Abstract
Aim
This review explores the molecular, neurological, and behavioural outcomes in animal models of uterine artery ligation. We analyse the relevance of this type of model to the pathological and functional phenotypes that are consistent with cerebral palsy and its developmental comorbidities in humans.
Method
A literature search of the PubMed database was conducted for research using the uterine artery ligation model published between 1990 and 2013. From the studies included, any relevant neuroanatomical and behavioural deficits were then summarized from each document and used for further analysis.
Results
There were 25 papers that met the criteria included for review, and several outcomes were summarized from the results of these papers. Fetuses with growth restriction demonstrated a gradient of reduced body weight with a relative sparing of brain mass. There was a significant reduction in the size of the somatosensory cortex, hippocampus, and corpus callosum. The motor cortex appeared to be spared of identifiable deficits. Apoptotic proteins were upregulated, while those important to neuronal survival, growth, and differentiation were downregulated. Neuronal apoptosis and astrogliosis occurred diffusely throughout the brain regions. White matter injury involved oligodendrocyte precursor maturation arrest, hypomyelination, and an aberrant organization of existing myelin. Animals with growth restriction demonstrated deficits in gait, memory, object recognition, and spatial processing.
Interpretation
This review concludes that neuronal death, white matter injury, motor abnormalities, and cognitive deficits are important outcomes of uterine artery ligation in animal models. Therefore, this is a clinically relevant type of model, as these findings resemble deficits in human cerebral palsy.
What this paper adds
• Uterine artery ligation in animal models resulted in apoptosis and astrogliosis.
• An important outcome of this model was white matter degeneration.
• Uterine artery ligation also resulted in abnormal cognitive function and motor coordination and memory.
• The outcomes of uterine artery ligation resulted in anatomical and functional phenotypes consistent with cerebral palsy.
Uterine artery ligation results in placental insufficiency and intrauterine growth restriction (IUGR).1 Animal models have demonstrated that this insult is associated with a host of metabolic and haemodynamic abnormalities2 and pathological outcomes are seen within respiratory,3 cardiac,4 renal,5 vascular,6 muscular,7 pancreatic,8 and neurological9–12 development.
The uterine artery ligation model has been aimed at replicating findings seen in human IUGR, a complication that occurs in an estimated 5% of human pregnancies.13 The most common cause of IUGR in developed countries is placental insufficiency,14 which is an important aetiology in cerebral palsy (CP).15,16 IUGR in children results in significant neurological, behavioural, and cognitive abnormalities. In order to understand the mechanisms, outcomes, and treatments for pathology associated with placental insufficiency and growth restriction, an appropriate animal model is required.
In this systematic review, we examine the molecular, neurological, and behavioural outcomes in animal uterine artery ligation experiments and relate these results to findings in human studies of IUGR. Our goal is to explore uterine artery ligation research in the several animal species that have been studied in order to provide a portrait of our current understanding of the outcomes of this model. This will allow us to assess the current validity of uterine artery ligation as a model of CP. In addition, it provides a basis for future research to explore and clarify deficits resulting from this injury, in the context of the current knowledge presented here. This will provide an opportunity to study the outcome of therapeutic approaches.
We explore multiple neuroanatomical outcomes of uterine artery ligation. This involves evaluation of resulting deficits in cellular populations such as neurons and their axons, mature and premature oligodendrocytes, and astrocytes. We outline the current understanding of region-specific outcomes, describe the molecular and biochemical mechanisms underlying these deficits, and explore the behavioural outcomes involving memory, cognition, and motor function.
Method
This literature search was conducted using the PubMed database. The following search was conducted within ‘title/abstract’ using the PubMed advanced search builder: ‘uterine’ AND ‘ligati*’ AND [‘behav*’ OR ‘brain’ OR ‘neuro*’ OR ‘cereb*’]. This search, conducted in July 2013, included papers published between 1990 and 2013 and yielded a total of 62 peer-reviewed papers (Fig.1). The abstracts and titles of these papers were assessed to identify if they did indeed look at the uterine artery ligation model; those that did not were excluded. This resulted in the exclusion of all human research involving such topics, for example hysterectomy and hypogastric artery ligation, as well as irrelevant research in animals. Only research articles that explored neurological or behavioural outcomes were included; thus, research on uterine artery ligation outcomes on cardiac, respiratory, musculoskeletal, renal, and other systems were excluded. A total of 25 peer-reviewed research publications met our criteria and were included in this review (TableI).
Figure 1.
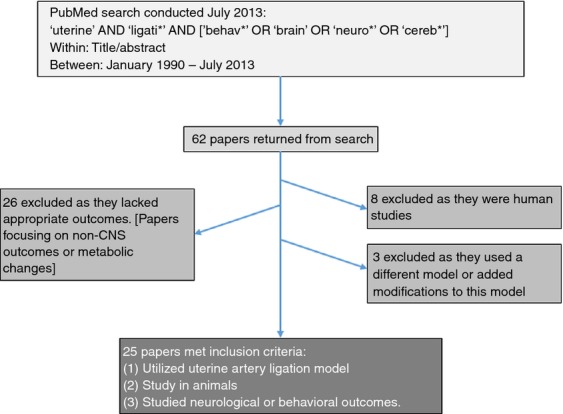
Summary of search terms and exclusion/inclusion criteria.
Table I.
