Abstract
In France, 190 306 patients were suffering from chronic hepatitis C in 2012. These patients have a decreased life expectancy and are susceptible to complications associated with chronic hepatitis. Current treatments are poorly tolerated and their effectiveness varies depending on the genotype of the virus. Sofosbuvir, a new class of treatment, has demonstrated in five phase III trials sustained viral response (SVR) rates of over 90% across genotypes, higher than current treatments and has a tolerance profile similar to placebo. The objective was to determine the cost-effectiveness of using sofosbuvir in the treatment of chronic HCV infection. A Markov model was used to compare treatment strategies with and without sofosbuvir. The model simulated the natural history of HCV infection. SVR rates were based on data from clinical trials. Utilities associated with different stages of disease were based on data from the literature. French direct medical costs were used. Price for sofosbuvir was the price used in the early access program for severe fibrosis stages. The incremental cost–effectiveness ratio for sofosbuvir versus current reference treatments was € 16 278/QALY and varied from 40 000 €/QALY for F0 stages to 12 080 €/QALY for F4 stages. The sensitivity analyses carried out confirmed the robustness of this result. Sofosbuvir is a cost-effective treatment option for patients with hepatitis C.
Keywords: cost-benefit analysis, France, healthcare costs, hepatitis C, sofosbuvir
Introduction
Chronic hepatitis C is due to a chronic infection by the hepatitis C virus (HCV). It develops in 75–85% of acute infections [1]. The prevalence of chronic hepatitis C was estimated in 2004 to 232 196 patients [2,3] and in 2012 to between 150 000 and 190 306 patients [4,5]. In the absence of an effective treatment, about 30% of infected patients develop cirrhosis and hepatocellular carcinoma after 20 years [6–8]. In contrast, patients with a sustained viral response (SVR) have a life expectancy similar to the general population [8].
Available treatments include pegylated interferon (PEGINF), ribavirin (RBV) and protease inhibitors (PIs). Recommended treatment regimens differ depending on the genotype of the virus, the level of liver fibrosis, HIV coinfection and the existence of prior treatments [9–11]. These treatments, although fairly effective, have some limitations. First, their associated SVR rates do not exceed 75% for genotype 1 infections in noncirrhotic naive patients [12], and for cirrhotic patients SVR drops to 62% at best. In addition, treatment durations are long, between 24 and 48 weeks. Third, they have tolerance issues with up to 26% premature discontinuation [13,14], that could result in reduced effectiveness in real life. Finally, all treatment regimens require the addition of pegylated interferon, which cannot be used in 15–45% of patients, because of tolerance issues and contraindications [15,16].
In this context, the arrival of a new therapeutic class, sofosbuvir (SOF), represents a new hope for many patients. Indeed, SOF-based treatments have shown SVR rates over 90% across genotypes, higher than the current treatments with tolerance profiles similar to placebo [17–21]. SOF treatments are also shorter than their counterpart. Given current healthcare budget constraints, we decided to assess the cost-effectiveness of SOF against current treatment options in the French context.
Method
Given the slow evolution of chronic HCV infections, and because treatments benefits are expected in the long term, a life-time horizon model was used. SOF treatments were compared with the recommended therapeutic strategies [9–11] for all chronically infected patients, regardless of their fibrosis stage, treatment history or HIV confection. G1/4 experienced patients and G4 coinfected patients were excluded because no clinical data were available for SOF. In addition, G3 naive coinfected patient was excluded because available clinical data (SOF + RBV treatment for 24 weeks) did not match the recommended SOF regimen (SOF + PEGINF + RBV for 12 weeks). Excluded patients accounted for approximately one quarter of chronic infection prevalence in France [1,15,16,22,23]. The population characteristics were based on French epidemiology [1,22,23] (Table 1).
Table 1.
