Abstract
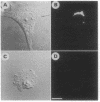
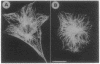
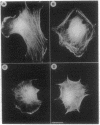
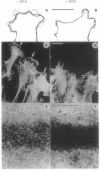
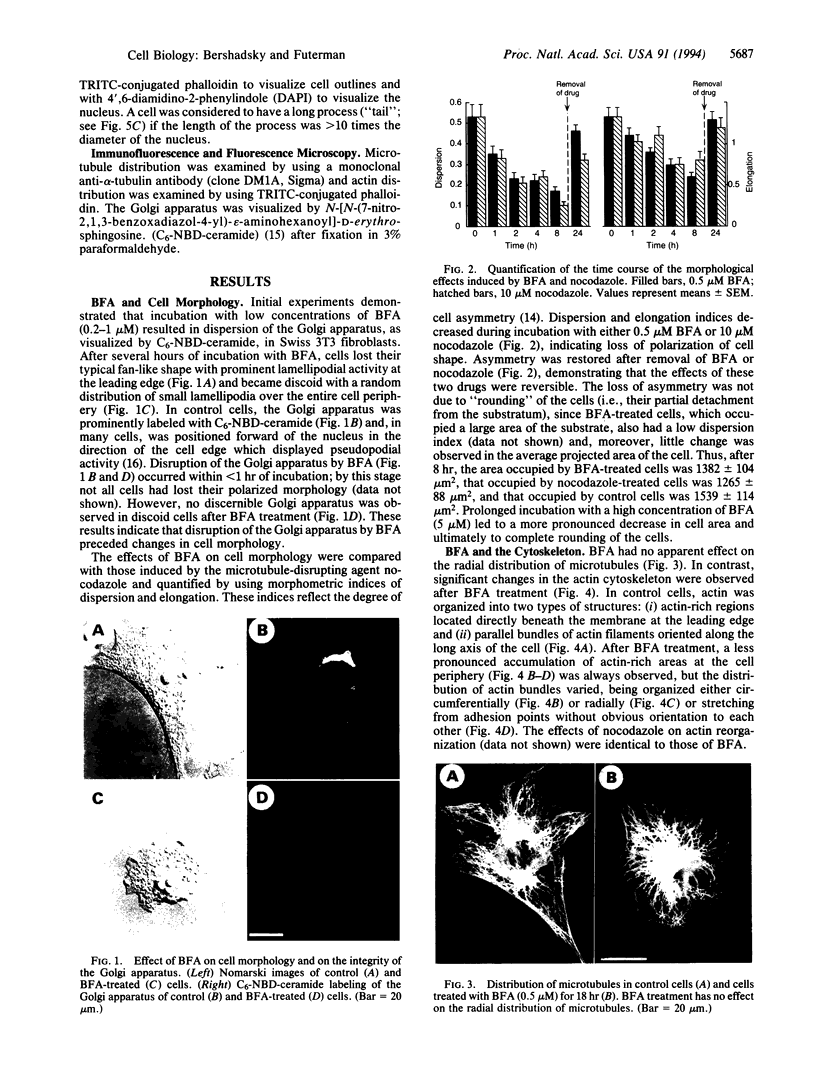
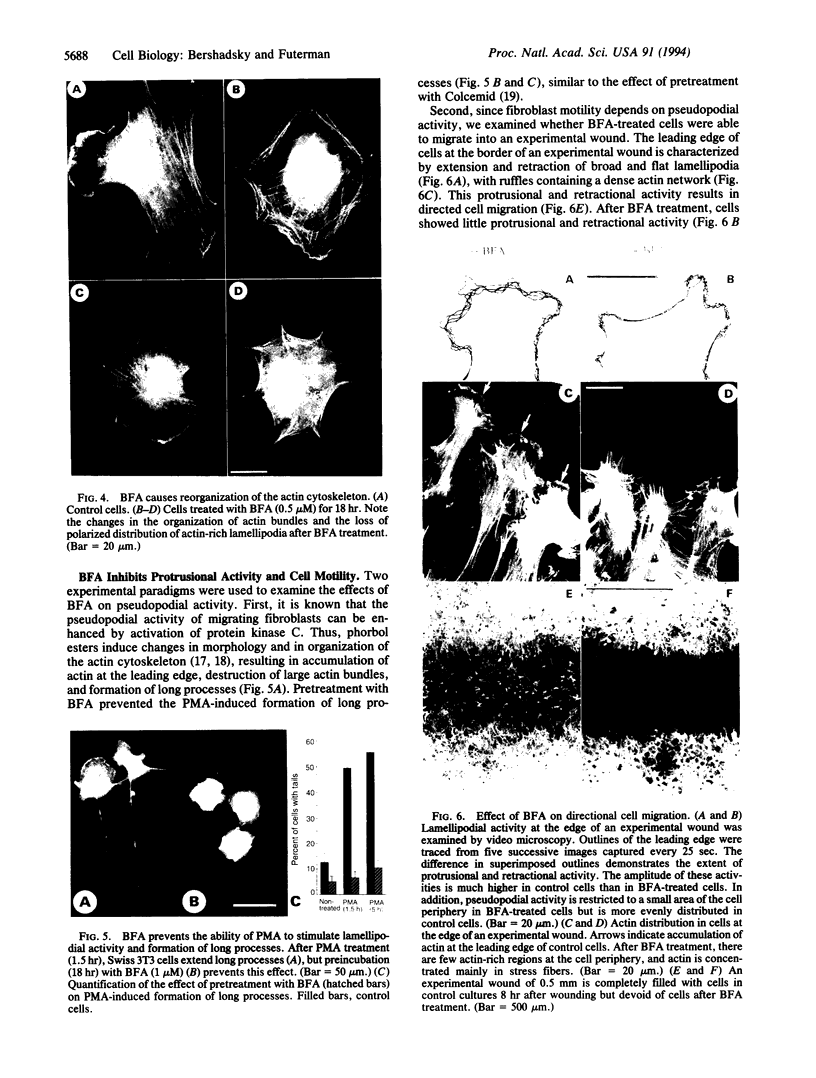
The role of the Golgi apparatus in the motile activity of fibroblasts was examined with brefeldin A (BFA), which disrupts the Golgi apparatus in a variety of cells. Upon incubation with BFA, Swiss mouse 3T3 fibroblasts lost their typical polarized morphology, in which the leading edge is characterized by intensive lamellipodia formation. BFA affected cell asymmetry as demonstrated by a decrease in the morphometric indices, dispersion, and elongation. After BFA treatment, cells showed little protrusional activity and did not form a dense actin network at the leading edge, and consequently the rate of cell migration into an experimental wound was significantly reduced. In addition, BFA prevented an increase in pseudopodial activity and prevented the formation of long processes induced by phorbol 12-myristate 13-acetate. The effects of BFA on cell shape and protrusional activity were quantitatively similar to those observed with the microtubule-disrupting agent nocodazole, although BFA had no effect on microtubule integrity. These results suggest that the integrity of both the Golgi apparatus and microtubules is necessary for the generation and maintenance of fibroblast asymmetry, which is a prerequisite for directed cell migration.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann J. E., Kupfer A., Singer S. J. Membrane insertion at the leading edge of motile fibroblasts. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1367–1371. doi: 10.1073/pnas.80.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A. D., Vaisberg E. A., Vasiliev J. M. Pseudopodial activity at the active edge of migrating fibroblast is decreased after drug-induced microtubule depolymerization. Cell Motil Cytoskeleton. 1991;19(3):152–158. doi: 10.1002/cm.970190303. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Skoufias D. A., Wilson L. Disruption of the Golgi apparatus with brefeldin A does not destabilize the associated detyrosinated microtubule network. Cell Motil Cytoskeleton. 