A summary of results of publications in this review
| Authors | Year | Major outcomes of uterine artery ligation |
|---|---|---|
| Reid et al.1 | 2012 | Oligodendrocyte differentiation was delayed until adulthood |
| MBP4 expression was upregulated in early life but normalized in adulthood | ||
| Adult females demonstrated behavioural deficits | ||
| Oxidative stress resulted in BMP4 upregulation and subsequent delayed myelination | ||
| Delcour et al.9 | 2012 | There was white matter damage and deficits in myelination |
| Astrogliosis, neuronal cell loss, and axonal degeneration occurred within white matter | ||
| There was normal neuronal density within the hippocampus and cingulate cortex | ||
| There were also behavioural deficits in locomotor and sensorimotor function, short-term memory, information coding, and sensory gating function | ||
| Spatial and working memory were spared | ||
| Rehn et al.10 | 2004 | Brain weight and basal ganglia volume were decreased |
| Lateral ventricles were enlarged | ||
| Catecholamine expression was unaffected | ||
| Prepulse inhibition was reduced into adulthood | ||
| Delcour et al.11 | 2012 | There was white matter damage within the corpus callosum, brainstem, hippocampus, and somatosensory cortex |
| But white matter was spared within the motor cortex | ||
| Motor maps were abnormal within the somatosensory cortex but were spared within the motor cortex | ||
| Spasticity and hyperactivity were present | ||
| There were short-term memory deficits | ||
| Mallard et al.12 | 2000 | Neuronal density within CA1 and the cerebellum was reduced |
| Volume of cerebellar white matter was reduced | ||
| Fung et al.14 | 2012 | There were reduced neuronal density and immature oligodendrocytes with astrogliosis in the hippocampus |
| ErbB-R expression was increased within the hippocampus | ||
| Tolcos et al.18 | 2011 | There was reduction in white matter in fetuses but not into adulthood |
| Myelinating oligodendrocytes (MBP, MAG, PLP) were reduced but normalized postnatally | ||
| Turner and Trudinger19 | 2009 | Body weight was reduced |
| There was a relative sparing of brain size | ||
| Dieni and Rees20 | 2005 | BDNF expression was reduced and TrkB expression was increased in the hippocampus |
| BDNF and TrkB expression in cerebellum were unaffected | ||
| Catteau et al.21 | 2011 | Birthweight was reduced |
| There was aberrant expression of vascular endothelial growth factor and NMDA receptors | ||
| Olivier et al.22 | 2005 | There were increased numbers of microglial cells and astrogliosis within the cingulate cortex and internal capsule |
| There was preoligodendrocyte apoptosis and subsequent scarcity | ||
| Hypomyelination was present until adulthood | ||
| Tashima et al.24 | 2001 | There was abnormal neuronal migration within the cerebral cortex |
| There were locomotor deficits in adult male rats but not in female rats | ||
| Mallard et al.25 | 1999 | Cerebral ventricles were enlarged |
| The cerebral cortex and hippocampal size were reduced | ||
| Olivier27 | 2007 | There was white matter damage, increased microglia, and astrogliosis |
| There was a reduction in preoligodendrocytes and myelination delay | ||
| Uysal29 | 2008 | Inducible NOS and p53 within the cortex were increased |
| Inducible NOS and endothelial NOS were increased and Bcl-2 expression decreased within the ventricular zone | ||
| Lane et al.30 | 2001 | Bcl-2 expression was decreased within the cortex |
| Bcl-2-associated X protein and caspase-3 activity were unaffected | ||
| Ke et al.31 | 2005 | There was increased p53 expression |
| Expression of murine double minute 2 was reduced | ||
| Olivier et al.37 | 2009 | Microglial activation and astrogliosis were present |
| There was a reduction in and delayed maturation of oligodendrocytes | ||
| Tolcos and Rees38 | 1997 | There were increased astrocyte populations within the vagus, nucleus tractus solitarius, and brain stem |
| Nitsos and Rees39 | 1990 | Hypomyelination was present within the cerebral cortex, corpus callosum, and cerebellum |
| There was astrocytosis within the cerebral cortex but not the cerebellum | ||
| Delayed myelination and hypomyelination were present but the total neuronal fibre count was unaffected | ||
| Tatli et al.42 | 2007 | There was lipid peroxidation and oxidative damage within the cerebellum and cerebral cortex |
| Dieni and Rees45 | 2003 | Dendritic branching patterns were reduced and abnormal within the hippocampus |
| Nishigori et al.46 | 2008 | There was abnormal expression of BDNF and TrkB throughout the brain |
| Schober et al.49 | 2009 | The hippocampi of males had a reduced NR1 expression and NR2A:NR2B ratio |
| There was reduced MBP within the hippocampus | ||
| O'Grady et al.61 | 2010 | There was an increased level of serum testosterone and reduced levels of hippocampal aromatase expression in males |
BDNF, brain-derived neurotrophic factor; BMP, bone morphogenetic protein; MAG, myelin-associated glycoprotein; MBP, myelin basic protein; NMDA, N-methyl-D-aspartate; NOS, Nitric oxide synthase; PLP, proteolipid protein; TrkB, tyrosine kinase receptor.
Results
The injury and gross outcomes
Uterine artery ligation was first described by Wigglesworth17 in pregnant rats. The pregnant females were deeply anaesthetized and then the uterine artery was ligated bilaterally or unilaterally on embryonic day 17 (E17)9 or day 19 (E19).1,14 This period represents the onset of rapid fetal growth and corresponds to the beginning of the third trimester in human pregnancy.17 Uterine artery ligation was performed at mid-gestation (28–30d) in guinea pigs18–20 and at embryonic day 13 (E13) in mice.21
The closer the fetus is to the ligation, the more significant the effect of the insufficiency and growth restriction. This leads to a gradient in the fetal size, with the smallest rats being closest to the ligation site and the largest being more distal.17,22,23 Reduced body weight was observed until postnatal day 21 (PN21) in rats24 and until PN10 in mice.21 In rats, placental insufficiency did not result in a concomitant reduction in brain weight and, thus, resulted in an increased ratio of brain weight to body weight.24 In guinea pigs fetuses with placental insufficiency, there was a significant reduction in whole brain weight, but this reduction was less than that seen in body weight.18–20 Thus, there was a relative sparing of the brain in this model. Ventriculomegaly25 and reduction in basal ganglia volume were observed within fetal and week 12 guinea pigs.10 Several studies have demonstrated that both behavioural and neurological deficits correlate with severity of growth restriction.9,14
Neuronal cell death and neuron density
Placental insufficiency resulted in neuronal cell death within several brain regions (TableII). Degenerative neural cell bodies have been found within the hippocampus (CA3 region) and within the medial and lateral entorhinal cortex, and the cingulate cortex of adult rats with placental insufficiency.9 Although there was reduced neuron density in the medial and lateral entorhinal cortices,11 the height of these cortices was not reduced.9 Neuron density was not reduced in other brain regions that demonstrated degenerative neural cell bodies. The parietal cerebral cortex experienced neuronal cell loss at E21 and persisted at 10 weeks after birth.24 Significant cell death occurred at PN3 within the white matter of rats with placental insufficiency.22
Table II.