Distributions of subgroups, comparators and treatment efficacy
| Subgroup | Proportion (%) | Cirrhotic (%) | Currently recommended treatment | SVR (cirrhotic/noncirrhotic) | Source | Sofosbuvir | SVR (cirrhotic/non cirrhotic) | Source |
|---|---|---|---|---|---|---|---|---|
| G1 naive | 56.8 | 40.0 | Télaprévir + PEGINF + RBV (48W) | 75.4/61.0 | Jacobson et al. 2011 [12] | SOF + PEGINF + RBV (12W) | 91.3/80.8 | Lalezary et al. 2013 [33] |
| G1 naive coinfected | 4.3 | 40.0 | Télaprévir + PEGINF + RBV (48W) | 72.1/61.0 | Sulkowski et al. AASLD 2012 | SOF + PEGINF + RBV (12W) | 77.1/60.0 | Sulkowski et al. 2013 [36] |
| G2 naive | 3.1 | 40.0 | PEGINF+RBV (24W) | 81.5/61.5 | Lawitz et al. 2013 [21] | SOF+RBV (12W) | 98.3/90.9 | Lawitz et al. 2013 [21] |
| G2 experience | 2.0 | 50.0 | PEGINF+RBV (48W) | 35.0/35.0 | Lagging et al. 2013 [37] | SOF+RBV (12W) | 91.0/87.5 | Zeuzem et al. 2013 [38] |
| G2 PEGINF ineligible | 2.9 | 40.0 | None | 0/0 | – | SOF+RBV (12W) | 91.8/93.3 | Jacobson et al. 2013 [17] |
| G2 naive coinfected | 0.4 | 40.0 | PEGINF+RBV (48W) | 86.0/61.1 | Labarga et al. 2012 [39] | SOF+RBV (12W) | 88.0/100.0 | Lalezary et al. 2013 [33] |
| G2 experienced coinfected | 0.4 | 50.0 | PEGINF+RBV (48W) | 86.0/61.1 | Labarga et al. 2012 [39] | SOF+RBV (12W) | 92.3/100.0 | Lalezary et al. 2013 [33] |
| G3 naive | 7.2 | 40.0 | PEGINF+RBV (24W) | 71.2/29.7 | Lawitz et al. 2013 [21] | SOF+ PEGINF + RBV (12W) | 100.0/83.3 | Lawitz et al. 2013 [40] |
| G3 experienced | 4.7 | 50.0 | PEGINF+RBV (48W) | 35.0/35.0 | Lagging et al. 2013 [37] | SOF+ PEGINF + RBV (12W) | 83.8/83.3 | Lawitz et al. 2013 [40] |
| G3 ineligible PEGINF | 6.2 | 40.0 | None | 0/0 | – | SOF+RBV (24W) | 85.0/60.0 | Zeuzem et al. 2013 [38] |
| G3 experienced coinfected | 0.5 | 50.0 | PEGINF+RBV (48W) | 86.0/61.1 | Labarga et al. 2012 [39] | SOF+RBV (24W) | 100.0/80.0 | Sulkowski et al. 2013 [36] |
| G4/5/6 naive | 11.6 | 40.0 | PEGINF+RBV (48W) | 50.0/38.6 | Manns et al. 2001 | SOF + PEGINF+ RBV (12W) | 100.0/50.0 | Lawitz et al. 2013 [20] |
W: Weeks, G: Genotype, PEGINF: pegylated interferons, RBV: ribavirine, SOF: sofosbuvir.
Model structure
A Markov model was used (Fig. 1). It is based on a previously published model that is described elsewhere [24]. Briefly, the model includes eleven states with two states for SVR patients, two absorbing states and one treatment state.
Fig 1.
