnight with tracer 125-I. All samples were assayed together. Radioactivity was measured by aγ-911 automatic RIA counter according to the manufacture’s instructions. The intra- and interassay variations were 3.39 and 4.42% for PRA, 2.55 and 4.54% for Ang I, 2.61 and 4.59% for Ang II, 2.89 and 3.18% for ALD, and 3.16 and 4.72% for ADH respectively. The hormone assays and data analysis were handled in a blind manner.
Serum BUN and creatinine were measured using automatic biochemical analyzer (Hitachi 7600-020). The assay experiments and data were also handled in a blind manner. An altered BUN:creatinine ratio may be due to a condition that causes a decrease in the flow of blood to the kidneys. Thus, the ratio was analyzed.
Real-time PCR
RNA was extracted from renal cortex tissues of fetal and offspring using TRIzol reagents (Invitrogen). mRNA abundance of renin, AGT, ACE, ACE2, AT1R, and AT2R was determined by real-time RT-PCR using an Icycler Thermal Cycler (Bio-Rad icycle iQ; Guan et al. 2009). Specific renin, AGT, ACE, ACE2, AT1, and AT2 primers were listed in Table 1. 18S was used as an internal control. All experiments were repeated three times for reliability. The amount of expressed target gene was normalized to 18S to obtain the relative threshold cycle (Dodic et al. 2002). The relative gene expression (RGE) was normalized to 18S to obtain the relative threshold cycle and calculated as RGE=2−(TΔCtNΔCt), where T represents the level of experimental groups, N represents the control group whose expression level was calculated, and ΔCt is the difference of threshold cycle (Ct) between the gene of interest and 18S.
Table 1.
Primers for real-time PCR analysis
| Name | Primer sequence |
|---|---|
| Renin | Forward: 5′-TGGATCTGGGAAGGTCAAAG-3′ |
| Reverse: 5′-GCGAGGATGTGGTCAAAGAC-3′ | |
| Angiotensinogen (AGT) | Forward: 5′-ATCACTCTCCCACGCTCACT-3′ |
| Reverse: 5′-ACCCCTTCATCTTTCCTTGG-3′ | |
| ACE | Forward: 5′-CCAAATATGTGGAGCTCACCAA-3′ |
| Reverse: 5′-GGAGTCCCCGCCATCC-3′ | |
| ACE2 | Forward: 5′-GCAGCCACACCTCACTATTTGA-3′ |
| Reverse: 5′-AGGAAGTTTATTTCTGTTTCATTGTCTTC-3′ | |
| AT1 | Forward: 5′-GGGCTGTCTACACTGCTATGGAA-3′ |
| Reverse: 5′-CCGGAAGCGATCTTACATAGGTA-3′ | |
| AT2 | Forward: 5′-TGTTCTGGCGTTCATCATTTG-3′ |
| Reverse: 5′-CCATCCAAGCTAGAGCATCCA-3′ |
Western-blotting
Renal cortical tissues were homogenized and prepared as reported previously (Mao et al. 2010). Polyclonal antibodies raised from rabbit against renin, AGT, ACE, ACE2, AT1, and AT2 (SC-22752, SC-20717, SC-20791, SC-20998SC-579, and SC-9040; 1:200–500, Santa Cruz Biotechnology) were used. Proteins were visualized using chemiluminescence reagents (Amersham Bioscience), and β-actin was blotted in the same membrane as an internal control for normalizing the relative density. Imaging signals were digitized and analyzed; data were quantified using a UVP imaging system (EC3-Imaging-System, Upland, CA, USA).
Statistical analysis
Statistical analysis was performed with SPSS software. Comparisons between NSD and HSD were determined with one-way ANOVA. All data were expressed as mean±s.e.m. P<0.05 or 0.01 was considered statistically significance.
Results
Kidney and body weight and ratio
There was no significant difference in body and kidney weights in the fetuses and offspring aged 15 days between the NSD and HSD groups. For offspring aged 90 days, there was no significant difference in kidney weight between the two groups, while body weight was higher in the HSD offspring. The kidney weight:body weight ratio in both the fetuses and offspring was significantly less in the HSD group than in the NSD group (P<0.05, Table 2).
Table 2.
Fetal and offspring kidney weights, body weights, and their ratios (mean±s.e.m.)
| Fetuses |
15-day-old offspring |
90-day-old offspring |
||||
|---|---|---|---|---|---|---|
| NSD (n=5) | HSD (n=5) | NSD (n=5) | HSD (n=6) | NSD (n=5) | HSD (n=7) | |
| Kidney weight (g) | 8.04±1.72 | 7.35±0.73 | 20.48±1.39 | 20.41±1.55 | 38.91±2.38 | 40.17±2.61 |
| Body weight (kg) | 2.51±0.46 | 2.88±0.14 | 6.50±0.41 | 7.71±0.52 | 20.15±0.72 | 27.01±2.42† |
| Kidney weight/body weight |
3.24±0.36 | 2.55±0.23* | 3.16±0.18 | 2.65±0.08* | 1.93±0.12 | 1.50±0.14† |
NSD, normal-salt diet; HSD, high-salt diet; HSD vs NSD.
P<0.05,
P<0.01.