A summary of neuroanatomical deficits resulting from uterine artery ligation. Changes in cell populations and brain regions are outlined
| Neuroanatomical deficit | Outcomes in uterine artery ligation |
|---|---|
| Neuronal cell death | Hippocampus (CA3 and dentate gyrus) |
| Medial and lateral entorhinal cortex | |
| Cingulate cortex | |
| Parietal cerebral cortex | |
| White matter | |
| Cerebellum | |
| Astrogliosis | Hippocampus |
| Medial and lateral entorhinal cortex | |
| Cingulated cortex | |
| Brain stem (dorsal motor nucleus of vagus and nucleus tractus solitarius) | |
| White matter | |
| Gross region size | Medial and lateral entorhinal cortices were unaffected |
| Motor cortex was unaffected | |
| Somatosensory cortex was reduced in height | |
| There was reduced hippocampal volume | |
| There was reduced corpus callosum thickness | |
| Motor cortex | No abnormalities present in astrocyte activation, axonal integrity, or cortical maps |
| Somatosensory cortex | Cellular degeneration, astrogliosis, and white matter damage were present |
| There was inhibitory GABAergic interneuron death | |
| There was an abnormal somatosensory map | |
| There was an abnormal stratification of cortical structures | |
| Hippocampus | The oligodendrocyte progenitor population was unaffected within the dentate gyrus and CA3 |
| There was abnormal dendritic morphology | |
| White matter injury | There was arrest of oligodendrocyte precursor maturation |
| Hypomyelination occurred within the spinal cord, cerebral cortex, corpus callosum, and cerebellum | |
| The number of mature oligodendrocytes was reduced | |
| There was no reduction in the number of oligodendrocyte precursors | |
| There was a reduction in MBP | |
| Degenerating axons were present | |
| There was poorly organized myelination with thinner myelin sheath |
MBP, myelin basic protein.
Although neuronal density within the CA1 region of the hippocampus was not reduced within PN2126 and adult rats, degenerating neurons9 and neuronal apoptosis11 were present. However, the neuronal population was reduced in the CA1 and CA3 regions in PN0 male rats.14 Neuron numbers within the dentate gyrus were decreased in PN0 female rats. Similarly, in fetal guinea pigs with placental insufficiency, there was a reduction in neuron number in the hippocampus and cerebellum.12 Existing cells demonstrated abnormal axon and dendrite growth.
The severity of neuron density loss within white matter was correlated with the extent of growth restriction. Placental insufficiency in rats with moderate growth restriction did not result in increased cell death within the white matter at PN3.27 However, levels of apoptosis were significantly higher in rats with severe restriction than in control rats.
Bcl-2 is an important anti-apoptotic protein, while p53 is an pro-apoptotic protein (TableIII).28 Studies of rats with placental insufficiency at birth demonstrated reduced Bcl-2 expression in the ventricular zone29 and reduced Bcl-2 mRNA levels.30 However, p53 (mRNA and protein) and activated phospho-p53 were upregulated within the cortical zone, CA1 region, subcortical and periventricular white matter, and the amygdala.29,31 Under physiological conditions, p53 should result in murine double minute 2 transcription, which functions to attenuate the apoptotic effect of p53.32 In contrast, rats which had suffered placental insufficiency had reduced levels of murine double minute 2 expression at birth.31
Table 3.
A summary of changes in molecular expression as a result of uterine artery ligation
| Molecule | Function | Expression in uterine artery ligation |
|---|---|---|
| Bcl-2 | Anti-apoptotic protein | Reduced (ventricular zone) |
| p53 | Pro-apoptotic protein | Increased (cortical zone, CA1 region, subcortical and periventricular white matter, the amygdala) |
| Phosphor-p53 | Pro-apoptotic protein | Increased (cortical zone, CA1 region, subcortical and periventricular white matter, the amygdala) |
| Murine double minute 2 | Mitigates apoptosis due to p53 | Reduced |
| Bcl-2-associated X protein | Pro-apoptotic protein | Increased |
| Caspase-3 | DNA fragmentation | Increased |
| Inducible NOS | Cerebral pathology associated with apoptosis | Increased (parietal cerebral cortex, ventricular zone) |
| Endothelial NOS | Cerebral pathology associated with apoptosis | Increased (ventricular zone) |
| BDNF | Support of neuronal survival, differentiation, and growth | Reduced (hippocampus) |
| TrkB receptor | Activated by BDNF to support neuronal growth/development | Reduced (hippocampus) |
| ErbB3 receptor | Cellular differentiation and proliferation | Reduced (hippocampus) |
| NMDA receptor subunits | Cognitive and memory development | Reduced NR1 expression and NR2A–NR2B ratio (hippocampus) |
Bcl-2, B-cell lymphoma 2; BDNF, brain-derived neurotrophic factor; NMDA, N-methyl-D-aspartate; NOS, nitric oxide synthase; TrkB, tyrosine kinase receptor.
Increased Bcl-2 expression due to placental insufficiency resulted in increased vulnerability to subsequent hypoxia-induced apoptosis. At birth, hypoxia following placental insufficiency resulted in increased levels of lipid peroxidation, Bcl-2-associated X protein mRNA expression, and caspase-3 activity.30 (Bcl-2-associated X protein is a mitochondrial-associated pro-apoptotic protein33 and caspase-3 is involved in DNA fragmentation.34,35) These markers of apoptosis were not increased in placental insufficiency or hypoxia alone.
Nitric oxide synthase (NOS) plays an important role in cerebral pathology. Placental insufficiency injury results in the upregulation of calcium-independent inducible NOS in the parietal cerebral cortex and ventricular zone at birth.29 Endothelial NOS is upregulated within the ventricular zone of these rats. It is likely that this activation of NOS played an important role in the apoptosis observed within the brains of rats with placental insufficiency.