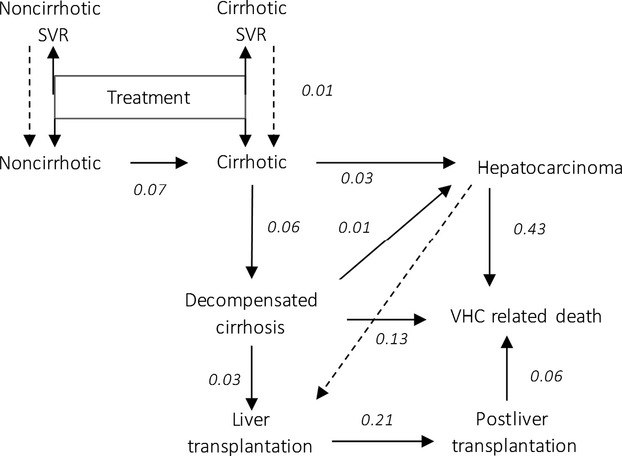
Markov tree.
Patients entered the model at treatment and were divided according to fibrosis stage, HCV genotype, prior treatment and coinfection distribution in the French population (Table 1). Patient could be treated by either currently recommended treatment [9–11] or by SOF. At the end of treatment, patients were moved either to an SVR state (SVR noncirrhotic, SVR cirrhotic) or a non-SVR state (noncirrhotic, cirrhotic) (Table 1). Retreatments, relapses and recurrences were not considered in the model. The impact of relapses and recurrences was tested in the sensitivity analysis (dotted arrow in Fig. 1). Fibrosis in SVR patients remained stable during lifetime and SVR patients had a mortality rate identical to that of the general population [8]. Fibrosis stage progressed in non-SVR patients and they could develop complications. At every stage, non-SVR patients had a background mortality rate based on the general population. In addition, patients with complications had a HCV-specific mortality rate.
The duration of the first eight cycles was three monthly to match treatment durations; subsequent treatment, cycles were yearly.
Clinical data
The proportions of patients in each subgroup, treatments, effectiveness and the duration are shown in Table 1. Serious adverse event rates (grade 3/4 WHO classification) of comparators and sofosbuvir were taken into account, and their frequencies were derived from clinical trial data (data not shown). Premature discontinuations due to adverse events were considered in the calculation of the effective treatment durations.
The transition probabilities of noncirrhotic to cirrhotic were calculated from the values used in a recent publication for the French population [25]. The transition probability from noncirrhotic to cirrhotic was extrapolated from this data using the stages distribution observed in the SOF clinical trials (77% of F0-F1-F2 patients and 23% of F3 patients). Other transition probabilities were based on the literature [26,27] (Fig. 1).
Utility data
In the absence of French utility data, utilities were derived from Hsu et al. 2012. The model also took into account the disutility related to treatment. These were determined from clinical trials sofosbuvir [20], telaprevir NICE notice [28] and the literature [29]. Data are presented in Table 2.
Table 2.
Parameters used in the model
| Parameter | Baseline value | Standard deviation | Distribution | Source |
|---|---|---|---|---|
| Utility (annual) | ||||
| Sofosbuvir | −0.08 | 0.054 | Uniform | Lawitz, 2013 [20] |
| PEGINF + RBV | −0.15 | 0.05 | Gamma | Wright 2006 [29] |
| Telaprevir | −0.14 | 0.01 | Gamma | NICE TA252 [28] |
| Noncirrhotic | 0.57 | 0.01 | Beta | PC Hsu, 2012 [41] |
| Cirrhotic | 0.51 | 0.01 | Beta | PC Hsu, 2012 [41] |
| SVR | +0.13 | 0.04 | Gamma | PC Hsu, 2012 [41] |
| Decompensated cirrhosis | 0.56 | 0.03 | Beta | PC Hsu, 2012 [41] |
| Hepatocarcinoma | 0.56 | 0.03 | Beta | PC Hsu, 2012 [41] |
| Liver transplantation | 0.56 | 0.03 | Beta | PC Hsu, 2012 [41] |
| Postliver transplantation | 0.64 | 0.03 | Beta | PC Hsu, 2012 [41] |
| Cost (annual) | ||||
| Noncirrhotic | 240 € | 397 € | Gamma | Schwarzinger 2013 [31] |
| Cirrhotic | 1081 € | 2642 € | Gamma | Schwarzinger 2013 [31] |
| Noncirrhotic SVR | – € | – € | Gamma | Assumption |
| Cirrhotic SVR | 178 € | 178 € | Gamma | Assumption |
| Decompensated cirrhosis | 11 719 € | 16 895 € | Gamma | Schwarzinger 2013 [31] |
| Hepatocarcinoma | 14 550 € | 19 770 € | Gamma | Schwarzinger 2013 [31] |
| Liver transplantation | 75 494 € | 89 294 € | Gamma | Schwarzinger 2013 [31] |
| Postliver transplantation | 3234 € | 7176 € | Gamma | Schwarzinger 2013 [31] |
| Sofosbuvir | 723 € | 46 € | Uniform | Assumption: 10% variation |
| Ribavirine | 223 € | 11 € | Uniform | Assumption: 10% variation |
| PEGINF 2a | 157 € | 8 € | Uniform | Assumption: 10% variation |
| Telaprevir | 2296 € | 115 € | Uniform | Assumption: 10% variation |
SVR: Sustained viral response, PEGINF: pegylated interferons, RBV: ribavirine.