Blood values
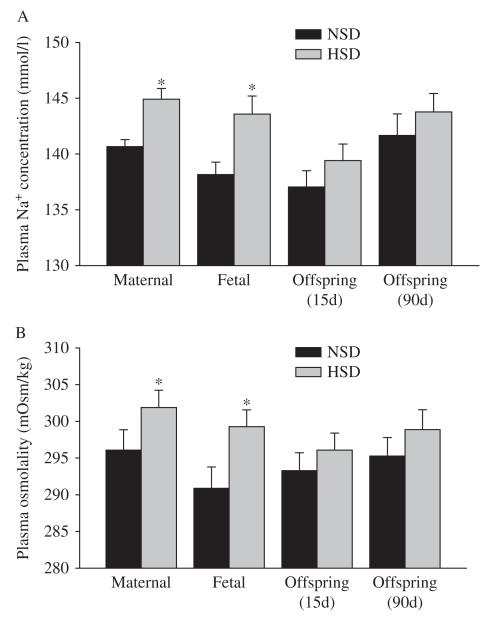
Maternal and fetal blood Na+ and osmolality levels were significantly increased by HSD (P<0.05). There was no difference in blood Na+ and osmolality levels in HSD and NSD offspring aged 15 and 90 days (Fig. 1).
Figure 1.
The effect of maternal HSD on plasma Na+ (A) and osmolality (B) levels (mean±s.e.m.). NSD, normal-salt diet; HSD, high-salt diet. HSD vs NSD: *P<0.05.
Fetal urine volume, electrolytes, and osmolality
Fetal urine flow (urine volume/60 min) was significantly decreased in response to maternal HSD (P<0.05). Fetal urine osmolality and Na+ and Cl− concentrations, as well as Na+:K+ ratio, were increased following maternal HSD (P<0.01, P<0.05). In addition, ratio of fetal urine electrolytes (Na+:Cl−) and urine flow rate was increased too. There was no significant difference in fetal urine K+ concentrations between the NSD and HSD groups (Fig. 2).
Figure 2.
The effect of maternal high salt on fetal urine volume (A), osmolality (B), and the electrolytes:volume ratio (C). NSD, normal-salt diet; HSD, high-salt diet. HSD vs NSD: **P<0.01.
Serum BUN and creatinine
There was no difference in fetal serum creatinine (CRE) between the NSD and HSD groups (P>0.05). However, fetal BUN was significantly increased in the HSD group (P<0.05). The BUN:CRE ratio in the fetuses was also significantly higher in the HSD group than in the NSD group (P<0.05). Serum BUN, CRE, and the BUN:CRE ratio in the mothers were the same between the HSD and NSD groups (P>0.05, Fig. 3A, B, and C).
Figure 3.
The effect of maternal high salt on maternal and fetal serum BUN (A), CRE (B), and their ratios (C). NSD, normal-salt diet; HSD, high-salt diet. HSD vs NSD: *P<0.05.
There was no difference in BUN and CRE in the offspring between the NSD and HSD groups at the age of either 15 or 90 days (P>0.05). However, the BUN:CRE ratio was still significantly higher in the HSD offspring (P<0.05, Fig. 4).
Figure 4.
The effect of maternal high salt on offspring serum BUN, CRE, and their ratios. (A) 15-day-old offspring; (B) 90-day-old offspring. NSD, normal-salt diet; HSD, high-salt diet. HSD vs NSD: *P<0.05.
Plasma hormones
Maternal and fetal plasma ADH concentrations were significantly increased in the HSD group (P<0.05). In addition, plasma ADH levels in the offspring at the age of 15 days, not 90 days, were also higher in the HSD group (Fig. 5A). Plasma Ang II levels were significantly decreased in the mothers and fetuses of the HSD group (P<0.05), but not in the offspring aged 15 and 90 days. Plasma ALD, renin activity, and Ang I levels were the same in the maternal, fetal, and offspring sheep between the NSD and HSD groups at 15 or 90 days of age (Fig. 5).
Figure 5.
The effect of maternal high salt on maternal and fetal plasma ADH (A), ALD (B) concentration, renin activity (PRA) (C), angiotensin I (D), and angiotensin II concentrations (E). NSD, normal-salt diet; HSD, high-salt diet. HSD vs NSD: *P<0.05.
Expression of mRNA in renal RAS elements
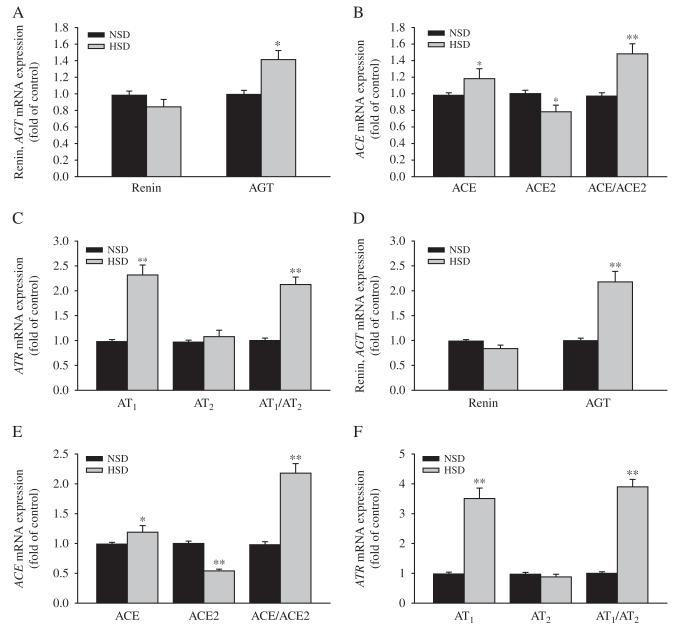
Expression levels of renal mRNA of AGT, AT1, AT2, ACE, and ACE:ACE2 ratio were significantly increased in the fetuses exposed to HSD. Fetal ACE2 mRNA expression was decreased in the HSD group, while renin and the AT1:AT2 mRNA expression ratio were the same between the two fetal groups (Fig. 6A, B, and C).