Astrogliosis
Astrogliosis was activated in hypoxia–ischaemia models.36 Uterine artery ligation resulted in astrogliosis within the hippocampus, medial and lateral entorhinal cortex, and cingulate cortex at PN14 and into adulthood in rats with placental insufficiency.9,11,37 The reactive astrocytes in these rats had larger cell bodies and more processes than control rats.37 The extent of astrogliosis was correlated with the severity of growth restriction. Rats with severe restriction (mass more than 2SD below the mean) had more significant astrogliosis within the cingulum than rats with moderate restriction (mass between 1SD and 2SD below the mean).27 Astrogliosis persisted until PN21 in rats with severe restriction, but only until PN14 in rats with moderate restriction.27 Fetal guinea pigs with placental insufficiency demonstrated increased astrocytes within the brain stem (dorsal motor nucleus of vagus, and nucleus tractus solitarius)38 and white matter.18,39 Astrogliosis was transient in guinea pigs, as it was in rats. Indeed, astrogliosis within the white matter of 60 day's gestation guinea pig fetuses resolved by week 1.10,18 However, other studies demonstrated persistent astrogliosis into adulthood.9
Specific studies of astrocyte populations within the hippocampus revealed increased levels of astrocytes within the dentate gyrus but a reduction in astrocytes within the CA3 region in male rats at PN0.14 In contrast, a study of male PN21 rats demonstrated increased astrocyte density within the CA3 region.26 However, in both sexes at PN0, there was no difference in hippocampus astrocyte number between the control rats and rats with placental insufficiency.14
Oxidative damage
The brain, and especially oligodendrocytes, are particularly susceptible to oxidative damage.1,40,41 This damage occurs when there is an imbalance in the free radical production and processes involved in free radical scavenging. Indeed, several markers of oxidative damage were observed within PN60 rats with placental insufficiency.42 There was an elevation in serum lipid peroxidation levels and protein oxidative damage within the cerebral cortex and cerebellum. There was a concomitant reduction in the activity of catalase and superoxide dismutase, both of which are important antioxidant enzymes.
Melatonin has been shown to have neuroprotective effects and it functions as an antioxidant. One study demonstrated that, in rats with placental insufficiency, myelination defects within the cingulate white matter were attenuated in animals with moderate growth restriction that were treated with melatonin.37 There was an increased density of myelin basic protein (MBP) immunoreactivity and improved organization and compaction of myelin sheets within these animals. Oligodendrocyte maturation was promoted, increasing the number of mature oligodendrocytes. In addition, this treatment reduced the activation of microglia resulting from placental insufficiency injury. However, melatonin did not appear to affect brain weight or oligodendrocyte proliferation.
Motor and somatosensory cortices
In adult rats with placental insufficiency, there seemed to be no abnormalities within the primary motor cortex anatomy. Indeed, neuronal density in these rats was similar to that in control animals and no astrocyte activation was detected.11 In addition, the primary motor cortex in these rats did not show axonal degeneration. The height of the motor cortex was equivalent to that seen in comparison animals.11 Evoked potentials were degraded in the motor cortex at PN10 but not at PN21, which was probably due to plasticity. Cortical maps within the primary motor cortex were not affected by placental insufficiency injury.
In contrast, the somatosensory cortex and parietal cerebral cortex were significantly damaged by placental insufficiency. In adult rats, the height of the somatosensory cortex was reduced when compared with control rats.11 This region underwent cellular degeneration, astrogliosis, and white matter damage (WMD).11 There was an increase in apoptotic neurons and a reduction in neuronal cell density, particularly of inhibitory GABAergic interneurons. It is likely that this lack of inhibitory signalling explains the increased size of receptive fields within the somatosensory cortex of rats with placental insufficiency. The topography of the somatosensory map of the lower limbs in these rats was found to lose its organization.11 At PN7 and PN49, there was a lack of the normal stratified structure of the parietal cerebral cortex.24
Hippocampal injury
The hippocampus was found to be particularly sensitive to metabolic injury in fetal development.43,44 Fetal guinea pigs with placental insufficiency had reduced hippocampal volume.25 Placental insufficiency resulted in a significant reduction in the volume and number of neurons within the dentate gyrus of female rats at PN0.14 In male rats, neuron volume and number were significantly reduced in the CA1 region.14 There were also changes to astrocyte populations within the hippocampus, as discussed in the section ‘Astrogliosis’, above. However, placental insufficiency did not alter the number of oligodendrocyte progenitors within the dentate gyrus or CA3 region in PN0 rats. There was an increase in the number of oligodendrocytes within the CA1 of female rats but a decrease within the CA1 of male rats. At PN21, oligodendrocyte density was decreased in the CA1, CA3, and dentate gyrus of male rats.26 This reduction in oligodendrocytes was not significant in female PN21 rats.
Dendrites within the hippocampus of guinea pig fetuses with placental insufficiency demonstrated abnormal morphology. Within the CA1 region, there was a significant reduction in dendritic elongation and an abnormal distribution of basal arbour branch point.45 Within both the dentate gyrus and CA1 regions, there was a significant reduction in dendritic outgrowth and a compensatory increase in dendritic spine density.45
Within the hippocampus of guinea pig fetuses with placental insufficiency (60d gestation), there was a reduction of brain-derived neurotrophic factor (BDNF) and a reactive increase in tyrosine kinase receptor (TrkB).20 This reduction in BDNF as a result of hypoperfusion was also seen in studies in sheep.46 BDNF was involved in cell growth and survival via activation of TrkB. BDNF reduction was associated with reduction in neuropil growth.20
Placental insufficiency also affected the expression of ErbB3 receptors, which play an important role in the development of neural stem cells into neurons and glial cells.47 ErbB3 receptor expression was reduced at PN21 in male and female rats.14
N-methyl-D-aspartate (NMDA) receptors play an important role in neurodevelopment and cognition. The activity of NMDA receptors facilitates stabilization of neuronal networks within the hippocampus that are important for learning and memory.26,48 Hypoxic–ischaemic injury resulted in neurotoxic cell death via overactivation of NMDA receptors. NR1, NR2A, and NR2B are subunits of the NMDA receptor that have been found to be altered by placental insufficiency.49 Specifically, NR1 expression and NR2A–NR2B ratio were reduced in the hippocampus of rat males at PN21. Previous work has demonstrated that an increase in the NR2A–NR2B ratio is important to the development of cognitive processes.50 Thus, cognitive deficits in rats with placental insufficiency may partly result from the reduction in this ratio.