1991;20(4):289–300. doi: 10.1002/cm.970200405. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Finazzi D., Klausner R. D. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992 Nov 26;360(6402):350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Dugina V. B., Svitkina T. M., Vasiliev J. M., Gelfand I. M. Special type of morphological reorganization induced by phorbol ester: reversible partition of cell into motile and stable domains. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4122–4125. doi: 10.1073/pnas.84.12.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G. A., Brown A. F. Alignment of fibroblasts on grooved surfaces described by a simple geometric transformation. J Cell Sci. 1986 Jul;83:313–340. doi: 10.1242/jcs.83.1.313. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Niclas J., Vale R. D., Banker G., Kosik K. S. Suppression of kinesin expression in cultured hippocampal neurons using antisense oligonucleotides. J Cell Biol. 1992 May;117(3):595–606. doi: 10.1083/jcb.117.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J. B., Rothman J. E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992 Nov 26;360(6402):352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- Hurtley S. M. Now you see it, now you don't: the Golgi disappearing act. Trends Biochem Sci. 1992 Sep;17(9):325–327. doi: 10.1016/0968-0004(92)90303-q. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Kronebusch P. J., Rose J. K., Singer S. J. A critical role for the polarization of membrane recycling in cell motility. Cell Motil Cytoskeleton. 1987;8(2):182–189. doi: 10.1002/cm.970080210. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Louvard D., Singer S. J. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2603–2607. doi: 10.1073/pnas.79.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Ishihara A., Jacobson K. How do cells move along surfaces? Trends Cell Biol. 1993 Nov;3(11):366–370. doi: 10.1016/0962-8924(93)90084-e. [DOI] [PubMed] [Google Scholar]