Figure 6.
The effect of maternal high salt on renin and AGT (A), ACE and ACE2 (B), AT1 and AT2 (C) mRNA expression in the fetal kidney. NSD, normal-salt diet; HSD, high-salt diet. HSD vs NSD: *P<0.05, **P<0.01.
ACE2 mRNA levels were significantly lower in the offspring aged both 15 and 90 days in the HSD group. AGT, ACE, AT1, and ACE:ACE2 and AT1:AT2 mRNA ratio were significantly increased in the HSD offspring (P<0.05, P<0.01). There was no significant change in renin and AT2 mRNA in the offspring (Fig. 7).
Figure 7.
The effect of maternal high salt on renin and AGT, ACE, ACE2, AT1, and AT2 mRNA expression in offspring kidney. NSD, normal-salt diet; HSD, high-salt diet. HSD vs NSD: *P<0.05, **P<0.01. (A, B, and C) 15-day-old offspring, (D, E, and F) 90-day-old offspring.
Protein of renal RAS elements
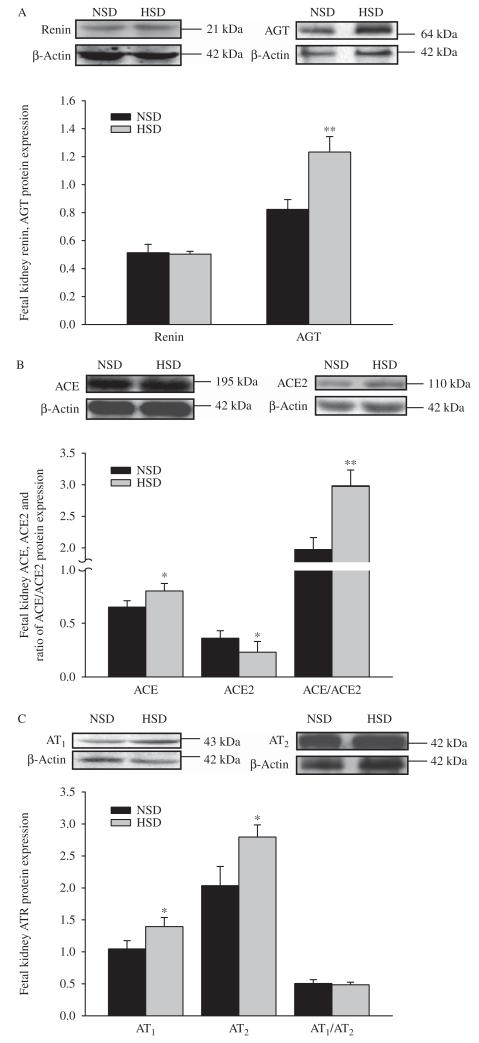
Levels of fetal renal protein expression of renin and AT1:AT2 ratio were the same between the control and experimental groups. Fetal renal AGT, ACE, AT1, AT2, and ACE:ACE2 protein expression ratio were significantly increased in the HSD group. However, ACE2 protein expression was significantly decreased in the HSD group (P<0.05, P<0.01, Fig. 8A, B, and C).
Figure 8.
The effect of maternal high salt on renin and AGT (A), ACE and ACE2 (B), AT1 and AT2 (C) protein expression in the fetal kidney. NSD, normal-salt diet; HSD, high-salt diet. HSD vs NSD: *P<0.05, **P<0.01.
There was no change in renin and AT2 protein expression in the offspring aged 15 and 90 days, while AGT, ACE, and ACE:ACE2 protein expression ratio were significantly increased in the HSD offspring. However, ACE2 protein was markedly reduced in the HSD offspring at both ages (P<0.05, P<0.01). AT1 protein expression was significantly increased in 15-day-old offspring, while unchanged in 90-day-old offspring, and AT1:AT2 ratio was significantly increased in the HSD offspring (P<0.05, P<0.01, Fig. 9).
Figure 9.
The effect of maternal high salt on renin, AGT, ACE, ACE2, AT1 and, AT2 protein expression in the offspring kidney. NSD, normal-salt diet; HSD, high-salt diet. HSD vs NSD. *P<0.05, **P<0.01. (A, B, and C) 15-day-old offspring, (D, E, and F) 90-day-old offspring.
Discussion
Intake of high salt is a risk in cardiovascular diseases (Alves da Silva et al. 2003). Accumulating evidence from clinical and experimental investigations in the last two decades demonstrated that renal and cardiovascular diseases, including hypertension, could be programmed in fetal origins (Guyton et al. 1972, Hoy et al. 1999, Woods 2000, Barker 2002, do Carmo Pinho et al. 2003, Logan 2006, Bagby 2007, Blache et al. 2007, Tay et al. 2007, 2012, Gluckman et al. 2008). The present study provided new information on the influence of high salt intake during pregnancy on fetal local renal RAS development in relation to the long-term impacts on the offspring.