Cost data
All costs were expressed in Euro 2013. Only direct medical costs were taken into account. Costs were estimated from a society point of view.
The price used for Sofosbuvir was the price in effect during the early access program for patients with severe fibrosis. The price of other treatments was based on the tax-inclusive retail price. The total cost of treatment took into account the average treatment duration.
The costs of serious adverse events were calculated based on their associated treatments strategies. Treatments strategies were based on telaprevir treatment guidelines, data from the literature [30] and expert opinions.
Monitoring costs were determined based on the recommended follow-up of chronically infected patients distinguishing cirrhotic patients from noncirrhotic [9–11]. The costs of laboratory tests, radiological examination and consultations were based on the prices fixed by the French national health insurance.
Management costs were based on a French cost study [31]. No cost was associated with SVR noncirrhotic patients as they were considered cured. Costs associated with the management of SVR cirrhotic patients were estimated based on follow-up recommendations from the French National Authority of Health (HAS) [32]. The cost data are presented in Table 2.
Analysis
The incremental cost–effectiveness ratio (ICER) was calculated for the life expectancy adjusted for quality of life (QALY). Costs and QALY were discounted at 2.5% per annum.
The robustness of the model was tested using probabilistic and deterministic sensitivity analysis. In the deterministic analysis, all the parameters were tested at −25% and +25% of baseline value. Only the ten parameters with the greatest influence on the results are presented. The results are presented with a Tornado graph (Fig. 2).
Fig 2.

Deterministic sensitivity analysis TP: transition probabilities, SVR: sustained viral response, HCC: Hepatocarcinoma, ICER: Incremental Cost–Effectiveness Ratio.
The probabilistic sensitivity analysis was conducted for utilities, costs, transition probabilities and treatment efficacy. It was based on a Monte-Carlo process with 1000 simulations. Parameters were varied randomly according to their associated distributions. The distributions used for the costs and utilities are shown in Table 2. For transition probabilities and efficacies a beta distribution was used, with a mean equal to the parameter baseline value and a standard deviation equal to 10% of the baseline value. The result is presented with an acceptability curve (Fig. 3).
Fig 3.
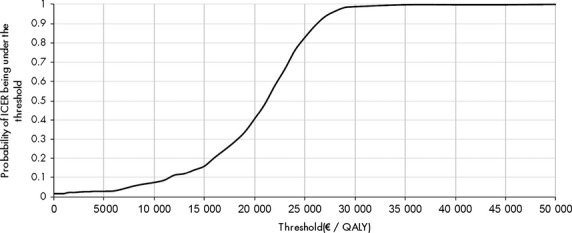
Acceptability curve.
Analysis was also performed for each fibrosis stage (F0 to F4), considering that all patient entered the model at the same stage. All parameters were unchanged, except for the proportion of cirrhotic patients and the noncirrhotic to cirrhotic transition probabilities. Transition probabilities were recalculated to match the fact that all patients entered the model at the same stage for F0 to F3. The analyses were performed using Excel version 2013 (Microsoft Corporation, Redmond, WA, USA).