White matter injury
White matter injury in uterine artery ligation involves damage to oligodendrocytes and defects in myelination and astrogliosis.51 The oxidative stress associated with injury is believed to disrupt oligodendrocyte maturation, partly because of changes in the oligodendrocyte gene expression41 and an increase in bone morphogenetic protein (BMP).1 Hypoxia/ischaemia and inflammatory and excitotoxic outcomes were associated with this oxidative stress.52
Oligodendrocyte precursors, which form white matter in the central nervous system, have been shown to be particularly susceptible to hypoxic and oxidative injury.1,41 This was a result of their low levels of endogenous glutathione and antioxidant enzymes,41,51 as well as the expression of Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors on the soma and NMDA receptors on processes.53 Uterine artery ligation during the perinatal period affected early brain development with oligodendrocyte precursors54 and can cause their maturation to arrest.55 As oligodendrocytes matured, they became less susceptible to oxidative damage.
Myelination defects after uterine artery ligation were found within the corpus callosum and the cingulate white matter at PN14 in rats with placental insufficiency.37 The severity of hypomyelination was correlated with the extent of growth restriction. Similarly, fetal guinea pigs with placental insufficiency (52d gestation, 62d gestation) demonstrated reduced myelination in the spinal cord, cerebral cortex, corpus callosum, and cerebellum.39 Myelination increased between 52 and 62 days' gestation in control guinea pigs, but remained reduced in guinea pigs with placental insufficiency.39
Histological studies in the rat model of uterine artery ligation have demonstrated that rats with growth restriction have decreased numbers of total oligodendrocytes and significantly reduced numbers of myelinating axons at PN14 compared with control rats.1,22,37 This injury caused a reduction in both MBP1 and mature oligodendrocytes.37 However, there seemed to be no significant reduction in the number of oligodendrocyte precursors or an increase in cell death in these animals. Thus, although there was a reduction in mature oligodendrocytes, the level of oligodendrocyte precursors remained normal.56,57 Significant astrogliosis, involving hypertrophy and hyperplasia, was also present within the corpus callosum1,9,11,12 and cingulate white matter,9 as seen in models of white matter disease.51,55 However, there seemed to be significant reversal of white matter injury, as differences between animals with placental insufficiency and sham animals are reduced at PN21 and insignificant at 8 weeks.1 In contrast other studies have demonstrated persistent myelination deficits in adult rats.22,37
Similar to findings in rats, guinea pig studies demonstrated transient effects of placental insufficiency on white matter. In IUGR fetuses (60d gestation), there was significant reduction of white matter volume12,18 and markers of early myelinating oligodendrocytes (MBP, MAG, PLP).18 However, total oligodendrocyte number was increased at this stage. The effects of placental insufficiency on white matter volume were normalized at 1 week and 8 weeks, although corpus callosum thickness remained reduced into adulthood.
Moderate growth restriction and severe growth restriction had significantly different long-term outcomes in white matter in rats with placental insufficiency.27 Severe growth restriction led to more significant and prolonged angiogenesis (until PN3 in animals with moderate growth restriction and until PN7 in animals with severe growth restriction). At PN7, preoligodendrocyte populations and MBP immunoreactivity were reduced in rats with moderate restriction, and even more so in rats with severe restriction. However, PN14 rats with moderate restriction had greater numbers of preoligodendrocytes and mature oligodendrocytes than control rats. This increase was a result of oligodendrocyte proliferation (5-bromo-2′-deoxyuridine immunoreactivity) within the white matter (corpus callosum, cingulum, and subventricular zone) of rats with moderate restriction. Indeed, at PN14 and into adulthood, rats with moderate restriction showed no difference in MBP immunostaining compared with control rats. In contrast, rats with severe restriction continued to demonstrate lower levels of oligodendrocytes and MBP-positive fibres within white matter into adulthood. In a ‘double hit’ protocol using uterine artery ligation and intracerebral ibotenate (NMDA antagonist) injection, moderate growth restriction was found to be neuroprotective against excitotoxic white matter lesions at PN10.27 Similarly, mice with placental insufficiency that were injected with ibotenate at PN2 experienced less cortical damage and WMD than comparison mice.21 However, rats with placental insufficiency were not protected, compared with sham animals, when injected with ibotenate at PN10. This suggests a transient neuroprotective effect of moderate IUGR.
On a more gross scale, fetal guinea pigs with placental insufficiency had reduced cross-sectional corpus callosum and striatum area.25 In rats with placental insufficiency, the thickness of the corpus callosum was found to be reduced into adulthood.9,11 Degenerating axons were found within the corpus callosum, white matter of the cingulate cortex, somatosensory cortex, pontocerebellar tract, and the internal and external capsules.9,11 Rats with more significant growth restriction demonstrated more degenerating axons. However, axonal degeneration was not present within the corticospinal tract of the rat brain.11
Electron microscopy of the cingulate gyrus in rats with placental insufficiency demonstrated poorly organized myelination. The existing myelin was uncompacted and swollen axons were present.37 Similar studies in fetal guinea pigs with placental insufficiency revealed a reduction in total number of myelinating fibres.39 Existing myelin produced significantly thinner myelin sheaths relative to axon thickness. There was also a significant delay in corticospinal tract myelination and a reduction in corticospinal tract cross-sectional area in these animals.39
Bone morphogenetic protein (BMP) inhibited oligodendrocyte differentiation and promoted formation of astrocytes.58 Indeed, at birth BMP levels decreased physiologically to allow for oligodendrocyte maturation.58 BMP is upregulated in models of demyelination in both neonatal and adult disease.59,60 BMP4, a BMP isoform, was significantly elevated in rats with placental insufficiency compared with sham animals at PN14 but not at 8 weeks.1 It is likely that these elevated levels of BMP4 resulted in the differentiation arrest of oligodendrocytes. The normalization of BMP4 into adulthood allowed previously arrested oligodendrocyte processors to mature and achieve normal myelination in adulthood.