Following 2 months of HSD intake during pregnancy, maternal plasma osmolality and Na+ levels were increased as those in adults (da Silva et al. 2003). Fetal plasma osmolality and Na+ were also enhanced by maternal salt intake during pregnancy. The observed fetal blood osmolality and Na+ levels could result from two major sources. One was the salt from the maternal circulation cross the placenta; the other could be related to fetal own regulations when Na+ levels were increased. Thus, we measured fetal urine values in the conscious and unstressed animals in utero. The results showed that fetal urine volume was significantly reduced, while urine osmolality, Na+ and Cl− concentrations, as well as Na+:K+ ratio were increased, the fetal urine Na+:Cl− ratio against flow rate were also enhanced, indicating that fetal renal systems have been functional in face of salt-loading challenge. Furthermore, the data on fetal renal excretion added information that maternal salt intake during pregnancy could affect the fetal renal functions.
In the present study, both fetal kidney and body weights were not statistically changed by HSDs; however, kidney weight:body weight ratio was significantly decreased, indicating a relatively poor development of the fetal kidney under the condition of high salt, although other possibilities may also cause a decrease in the ratio. Previous studies showed that other environmental insults during pregnancy could cause small-sized or poorly developed fetal kidneys (Digby et al. 2010, Mao et al. 2010). In future studies regarding development, it is worth considering measuring food intake. While testing functional influence of salt intake, changes in urine volume and electrolytes seem to be acute protective mechanisms to remove excess salt from the fetal body. In the present study, although fetal serum CRE was not significantly changed, BUN concentrations and BUN:CRE ratio were increased. BUN and CRE, commonly used indicators of renal functions (Mao et al. 2010), can reflect glomerular filtration and renal functions. Notably, fetal BUN was increased while the maternal BUN was not in face of high salt intake in the present study. This could be related to the fact that fetal renal functions were immature (Drukker & Guignard 2002). Together with the evidence of reduced kidney weight:body weight ratio, the data suggest that fetal renal development could be affected by maternal salt intake and fetal renal capability in response to salt intake was different from that of the mothers or fetal renal functions are more vulnerable to environmental insults.
Endocrine pathway is a key mechanism in the control of renal functions. It is not strange that maternal plasma ADH was increased following high salt intake (Kjeldsen et al. 1985). Although it is well known that an increased osmolality can stimulate ADH release, it was new evidence that fetal plasma ADH could be significantly increased by maternal salt intake. ADH plays an important role in body fluid balance via renal excretion (Ranadive & Rosenthal 2011). This neurohypophysial hormone is responsible for increasing water absorption in collecting ducts of the kidney, via increasing water permeability by inducing translocation of aquaporin-CD water channels in collecting ducts (Nielsen et al. 1995). This can explain why fetal urine volume was reduced during maternal high salt intake in the present study. We also measured ALD levels because this steroid hormone acts on renal distal tubules and collecting ducts to cause conservation of sodium and retention of water. Plasma ALD remained unchanged while ADH was elevated, indicating ADH-mediated fetal renal regulations during maternal salt intake.
Renin–angiotensin–ALD system is an important endocrine pathway in the control of renal functions (Fitzsimons 1998). High salt intake not only decreased maternal plasma Ang II as shown in adult models (Ding et al. 2010) but also reduced fetal Ang II concentrations in the circulation. Together with the evidence of unchanged fetal PRA and renal renin mRNA and protein, the data suggest that mechanisms for the reduction of plasma Ang II could be outside of the kidney. One possibility in the pathway may be reduced hepatic AGT that is the primary source for the precursor of plasma Ang II (Fitzsimons 1998). However, this assumption is not supported by the fact that both maternal and fetal Ang I levels were not changed following high salt intake. As ACEs are critical in turning Ang I into Ang II or Ang(1–7), it is very possible that the underlying mechanism of high salt intake-reduced fetal Ang II levels was related to those converting enzymes affected. Previous study (Stevens & Lumbers 1986, Boyce et al. 2008) showed that high salt decreased renin in the adult sheep. The difference in renin results could be related to different conditions for HSDs used in our and their experiments. Stevens & Lumbers (1986) and Boyce et al. (2008) showed almost no difference in fetal plasma sodium or plasma osmolality in the HSD group and fetal urinary sodium excretion as well as urinary osmolality were not altered. However, a HSD (40 days of 210 g/day of Na+) was associated with an increase in fetal plasma sodium and osmolality in cows (Rouffett et al. 1990). In pregnant rats, Deloof et al. (2000)) found an increase in fetal plasma sodium with maternal HSD. In the present study, maternal and fetal renin levels are similar, and high-salt intake was associated with increased osmolality and Na+. The various results among those studies may be related to the degree and duration of salt loading. In addition, previous study suggested that decreased plasma Ang II levels would lead to a decrease in ALD. In the present study, plasma ALD was unchanged associated with an increase in plasma ADH as well as fetal urine Na+/K+. It is possible that other body fluid regulatory mechanisms, except Ang II, may also be involved in the regulation of ALD levels.