- Lyass L. A., Bershadsky A. D., Vasiliev J. M., Gelfand I. M. Microtubule-dependent effect of phorbol ester on the contractility of cytoskeleton of cultured fibroblasts. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9538–9541. doi: 10.1073/pnas.85.24.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness M. P., Orth J. M. Gonocytes of male rats resume migratory activity postnatally. Eur J Cell Biol. 1992 Oct;59(1):196–210. [PubMed] [Google Scholar]
- Miller S. G., Carnell L., Moore H. H. Post-Golgi membrane traffic: brefeldin A inhibits export from distal Golgi compartments to the cell surface but not recycling. J Cell Biol. 1992 Jul;118(2):267–283. doi: 10.1083/jcb.118.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano R. E., Sepanski M. A., Martin O. C. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J Cell Biol. 1989 Nov;109(5):2067–2079. doi: 10.1083/jcb.109.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Multiple targets for brefeldin A. Cell. 1991 Nov 1;67(3):449–451. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]
- Rodionov V. I., Gyoeva F. K., Tanaka E., Bershadsky A. D., Vasiliev J. M., Gelfand V. I. Microtubule-dependent control of cell shape and pseudopodial activity is inhibited by the antibody to kinesin motor domain. J Cell Biol. 1993 Dec;123(6 Pt 2):1811–1820. doi: 10.1083/jcb.123.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M., Nakamura T., Porter K. R., Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J Cell Biol. 1984 Sep;99(3):1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scliwa M., Höner B. Microtubules, centrosomes and intermediate filaments in directed cell movement. Trends Cell Biol. 1993 Nov;3(11):377–380. doi: 10.1016/0962-8924(93)90086-g. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Kupfer A. The directed migration of eukaryotic cells. Annu Rev Cell Biol. 1986;2:337–365. doi: 10.1146/annurev.cb.02.110186.002005. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Vasiliev J. M., Gelfand I. M., Domnina L. V., Ivanova O. Y., Komm S. G., Olshevskaja L. V. Effect of colcemid on the locomotory behaviour of fibroblasts. J Embryol Exp Morphol. 1970 Nov;24(3):625–640. [PubMed] [Google Scholar]
- Walker R. A., Sheetz M. P. Cytoplasmic microtubule-associated motors. Annu Rev Biochem. 1993;62:429–451. doi: 10.1146/annurev.bi.62.070193.002241. [DOI] [PubMed] [Google Scholar]