Sex differences
Studies have demonstrated that male and female brains respond differently to placental insufficiency, as exemplified by the hippocampus.14 NMDA receptor expression and oligodendrocyte density in the hippocampus were more significantly affected by placental insufficiency injury in male than in female rats.49 A possible explanation for these differences involves differential hormonal expression in male and female rats affected by IUGR. For example, male IUGR rats at PN0 had an increased level of serum testosterone but reduced levels of hippocampal aromatase expression.61 Thus, it is likely that the CA1 and CA3 regions within the male brain would develop under an abnormal testosterone–oestradiol imbalance.
Behavioural deficits
Neurobehavioural deficits were an important part of an accurate model of IUGR (TableIV). Rats with uterine artery ligation were found to have decreased strength and coordination at P14.1 Although these deficits persisted into adulthood, the difference between placental insufficiency and sham animals was decreased. Thus, even though myelination may normalize into adulthood, behavioural deficits remain.
Table IV.
Behavioural deficits detected with uterine artery ligation
| Motor | Cognitive/memory |
|---|---|
| Motor hyperactivity and decreased strength and coordination are present | Deficits are present in short-term memory, spatial encoding, and long-term memory |
| There is a deficit in prepulse inhibition | There is reduced object recognition |
| There are mild gait and posture abnormalities | Working and reference memory are not affected |
Rats with placental insufficiency demonstrated motor hyperactivity and spontaneous exploration in an open field.9,11 It is likely that this partially resulted from damage seen within the CA1 region of the hippocampus9,62 and increased inhibition of the prefrontal cortex.11 In week 12 guinea pigs with placental insufficiency, there was a deficit in prepulse inhibition which related to sensorimotor gating deficits observed in human patients.10
This injury resulted in deficits in short-term memory, spatial encoding, and long-term memory. Object recognition memory was also impaired in adulthood and correlated with increased prefrontal inhibitory GABAergic neuron density.11 Spatial deficits were related to WMD.9 Deficits in memory were related to damage in the hippocampus11 and parahippocampal9 regions. The severity of behavioural and cognitive deficits was found to correlate with the extent of growth restriction (indicated by birthweight).9 However, working memory and reference memory did not seem to be affected in this model. This may be related to the maintained neuronal density within the hippocampus, which may have compensated for abnormalities within the prefrontal cortex.
Rats with placental insufficiency at PN65 demonstrated mild abnormalities in gait and posture when compared with control rats.11 Muscle hypertrophy at the level of the myofibril and lower limb velocity-dependent resistance to passive motion was observed in these rats.11 At PN49, male rats with placental insufficiency demonstrated locomotor disturbances on an open field while females do not.24
Discussion
Cerebral palsy has recently been defined as ‘a group of permanent disorders of the development of movement and posture … attributed to non-progressive disturbances in the developing fetal or infant brain'63 ‘… often accompanied by disturbances of sensation, perception, cognition, communication and behavior …’.64 In this review, we have yielded a comprehensive perspective of behavioural and neurological outcomes of uterine artery ligation in the animal model that significantly reflects that seen in CP in the human.
While the aetiologies for CP in humans are broad, and it is likely that no single animal model can reflect all of the causes and pathological markers of this disorder, there are many parallels between the uterine artery ligation model in the rodent, and the behavioural and anatomical outcomes seen in the newborn human infant.65,66
In this regard, it has been clearly shown that the majority of insults resulting in the CP phenotype occur before the onset of labour and delivery.67,68 Moreover, the underlying aetiology for CP is frequently associated with a final common pathway leading to vascular insufficiency of the fetus.69 In addition, there is a sex bias in children with CP, with males being more significantly affected than females.69–71 The latter is observed more frequently in animal models of CP and in those utilizing the uterine ligation model.72 Although no one aetiological factor is uniquely associated with CP, several ‘risk factors’ consistently yield a higher incidence of CP in the epidemiology literature. Badawi et al.73 found a 40-fold increase in terminal hypoxic insults in newborn infants with fetal growth restriction. Dahlseng et al.16 and others74,75 have similarly found significant correlations between fetal growth in the newborn infant and the risk of CP.
Human infants who are small for gestational age demonstrate reduced body weight and brain weight, as do animal models.76 Although there are a number of underlying causes for growth restriction in humans in developing countries, placental insufficiency is certainly the most common cause of IUGR, and is associated with significant fetal and infant morbidity and mortality.77 Placental insufficiency, visualized on antenatal Doppler scanning, has been shown to be associated with reductions in cortical grey matter volume and overall brain volume on magnetic resonance imaging.77 These findings are suggestive of an anatomical biomarker of IUGR secondary to placental insufficiency, with later developmental disabilities. In animal models of uterine artery ligation, both diffuse brain mass reduction and specific brain region volume deficits have been shown, which correlate with the human condition.
Infants with CP have reduced attention–interaction capacity, as do children who were growth restricted at birth. Studies in humans have now begun to recognize the overlapping nature of several of growth restriction, CP, and developmental disabilities including attention deficit and even autism.62,78–82 As it is well known that white matter injury is the recognized anatomical hallmark associated with CP in preterm or term-born children,83–87 the further association of abnormalities in human connectivity with behavioural and mental health and fetal growth restriction is yet another link tying these disorders together, highlighting the relevance of the placental insufficiency and growth restriction models. Eixarch et al.88 studied IUGR in fetal rabbits with diffusion tensor imaging after birth and found indications of abnormal connectivity and significant abnormalities of the grey and white matter, as measured by fractional anisotropy. In humans, abnormalities of white matter connectivity have been found in attention-deficit–hyperactivity disorder and autism, again providing evidence that white matter abnormalities arising from birth are a common theme amongst the developmental disabilities, of which CP is the recognized hallmark.89–91 Uterine artery ligation as a result of placental insufficiency produces both motor and cognitive deficits, including deficits of working memory, spatial encoding, and hyperactivity.
In the animal model of growth restriction caused by uterine artery ligation, WMD is an important outcome. Diffuse WMD has been demonstrated within the corpus callosum, brain stem, hippocampus, and somatosensory cortex. These brain regions are hypomyelinated and have a reduced number of oligodendrocytes. Existing myelin in these brains is thin and aberrantly organized. Preterm infants with WMD present with cognitive, behavioural, and neurodevelopmental abnormalities.85,92–94 Associated deficits in working memory, short-term memory, and long-term memory are also demonstrated within animals with placental insufficiency.