Whether those fetal alterations are related to long-term influence is an important question. Our data showed that when the maternal and offspring sheep returned to NSDs after birth, blood Na+ and osmolality levels as well as BUN and CRE concentrations were the same between the control and experimental offspring at the age of 15 and 90 days. The body and kidney weights (except body weight at 90 days), plasma Ang I, Ang II, ALD, and ADH levels at 90 days were also not changed. However, kidney weight: body weight ratio was still significantly lower, and BUN:CRE ratio was higher at both the 15- and 90-day-old offspring exposed to prenatal high salt. This indicates that some fetal changes in utero by environmental insults could be reversed after birth if postnatal conditions were corrected, while other alterations appeared with long-term influence, adding new information on the relationship between prenatal renal alterations and postnatal health.
Local renal RAS plays an important role in cellular proliferation and apoptosis (Xu et al. 2009), and intrarenal RAS components mediate nephrogenesis (Woods & Rasch 1998, Guron & Friberg 2000), as well as renal functions. Growth of the fetal kidney can be regulated via AT1 receptors (Guron & Friberg 2000, Xu et al. 2009). Dietary sodium manipulation induced organ-specific modulation of AT1 and AT2 expression in the kidney (Ruan et al. 1997). The present study was the first to assess influence of prenatal high salt intake on expression of the key elements of local RAS (renin, AGT, ACE, ACE2, AT1, and AT2) in the fetal kidney. The results showed that AGT mRNA and protein expressions were significantly increased in the kidney of the fetuses and offspring exposed to HSD, while renal renin mRNA and protein levels in the fetuses and offspring were not changed. In the systemic RAS, AGT is mainly from the liver while renin is mainly from the kidney (Pereira et al. 2009, Urushihara & Kobori 2011). The fact that both renin mRNA and protein levels unchanged further supported the endocrine finding that plasma renin remained unchanged in the present study. AGT is a precursor of angiotensin peptides such as renin and ACE, which are two key enzymes for the production of different RAS peptides (Shi et al. 2010). Although renin was unchanged, ACE mRNA and protein levels in the fetal and offspring kidneys were markedly altered. ACE turns Ang I into Ang II, while ACE2 plays a critical role in the formation of Ang(1–7; Shi et al. 2010). Notably, the increase of renal ACE and ACE:ACE2 ratio as well as a decrease of ACE2 in the present study strongly suggests a possibility of increase of renal local Ang II and decrease of Ang(1–7). In adults, both Ang II and Ang(1–7) play important roles in regulating renal hemodynamics. ACE and ACE2 are counter-regulatory enzymes in the control of levels of angiotensin peptides (Shi et al. 2010). Our finding suggests a shift toward greater renal ACE synthesis and reduced metabolism of Ang II via ACE2, as well as reduced Ang(1–7) in the kidney. ACE inhibitors or AT1 receptor blockers used in pregnancy lead to fetal renal dysplasia, indicating that an intact and balanced RAS is critical to normal renal development (Jones et al. 1990). Reduction of renal ACE2 as well as increase of ACE:ACE2 ratio by prenatal high salt could be a likely risk in renal and cardiovascular diseases.
In the present study, following exposure to prenatal HSDs, AT1 expression in both mRNA and protein was significantly increased in the fetal and offspring kidney except its protein at 90 days of age, and AT2 expression was significantly increased in the fetal kidney, although such alterations disappeared in the offspring. However, AT1:AT2 mRNA and protein expression ratio in the offspring kidney was significantly increased. An absolute or relative increase of AT1 may contribute to cell growth and apoptosis (Fitzsimons 1998, Mao et al. 2009) in the kidney. The present study demonstrated that renal AT1/AT2 could be significantly altered in the kidney by prenatal HSDs. In addition, we noted that altered fetal renal AT2 expression as well as AT1 protein at 90 days could disappear in the offspring, indicating again that some fetal alterations in utero by environmental influence could be reversed after birth, and different postnatal kidneys responding to differential environments may be explanations. Significance of this finding is that it provides meaningful information for early prevention of adult diseases in fetal origins.
Furthermore, outgrowth and branching of ureteric bud could be augmented by Ang II via stimulating AT1/AT2 receptors, indicating roles of RAS in regulating renal development (Esther et al. 1996, Guron et al. 1999, Guron & Friberg 2000). Growth retardation of developing kidney during gestation induced by AT1 receptor antagonists was associated with an increased expression of the angiotensin receptor (Kriegsmann et al. 2000), suggesting that growth of fetal kidneys was also regulated by AT1 receptor. It is reasonable to consider that Ang II might either act as a partner of growth signaling or independently in regulating kidney development as well as influencing renal functions. Whether reduced fetal kidney/body weight following HSDs was related to the altered expression of the key elements of local renal RAS and their receptors is worth further investigations.
In conclusion, the present study demonstrated that HSDs during pregnancy could affect fetal urine excretion via ADH signaling and fetal renal functions, in association with significant changes in expression of mRNA and protein in several key components of local renal RAS and their receptors. The findings provide information on subtle pathophysiological changes in the kidney due to chronic exposure to HSDs during gestation. Further investigations on molecular targets responsible for the observed changes may lead to new approaches in early prevention and treatment of renal and cardiovascular diseases in fetal origins.