Results
Main analysis
The nonactualized and actualized results are shown in Table 3. ICER of sofosbuvir compared to currently recommended treatments was estimated at € 16 278 €/QALY. Results for each fibrosis stage are shown in Table 4.
Table 3.
Results of the cost-effectiveness analysis
| Sofosbuvir | Comparators | |
|---|---|---|
| Nonactualized | ||
| Cost of treatment | 67 291 € | 24 186 € |
| Cost of care | 7239 € | 23 772 € |
| Total cost | 74 531 € | 47 958 € |
| Life expectancy (years) | 33.8 | 30.2 |
| Quality adjusted life expectancy (QALY) | 22.9 | 19.4 |
| Actualized | ||
| Total cost | 72 213 € | 39 789 € |
| Life expectancy (years) | 22.3 | 20.4 |
| QALY | 15.0 | 13.0 |
| Sofosbuvir versus Comparators | ||
| Incremental cost | 32 423 € | |
| Incremental life expectancy | 1.8 | |
| Incremental QALY | 2.0 | |
| ICER (life expectancy) | 17 817 €/LY | |
| ICER (QALY) | 16 278 €/QALY | |
ICER: Incremental Cost–Effectiveness Ratio, LY: Life Year.
Table 4.
ICER by Stage
| ICER by stage | |
|---|---|
| F0 | 40 653 €/QALY |
| F1 | 31 348 €/QALY |
| F2 | 17 651 €/QALY |
| F3 | 11 359 €/QALY |
| F4 | 12 080 €/QALY |
Sensitivity analyses
Figure 2 shows the deterministic sensitivity analyses for the ten parameters that have the greatest impact on the incremental cost–effectiveness ratio. Overall, ICER varied at most between 5000 €/QALY and 24 000 €/QALY for a 25% variation of baseline parameters.
Figure 3 shows the probability that the ICER is below a given threshold. The probability that the ICER of treatment sofosbuvir is <€ 40 000/QALY was 99.5%.
Discussion
This study showed that sofosbuvir, at the early access program price, is a cost-effective strategy in chronic HCV infection treatments at a commonly accepted threshold of € 40 000/QALY.
Sensitivity analyses confirmed the robustness of these results. Probabilistic analyses showed that close to a 100% of simulations were below 40 000 € per QALY. In our model, using sofosbuvir lead to a lower overall cost of the disease as more patients were cured leading to fewer patients developing expensive-to-treat advance liver conditions. This reduction in overall disease cost is the main factor explaining the observed small ICER.
The model was based on the most robust data available in the literature. However, the lack of available data for some parameters led us to make several assumptions. Firstly, the model does not discern F0 to F3 stage. Indeed, the efficacy of sofosbuvir in clinical trials of over 90% SVR was available only for the group of noncirrhotic patients without differentiating stage of fibrosis. We hypothesized that treatment efficacy was similar between F0, F1, F2 and F3 due to the high cure rates. Moreover, transition probabilities took into account the distribution of fibrosis stage observed in sofosbuvir clinical trials in order to be as close as possible to the clinical trial data, and thus have a higher confidence in the efficacy rates that were used. Furthermore, by stage analysis shows that ICERs are lower in higher stages. Considering that higher stages have a higher probability of reaching F4, this is coherent with the determinist analysis that showed that higher transition rates are associated with lower ICER. Secondly, in the absence of a comparator arm in the G1 naive sofosbuvir clinical trial, the effectiveness of telaprevir was based on clinical trial data available from the literature. This was deemed conservative as the population of SOF trial contained more difficult to treat patients with advanced disease, high BMI, low platelets and black ethnic status than the pivotal trial evaluating telaprevir [12,33]. Thirdly, the baseline model did not take into account the risk of reinfection and recurrence. However, this hypothesis was tested in the deterministic analysis and was not found to have an important impact on the ICER. Finally, genotype 3 naive coinfected patients were excluded from the analysis because available data for these patients does not match recommended regimen in sofosbuvir authorization. However, the overall impact on the ICER of including this population would have been negligible in view of the very limited size of that specific population.