Spastic diplegia and abnormal networks within the somato-motor attention areas are observed in humans.87 The extent of WMD in affected infants correlates with the severity of impairments in planning, cognitive flexibility, and hyperactivity.95 Interestingly, this correlates with the significant deficits observed in animal somatosensory cortex after placental insufficiency, with sparing of the motor cortex. Abnormal somatosensory mapping and stratification is an important outcome. Within this region there is also significant cellular degeneration and astrogliosis.
Studies involving functional magnetic resonance imaging in children with CP demonstrate abnormal activity within the parietal somatosensory cortex and cortical areas involved in goal-directed behaviour.96 It is likely that this activity underlies deficits in tactile discrimination and shape recognition. Abnormal somatosensory activation is thought to contribute to motor deficits in CP. Behavioural testing in the uterine artery ligation model has also demonstrated deficits in object recognition and spatial reasoning.
Conclusion
The neurological outcomes of placental insufficiency in uterine artery ligation demonstrate important outcomes with regards to astrogliosis, neuronal cell death, and WMD. The gross neurological damage in this model resembles the findings on imaging studies of IUGR humans. The cognitive, motor, and behavioural outcomes associated with IUGR in human children are also replicated in animal models. For this reason, uterine artery ligation may provide a promising model for studies of diseases such as CP and other pathological outcomes of placental insufficiency in human infants. Emerging therapies for neurological damage associated with placental insufficiency should be tested in this model to evaluate outcomes of these treatments.
Acknowledgments
We would like to gracefully acknowledge Dr Michael Fehlings' Gerald and Tootsie Halbert Chairship in Neural Repair and Regeneration and funding from NeuroDevNet, the Ontario Brain Institute (OBI), and The Freedman Family Foundation.
Glossary
- Bcl-2
B-cell lymphoma 2
- BDNF
Brain-derived neurotrophic factor
- BMP
Bone morphogenetic protein
- IUGR
Intrauterine growth restriction
- MAG
Myelin-associated glycoprotein
- MBP
Myelin basic protein
- MDM2
Murine double minute 2
- NMDA
N-methyl-D-aspartate
- NOS
Nitric oxide synthase
- PLP
Proteolipid protein
- TrkB
Tyrosine kinase receptor
- WMD
White matter damage
References
- 1.Reid MV, Murray KA, Marsh ED, Golden JA, Simmons RA, Grinspan JB. Delayed myelination in an intrauterine growth retardation model is mediated by oxidative stress upregulating bone morphogenetic protein 4. J Neuropathol Exp Neurol. 2012;71:640–53. doi: 10.1097/NEN.0b013e31825cfa81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry JS, Rozance PJ, Anthony RV. An animal model of placental insufficiency-induced intrauterine growth restriction. Semin Perinatol. 2008;32:225–30. doi: 10.1053/j.semperi.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Joss-Moore L, Carroll T, Yang Y, et al. Intrauterine growth restriction transiently delays alveolar formation and disrupts retinoic acid receptor expression in the lung of female rat pups. Pediatr Res. 2013;73:612–20. doi: 10.1038/pr.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadley GD, McConell GK, Goodman CA, Siebel AL, Westcott KT, Wlodek ME. Growth restriction in the rat alters expression of metabolic genes during postnatal cardiac development in a sex-specific manner. Physiol Genomics. 2013;45:99–105. doi: 10.1152/physiolgenomics.00095.2012. [DOI] [PubMed] [Google Scholar]
- 5.Gallo LA, Tran M, Moritz KM, et al. Cardio-renal and metabolic adaptations during pregnancy in female rats born small: implications for maternal health and second generation fetal growth. J Physiol. 2012;590:617–30. doi: 10.1113/jphysiol.2011.219147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tare M, Parkington HC, Bubb KJ, Wlodek ME. Uteroplacental insufficiency and lactational environment separately influence arterial stiffness and vascular function in adult male rats. Hypertension. 2012;60:378–86. doi: 10.1161/HYPERTENSIONAHA.112.190876. [DOI] [PubMed] [Google Scholar]
- 7.Laker RC, Wlodek ME, Wadley GD, Gallo LA, Meikle PJ, McConell GK. Exercise early in life in rats born small does not normalize reductions in skeletal muscle PGC-1α in adulthood. Am J Physiol Endocrinol Metab. 2012;302:E1221–30. doi: 10.1152/ajpendo.00583.2011. [DOI] [PubMed] [Google Scholar]
- 8.Thompson RF, Fazzari MJ, Niu H, Barzilai N, Simmons RA, Greally JM. Experimental intrauterine growth restriction induces alterations in DNA methylation and gene expression in pancreatic islets of rats. J Biol Chem. 2010;285:15111–8. doi: 10.1074/jbc.M109.095133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delcour M, Russier M, Amin M, et al. Impact of prenatal ischemia on behavior, cognitive abilities and neuroanatomy in adult rats with white matter damage. Behav Brain Res. 2012;232:233–44. doi: 10.1016/j.bbr.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Rehn AE, Van Den Buuse M, Copolov D, Briscoe T, Lambert G, Rees S. An animal model of chronic placental insufficiency: relevance to neurodevelopmental disorders including schizophrenia. Neuroscience. 2004;129:381–91. doi: 10.1016/j.neuroscience.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 11.Delcour M, Olivier P, Chambon C, et al. Neuroanatomical, sensorimotor and cognitive deficits in adult rats with white matter injury following prenatal ischemia. Brain Pathol. 2012;22:1–16. doi: 10.1111/j.1750-3639.2011.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallard C, Loeliger M, Copolov D, Rees S. Reduced number of neurons in the hippocampus and the cerebellum in the postnatal guinea-pig following intrauterine growth-restriction. Neuroscience. 2000;100:327–33. doi: 10.1016/s0306-4522(00)00271-2. [DOI] [PubMed] [Google Scholar]