Acknowledgments
Funding
This study is partly supported by National key scientific research projects (2013BAI04B05, 2012CB947600), NIH grant (HL090920), National Nature and Science Foundation of China (81070540, 81030006), Jiangsu Province’s Key Discipline/Laboratory of Fetal Medicine and Human Assisted Reproduction, and Jiangsu Province’s ‘Chuang Xin Tuan Dui’ of Fetal Medicine.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Alves da Silva A, de Noronha IL, de Oliveira IB, Malheiros DM, Heimann JC. Renin–angiotensin system function and blood pressure in adult rats after perinatal salt overload. Nutrition, Metabolism, and Cardiovascular Diseases. 2003;13:133–139. doi: 10.1016/s0939-4753(03)80172-2. doi:10.1016/S0939-4753(03)80172-2. [DOI] [PubMed] [Google Scholar]
- Bagby SP. Maternal nutrition, low nephron number, and hypertension in later life: pathways of nutritional programming. Journal of Nutrition. 2007;137:1066–1072. doi: 10.1093/jn/137.4.1066. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The fetal origins of adult hypertension. Journal of Hypertension. Supplement. 1992;10:S39–S44. doi:10.1097/00004872-199212007-00003. [PubMed] [Google Scholar]
- Barker DJ. Fetal programming of coronary heart disease. Trends in Endocrinology and Metabolism. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. doi:10.1016/S1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- Barron LA, Giardina JB, Granger JP, Khalil RA. High-salt diet enhances vascular reactivity in pregnant rats with normal and reduced uterine perfusion pressure. Hypertension. 2001;38:730–735. doi: 10.1161/01.hyp.38.3.730. doi:10.1161/01.HYP.38.3.730. [DOI] [PubMed] [Google Scholar]
- Blache D, Grandison MJ, Masters DG, Dynes RA, Blackberry MA, Martin GB. Relationships between metabolic endocrine systems and voluntary feed intake in Merino sheep fed a high salt diet. Australian Journal of Agricultural Research. 2007;47:544–550. [Google Scholar]
- Boero R, Pignataro A, Quarello F. Salt intake and kidney disease. Journal of Nephrology. 2002;15:225–229. [PubMed] [Google Scholar]
- Bowen DJ. Taste and food preference changes across the course of pregnancy. Appetite. 1992;19:233–242. doi: 10.1016/0195-6663(92)90164-2. doi:10.1016/0195-6663(92)90164-2. [DOI] [PubMed] [Google Scholar]
- Boyce AC, Gibson KJ, Thomson CL, Lumbers ER. Interactions between maternal subtotal nephrectomy and salt:effects on renal function and the composition of plasma in the late gestation sheep fetus. Experimental Physiology. 2008;93:262–270. doi: 10.1113/expphysiol.2007.039149. doi:10.1113/expphysiol.2007.039149. [DOI] [PubMed] [Google Scholar]
- Brown JE, Toma RB. Taste changes during pregnancy. American Journal of Clinical Nutrition. 1986;43:414–418. doi: 10.1093/ajcn/43.3.414. [DOI] [PubMed] [Google Scholar]
- du Cailar G, Ribstein J, Mimran A. Dietary sodium and target organ damage in essential hypertension. American Journal of Hypertension. 2002;15:222–229. doi: 10.1016/s0895-7061(01)02287-7. doi:10.1016/S0895-7061(01)02287-7. [DOI] [PubMed] [Google Scholar]
- do Carmo Pinho FM, Nigro D, Fortes ZB, Tostes RC, Carvalho MH, Lucas SR, Gomes GN, Coimbra TM, Gil FZ. Intrauterine undernutrition – renal and vascular origin of hypertension. Cardiovascular Research. 2003;60:228–234. doi: 10.1016/s0008-6363(03)00541-8. doi:10.1016/S0008-6363(03)00541-8. [DOI] [PubMed] [Google Scholar]
- Coelho MS, Passadore MD, Gasparetti AL, Bibancos T, Prada PO, Furukawa LL, Furukawa LN, Fukui RT, Casarini DE, Saad MJ, et al. High- or low-salt diet from weaning to adulthood: effect on body weight, food intake and energy balance in rats. Nutrition, Metabolism, and Cardiovascular Diseases. 2006;16:148–155. doi: 10.1016/j.numecd.2005.09.001. doi:10.1016/j.numecd.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Deloof S, De Seze C, Montel V, Chatelain A. Atrial natriuretic peptide and aldosterone secretions, and atrial natriuretic peptide-binding sites in kidneys and adrenal glands of pregnant and fetal rats in late gestation in response to a high-salt diet. European Journal of Endocrinology. 2000;142:524–532. doi: 10.1530/eje.0.1420524. doi:10.1530/eje.0.1420524. [DOI] [PubMed] [Google Scholar]
- De Wardener HE, MacGregor GA. Sodium and blood pressure. Current Opinion in Cardiology. 2002;17:360–367. doi: 10.1097/00001573-200207000-00007. doi:10.1097/00001573-200207000-00007. [DOI] [PubMed] [Google Scholar]
- Digby S, Masters DG, Blache D, Hynd PI, Revell DK. Offspring born to ewes fed high salt during pregnancy have altered responses to oral salt loads. Animal. 2010;4:81–88. doi: 10.1017/S1751731109990772. doi:10.1017/S1751731109990772. [DOI] [PubMed] [Google Scholar]