Thus, these analyses showed that the use of sofosbuvir in the French context to treat chronically HCV infected patient is more effective and efficient than other available options defined by PIs and PEGINF. The relatively low ICER resulted from the higher SVR achieved and the subsequent reduction in the cost of the disease. This can be seen in the 12% gain in life expectancy in the sofosbuvir arm in the model. Those results are based on clinical trial data, which usually show better efficacy and safety. Indeed, recent results for protease inhibitors have shown that real life treatment completion was much lower than in clinical trials [34]. Similar results have also been observed for INFPEG/RBV treatments [35]. However, we expect that sofosbuvir, because of shorter treatment duration, better safety profile, inclusion of difficult to treat patient populations within the trials, PEGINF-free regimen and all oral treatments will result in a similar efficacy in real life. Furthermore, these results are probably conservative because no account was taken of comorbidities and extra hepatic manifestations associated with HCV such as diabetes, high blood pressure, kidney failure that would also be reduced with a more effective treatment. Thus, we expect even lower ICER in real life.
In conclusion, these analyses suggest that sofosbuvir is a cost-effective option for treating chronic hepatitis C regardless of fibrosis stage in French patients. Given these results, its use should be recommended in every eligible patients awaiting treatment.
Glossary
- HCV
hepatitis C virus
- ICER
incremental cost–effectiveness ratio
- PEGINF
pegylated interferon
- QALY
quality of life
- RBV
ribavirin
- SOF
sofosbuvir
- SVR
sustained viral response
Conflict of Interests
HL and MB have served as consultants for Gilead, Janssen, Ipsen and Lundbeck. This study was funded in full by Gilead.
References
- 1.Alter MJ. Epidemiology of hepatitis C. Hepatol Baltim Md. 1997;26(3 Suppl 1):62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 2.INVS. 2009. [National reference lab data for hepatitis C, 2001-2007] Surveillance nationale de l'hépatite C à partir des pôles de référence, données épidémiologiques 2001-2007. Saint-Maurice Cedex, France Institut de Veille Sanitaire.
- 3.Meffre C, Le Strat Y, Delarocque-Astagneau E, et al. Prevalence of hepatitis B and hepatitis C virus infections in France in 2004: social factors are important predictors after adjusting for known risk factors. J Med Virol. 2010;82(4):546–555. doi: 10.1002/jmv.21734. [DOI] [PubMed] [Google Scholar]
- 4.Deuffic-Burban S, Mathurin P, Pol S, et al. Impact of hepatitis C triple therapy availability upon the number of patients to be treated and associated costs in France: a model-based analysis. Gut. 2012;61(2):290–296. doi: 10.1136/gutjnl-2011-300586. [DOI] [PubMed] [Google Scholar]
- 5.Henri L, Homie R, Chris E, Christophe H, Martin B, Francoise R-T. Modeling the epidemiological impact of upcoming direct antiviral agents in hepatitis C treatment. Hepatology. 2013;58:208A–1309A. [Google Scholar]
- 6.Lee LY, Tong CYW, Wong T, Wilkinson M. New therapies for chronic hepatitis C infection: a systematic review of evidence from clinical trials. Int J Clin Pract. 2012;66(4):342–355. doi: 10.1111/j.1742-1241.2012.02895.x. [DOI] [PubMed] [Google Scholar]
- 7.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA, J Am Med Assoc. 2012;308(24):2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 8.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349(9055):825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 9.Association Francaise pour l'Etude du Foie (AFEF) 2011. Prise de position de l'Association Française pour l'Etude du Foie (AFEF) sur les trithérapies (Peg-IFN + ribavirine + inhibiteur de protéase) dans la prise en charge des malades atteints d'hépatite chronique C [Internet]. Available from: http://www.afef.asso.fr/rc/org/afef/htm/Article/2011/htm-20110414-094626-465/src/htm_fullText/fr/reco%20afef%20V2%2030%2011%2011.pdf.