- Ding Y, Lv J, Mao C, Zhang H, Wang A, Zhu L, Zhu H, Xu Z. High salt diet during pregnancy and angiotensin-related cardiac changes. Journal of Hypertension. 2010;28:1290–1297. doi: 10.1097/HJH.0b013e328337da8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodic M, Hantzis V, Duncan J, Rees S, Koukoulas I, Johnson K, Wintour EM, Moritz K. Programming effects of short prenatal exprosure to cortisol. FASEB Journal. 2002;16:1017–1026. doi: 10.1096/fj.01-1045com. doi:10.1096/fj.01-1045com. [DOI] [PubMed] [Google Scholar]
- Drukker A, Guignard JP. Renal aspects of the term and preterm infant: a selective update. Current Opinion in Pediatrics. 2002;14:175–182. doi: 10.1097/00008480-200204000-00006. doi:10.1097/00008480-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Esther CR, Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Laboratory Investigation. 1996;74:953–965. [PubMed] [Google Scholar]
- Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiological Reviews. 1998;78:585–589. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. doi:10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J, Mao C, Xu F, Geng C, Zhu L, Wang A, Xu Z. Prenatal dehydration affected renin–angiotensin system associated with angiotensin-increased blood pressure in young offspring. Hypertension Research. 2009;32:1104–1111. doi: 10.1038/hr.2009.155. doi:10.1038/hr.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guron G, Friberg P. An intact renin–angiotensin system is a prerequisite for normal renal development. Journal of Hypertension. 2000;18:123–137. doi: 10.1097/00004872-200018020-00001. doi:10.1097/00004872-200018020-00001. [DOI] [PubMed] [Google Scholar]
- Guron G, Nilsson A, Nitescu N, Nielsen S, Sundelin B, Frøkiaer J, Friberg P. Mechanisms of impaired urinary concentrating ability in adult rats treated neonatally with enalapril. Acta Physiologica Scandinavica. 1999;165:103–112. doi: 10.1046/j.1365-201x.1999.00477.x. doi:10.1046/j.1365-201x.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Coleman TG, Cowley AJ, Cowley AV, Jr, Scheel KW, Manning RD, Jr, Norman RA., Jr Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. American Journal of Medicine. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. doi:10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- Hettinger U, Lukasova M, Lewicka S, Hilgenfeldt U. Regulatory effects of salt diet on renal renin–angiotensin–aldosterone, and kallikrein–kinin systems. International Immunopharmacology. 2002;2:1975–1980. doi: 10.1016/s1567-5769(02)00163-7. doi:10.1016/S1567-5769(02)00163-7. [DOI] [PubMed] [Google Scholar]
- Hoy WE, Rees M, Kile E, Mathews JD, Wang Z. A new dimension to the Barker hypothesis: low birth weight and susceptibility to renal disease. Kidney International. 1999;56:1072–1077. doi: 10.1046/j.1523-1755.1999.00633.x. doi:10.1046/j.1523-1755.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- Jones CA, Sigmund CD, McGowan RA, Kane-Haas CM, Gross KW. Expression of murine renin genes during fetal development. Molecular Endocrinology. 1990;4:375–383. doi: 10.1210/mend-4-3-375. doi:10.1210/mend-4-3-375. [DOI] [PubMed] [Google Scholar]
- Kjeldsen SE, Os I, Forsberg G, Aakesson I, Skjøtø J, Frederichsen P, Fønstelien E, Eide I. Dietary sodium intake increases vasopressin secretion in man. Journal of Clinical Hypertension. 1985;1:123–131. [PubMed] [Google Scholar]
- Kriegsmann J, Coerdt W, Kommoss F, Beetz R, Hallermann C, Müntefering H. Renal tubular dysgenesis (RTD) – an important cause of the oligohydramnion-sequence. Report of 3 cases and review of the literature. Pathology, Research and Practice. 2000;196:861–865. doi: 10.1016/s0344-0338(00)80090-4. doi:10.1016/S0344-0338(00)80090-4. [DOI] [PubMed] [Google Scholar]
- Logan AG. Dietary sodium intake and its relation to human health: a summary of the evidence. Journal of the American College of Nutrition. 2006;25:165–169. doi: 10.1080/07315724.2006.10719528. [DOI] [PubMed] [Google Scholar]
- Mao C, Shi L, Xu F, Zhang L, Xu Z. Development of fetal brain renin–angiotensin system and hypertension programmed in fetal origins. Progress in Neurobiology. 2009;87:252–263. doi: 10.1016/j.pneurobio.2008.12.001. doi:10.1016/j.pneurobio.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Hou J, Ge J, Hu Y, Ding Y, Zhou Y, Zhang H, Xu Z, Zhang L. Changes of renal AT1/AT2 receptors and structures in ovine fetuses following exposure to long-term hypoxia. American Journal of Nephrology. 2010;31:141–150. doi: 10.1159/000259901. doi:10.1159/000259901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. PNAS. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. doi:10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira RM, dos Santos RAS, da Costa Dias FL, Mauro Martins Teixeira MM, Simôes e Silva AC. Renin–angiotensin system in the pathogenesis of liver fibrosis. World Journal of Gastroenterology. 2009;15:2579–2586. doi: 10.3748/wjg.15.2579. doi:10.3748/wjg.15.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranadive SA, Rosenthal SM. Pediatric disorders of water balance. Pediatric Clinics of North America. 2011;58:1271–1280. doi: 10.1016/j.pcl.2011.07.013. doi:10.1016/j.pcl.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Resende MM, Mill JG. Effect of high salt intake on local renin–angiotensin system and ventricular dysfunction following myocardial infarction in rats. Clinical and Experimental Pharmacology & Physiology. 2007;34:274–279. doi: 10.1111/j.1440-1681.2007.04556.x. doi:10.1111/j.1440-1681.2007.04556.x. [DOI] [PubMed] [Google Scholar]
- Rouffett J, Dalle M, Tournaire C, Barlet JP, Delost P. Sodium intake by pregnant cows and plasma aldosterone and cortisol concentrations in the fetus during late pregnancy. Journal of Dairy Science. 1990;73:1762–1765. doi: 10.3168/jds.s0022-0302(90)78854-6. doi:10.3168/jds.S0022-0302(90)78854-6. [DOI] [PubMed] [Google Scholar]
- Ruan X, Wagner C, Chatziantoniou C, Kurtz A, Arendshorst WJ. Regulation of angiotensin II receptor AT1 subtypes in renal afferent arterioles during chronic changes in sodium diet. Journal of Clinical Investigation. 1997;99:1072–1081. doi: 10.1172/JCI119235. doi:10.1172/JCI119235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MW, Fazzi GE, Janssen GMJ, Blanco CE, De Mey JG. High sodium intake increases blood pressure and alters renal function in intrauterine growth retarded rats. Hypertension. 2005;46:71–75. doi: 10.1161/01.HYP.0000171475.40259.d1. doi:10.1161/01.HYP.0000171475.40259.d1. [DOI] [PubMed] [Google Scholar]
- Schunkert H, Ingelfinger JR, Dzau VJ. Evolving concepts of the intrarenal renin–angiotensin system in health and disease: contributions of molecular biology. Renal Physiology and Biochemistry. 1991;14:146–154. doi: 10.1159/000173400. [DOI] [PubMed] [Google Scholar]
- Shi L, Mao C, Zhang L, Xu Z. Two angiotensin converting enzymes and their roles in the cardiovascular diseases. Drug Discovery Today. 2010;15:332–341. doi: 10.1016/j.drudis.2010.02.003. doi:10.1016/j.drudis.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva AA, de Noronha IL, de Oliveira IB, Malheiros DM, Heimann JC. Renin–angiotensin system function and blood pressure in adult rats after perinatal salt overload. Nutrition, Metabolism, and Cardiovascular Diseases. 2003;13:133–139. doi: 10.1016/s0939-4753(03)80172-2. doi:10.1016/S0939-4753(03)80172-2. [DOI] [PubMed] [Google Scholar]
- Stevens AD, Lumbers ER. Effect on maternal and fetal renal function and plasma renin activity of a high salt intake by the ewe. Journal of Developmental Physiology. 1986;8:267–275. [PubMed] [Google Scholar]
- Tay S, Digby S, Pain S, Blache D, Revell DK. High-salt intake during pregnancy in ewes altersorgan weights and aldosterone concentration in adult offspring. Early Human Development. 2007;83:S170–S130. doi:10.1016/S0378-3782(07)70477-2. [Google Scholar]
- Tay SH, Blache D, Gregg K, Revell DK. Consumption of a high-salt diet by ewes during pregnancy alters nephrogenesis in five-month-old offspring. Animal. 2012;11:1803–1810. doi: 10.1017/S1751731112000584. doi:10.1017/S1751731112000584. [DOI] [PubMed] [Google Scholar]
- Thomson SC, Deng A, Wead L, Richter K, Blantz RC, Vallon V. An unexpected role for angiotensin II in the link between dietary salt and proximal reabsorption. Journal of Clinical Investigation. 2006;116:1110–1116. doi: 10.1172/JCI26092. doi:10.1172/JCI26092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara M, Kobori H. Angiotensinogen expression is enhanced in the progression of glomerular disease. International Journal of Clinical Medicine. 2011;2:378–387. doi: 10.4236/ijcm.2011.24064. doi:10.4236/ijcm.2011.24064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods LL. Fetal origins of adult hypertension: a renal mechanism? Current Opinion in Nephrology and Hypertension. 2000;9:419–425. doi: 10.1097/00041552-200007000-00014. doi:10.1097/00041552-200007000-00014. [DOI] [PubMed] [Google Scholar]
- Woods LL, Rasch R. Perinatal Ang II programs adult blood pressure, glomerular number, and renal function in rats. American Journal of Physiology. 1998;275:R1593–F1599. doi: 10.1152/ajpregu.1998.275.5.R1593. [DOI] [PubMed] [Google Scholar]
- Xu Z, Glenda C, Day L, Yao J, Ross MG. Central angiotensin induction of fetal brain c-fos expression and swallowing activity. American Journal of Physiology. 2001;280:R1837–R1843. doi: 10.1152/ajpregu.2001.280.6.R1837. [DOI] [PubMed] [Google Scholar]
- Xu F, Mao C, Liu Y, Wu L, Xu Z, Zhang L. Losartan chemistry and its effects via AT1 mechanisms in the kidney. Current Medicinal Chemistry. 2009;16:3701–3715. doi: 10.2174/092986709789105000. doi:10.2174/092986709789105000. [DOI] [PMC free article] [PubMed] [Google Scholar]