- 10.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55(2):245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Arvieux C, Bourlière M, Cacoub P, et al. 2013. L'utilisation des inhibiteurs de protéase du VHC de première génération chez les patients co-infectés par le VIH et le VHC, de génotype 1 [cited 2014 Feb 19]; Available from: http://www.vih.org/sites/default/files/RecomandationsIPantiVHC%20coinfection%20VIHVHC220313.pdf.
- 12.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 13.Hézode C, Fontaine H, Dorival C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol. 2013;59:434–441. doi: 10.1016/j.jhep.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 14.Bota S, Sporea I, Sirli R, et al. Severe adverse events during antiviral therapy in hepatitis C virus cirrhotic patients: a systematic review. World J Hepatol. 2013;5(3):120–126. doi: 10.4254/wjh.v5.i3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pariente A, Lahmek P, Duprat C, et al. Treatment of chronic hepatitis C with pegylated interferon and ribavirin in treatment-naive patients in “true life”: a plea in favor of independent postmarketing evaluations. Eur J Gastroenterol Hepatol. 2010;22(11):1297–1302. doi: 10.1097/meg.0b013e32833bb4b0. [DOI] [PubMed] [Google Scholar]
- 16.Falck-Ytter Y, Kale H, Mullen KD, Sarbah SA, Sorescu L, McCullough AJ. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002;136(4):288–292. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 18.Gane EJ, Stedman CA, Hyland RH, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368(1):34–44. doi: 10.1056/NEJMoa1208953. [DOI] [PubMed] [Google Scholar]
- 19.Kowdley KV, Lawitz E, Crespo I, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381(9883):2100–2107. doi: 10.1016/S0140-6736(13)60247-0. [DOI] [PubMed] [Google Scholar]
- 20.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 21.Lawitz E, Lalezari JP, Hassanein T, et al. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis. 2013;13(5):401–408. doi: 10.1016/S1473-3099(13)70033-1. [DOI] [PubMed] [Google Scholar]
- 22.Bourlière M, Ouzan D, Rosenheim M, et al. Pegylated interferon-α2a plus ribavirin for chronic hepatitis C in a real-life setting: the Hepatys French cohort (2003-2007) Antivir Ther. 2012;17(1):101–110. doi: 10.3851/IMP1935. [DOI] [PubMed] [Google Scholar]
- 23.Marcellin P, Chousterman M, Fontanges T, et al. Adherence to treatment and quality of life during hepatitis C therapy: a prospective, real-life, observational study. Liver Int. 2011;31(4):516–524. doi: 10.1111/j.1478-3231.2011.02461.x. [DOI] [PubMed] [Google Scholar]
- 24.Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon-α2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127(10):855–865. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 25.Deuffic-Burban S, Deltenre P, Buti M, et al. HCV burden in Europe: impact of national treatment practices on future HCV-related morbidity and mortality through a modeling approach. J Hepatol. 2011;54:S54. [Google Scholar]
- 26.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112(2):463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 27.Shepperd S, Richards S. Continuity of care – a chameleon concept. J Health Serv Res Policy. 2002;7(3):130–132. doi: 10.1258/135581902760082427. [DOI] [PubMed] [Google Scholar]
- 28.Janssen pharmaceutical companies of Johnson&Johnson. Telaprevir for the Treatment of Genotype 1 Chronic Hepatitis C. London: NICE; 2011. [Google Scholar]
- 29.Wright TL. Treatment of patients with hepatitis C and cirrhosis. Hepatology. 2002;36(S1):S185–S194. doi: 10.1053/jhep.2002.36812. [DOI] [PubMed] [Google Scholar]
- 30.Gao X, Stephens JM, Carter JA, Haider S, Rustgi VK. Impact of adverse events on costs and quality of life in protease inhibitor-based combination therapy for hepatitis C. Expert Rev Pharmacoecon Outcomes Res. 2012;12(3):335–343. doi: 10.1586/erp.12.10. [DOI] [PubMed] [Google Scholar]
- 31.Schwarzinger M, Deuffic-Burban S, Mallet V, et al. Lifetime costs attributable to chronic hepatitis C from the French healthcare perspective. J Hepatol. 2013;58(Supplement 1):S21–S22. [Google Scholar]
- 32.HAS. 2006. Hépatite Chronique C [Internet]. Saint-Denis HAS; Mai. Available at: http://www.has-sante.fr/portail/upload/docs/application/pdf/06-072_hepat-c_internet_sans_liste.pdf.
- 33.Lalezari JP, Nelson DR, Hyland RH, et al. Once daily sofosbuvir plus ribavirin for 12 and 24 weeks in treatment-naïve patients with HCV infection: the quantum study. J Hepatol. 2013;58:S346. [Google Scholar]
- 34.Vutien P, Kim Y, Brooks L, Livornese R, Nguyen MH. Low Treatment Rates and Suboptimal Treatment Completion Rates to Hepatitis C Virus (HCV) Therapy: A Real-World Analysis of a Large US Cohort. Gastroenterology. 2014;146(5):S–920. May 1. [Google Scholar]
- 35.Beste LA, Ioannou GN, Larson MS, Chapko M, Dominitz JA. Predictors of early treatment discontinuation among patients with genotype 1 hepatitis C and implications for viral eradication. Clin Gastroenterol Hepatol. 2010;8(11):972–978. doi: 10.1016/j.cgh.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Sulkowski MS, Rodriguez-Torres M, Lalezari JP, Fessel W, Mounzer K, Shuhart M. All-oral therapy with sofosbuvir plus ribavirin for the treatment of HCV genotype 1, 2, and 3 infection in patients co-infected with HIV (PHOTON-1) Hepatology. 2013;58(S1):313–4A. [Google Scholar]
- 37.Lagging M, Rembeck K, Rauning BM, et al. Retreatment with peg-interferon and ribavirin in patients with chronic hepatitis C virus genotype 2 or 3 infection with prior relapse. Scand J Gastroenterol. 2013;48(7):839–847. doi: 10.3109/00365521.2013.793389. [DOI] [PubMed] [Google Scholar]
- 38.Zeuzem S, Dusheiko GM, Salupere R. 2013. pp. 1–5. Sofosbuvir+ ribavirin for 12 or 24 weeks for patients with HCV genotype 2 or 3: the VALENCE Trial. 64th Annu Meet Am Assoc Study Liver Dis.
- 39.Labarga P, Barreiro P, da Silva A, et al. Comparison of high ribavirin induction versus standard ribavirin dosing, plus peginterferon-α for the treatment of chronic hepatitis C in HIV-infected patients: the PERICO trial. J Infect Dis. 2012;206(6):961–968. doi: 10.1093/infdis/jis449. [DOI] [PubMed] [Google Scholar]
- 40.Lawitz E, Poordad F, Brainard DM, Hyland RH, An D, Symonds WT, et al. 2013. pp. 1380A–1380A. Sofosbuvir in Combination With PegIFN and Ribavirin for 12 Weeks Provides High SVR Rates in HCV-Infected Genotype 2 or 3 Treatment Experienced Patients with and without Compensated Cirrhosis: Results from the LONESTAR-2 Study. Hepatology. Hoboken, NJ, USA Wiley-Blackwell.
- 41.Hsu PC, Federico CA, Krajden M, et al. Health utilities and psychometric quality of life in patients with early-and late-stage hepatitis C virus infection. J Gastroenterol Hepatol. 2012;27:149–157. doi: 10.1111/j.1440-1746.2011.06813.x. [DOI] [PubMed] [Google Scholar]