- 13.Mandruzzato G, Antsaklis A, Botet F, et al. Intrauterine restriction (IUGR) J Perinat Med. 2008;36:277–81. doi: 10.1515/JPM.2008.050. [DOI] [PubMed] [Google Scholar]
- 14.Fung C, Ke X, Brown AS, Yu X, McKnight RA, Lane RH. Uteroplacental insufficiency alters rat hippocampal cellular phenotype in conjunction with ErbB receptor expression. Pediatr Res. 2012;72:2–9. doi: 10.1038/pr.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobsson B, Ahlin K, Francis A, Hagberg G, Hagberg H, Gardosi J. Cerebral palsy and restricted growth status at birth: population-based case–control study. BJOG. 2008;115:1250–5. doi: 10.1111/j.1471-0528.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 16.Dahlseng MO, Andersen GL, Irgens LM, Skranes J, Vik T. Risk of cerebral palsy in term-born singletons according to growth status at birth. Dev Med Child Neurol. 2014;56:53–8. doi: 10.1111/dmcn.12293. [DOI] [PubMed] [Google Scholar]
- 17.Wigglesworth JS. Experimental growth retardation in the foetal rat. J Pathol Bacteriol. 1964;88:1–13. [PubMed] [Google Scholar]
- 18.Tolcos M, Bateman E, O'Dowd R, et al. Intrauterine growth restriction affects the maturation of myelin. Exp Neurol. 2011;232:53–65. doi: 10.1016/j.expneurol.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Turner AJ, Trudinger BJ. A modification of the uterine artery restriction technique in the guinea pig fetus produces asymmetrical ultrasound growth. Placenta. 2009;30:236–40. doi: 10.1016/j.placenta.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Dieni S, Rees S. BDNF and TrkB protein expression is altered in the fetal hippocampus but not cerebellum after chronic prenatal compromise. Exp Neurol. 2005;192:265–73. doi: 10.1016/j.expneurol.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Catteau J, Gernet J-I, Marret S, et al. Effects of antenatal uteroplacental hypoperfusion on neonatal microvascularisation and excitotoxin sensitivity in mice. Pediatr Res. 2011;70:229–35. doi: 10.1203/PDR.0b013e318224285f. [DOI] [PubMed] [Google Scholar]
- 22.Olivier P, Baud O, Evrard P, Gressens P, Verney C. Prenatal ischemia and white matter damage in rats. J Neuropathol Exp Neurol. 2005;64:998–1006. doi: 10.1097/01.jnen.0000187052.81889.57. [DOI] [PubMed] [Google Scholar]
- 23.Nüsken K-D, Warnecke C, Hilgers KF, Schneider H. Intrauterine growth after uterine artery ligation in rats: dependence on the fetal position in the uterine horn and need for prenatal marking of the animals. J Hypertens. 2007;25:247–8. doi: 10.1097/01.hjh.0000254371.70823.d4. [DOI] [PubMed] [Google Scholar]
- 24.Tashima L, Nakata M, Anno K, Sugino N, Kato H. Prenatal influence of ischemia-hypoxia-induced intrauterine growth retardation on brain development and behavioral activity in rats. Biol Neonate. 2001;80:81–7. doi: 10.1159/000047125. [DOI] [PubMed] [Google Scholar]
- 25.Mallard EC, Rehn A, Rees S, Tolcos M, Copolov D. Ventriculomegaly and reduced hippocampal volume following intrauterine growth-restriction: implications for the aetiology of schizophrenia. Schizophr Res. 1999;40:11–21. doi: 10.1016/s0920-9964(99)00041-9. [DOI] [PubMed] [Google Scholar]
- 26.Brooks WJ, Weeks AC, Leboutillier JC, Petit TL. Altered NMDA sensitivity and learning following chronic developmental NMDA antagonism. Physiol Behav. 1997;62:955–62. doi: 10.1016/s0031-9384(97)00169-8. [DOI] [PubMed] [Google Scholar]
- 27.Olivier P. Moderate growth restriction: deleterious and protective effects on white matter damage. Neurobiol Dis. 2007;26:253–63. doi: 10.1016/j.nbd.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem Soc Trans. 2001;29:684. doi: 10.1042/0300-5127:0290684. [DOI] [PubMed] [Google Scholar]
- 29.Uysal A. Quantitative immunohistochemical analysis of nitric oxide synthases and apoptosis regulator proteins in the fetal rat brain following maternal uterine artery ligation. Int J Neurosci. 2008;118:891–901. doi: 10.1080/00207450701769364. [DOI] [PubMed] [Google Scholar]
- 30.Lane RH, Ramirez RJ, Tsirka AE, et al. Uteroplacental insufficiency lowers the threshold towards hypoxia-induced cerebral apoptosis in growth-retarded fetal rats. Brain Res. 2001;895:186–93. doi: 10.1016/s0006-8993(01)02074-1. [DOI] [PubMed] [Google Scholar]
- 31.Ke X, McKnight RA, Wang Z-M, et al. Nonresponsiveness of cerebral p53-MDM2 functional circuit in newborn rat pups rendered IUGR via uteroplacental insufficiency. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1038–45. doi: 10.1152/ajpregu.00701.2004. [DOI] [PubMed] [Google Scholar]
- 32.Wadgaonkar R, Collins T. Murine Double Minute (MDM2) blocks p53-coactivator interaction, a new mechanism for inhibition of p53-dependent gene expression. J Biol Chem. 1999;274:13760–7. doi: 10.1074/jbc.274.20.13760. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Zhu XZ. Roles of p53, c-Myc, Bcl-2, Bax and caspases in serum deprivation-induced neuronal apoptosis: a possible neuroprotective mechanism of basic fibroblast growth factor. NeuroReport. 1999;10:3087–91. doi: 10.1097/00001756-199909290-00039. [DOI] [PubMed] [Google Scholar]
- 34.Jänicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–60. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 35.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 36.Vannucci RC, Vannucci SJ. Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Dev Neurosci. 2005;27:81–6. doi: 10.1159/000085978. [DOI] [PubMed] [Google Scholar]
- 37.Olivier P, Fontaine RH, Loron G, et al. Melatonin promotes oligodendroglial maturation of injured white matter in neonatal rats. PLoS ONE. 2009;4:e7128. doi: 10.1371/journal.pone.0007128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolcos M, Rees S. Chronic placental insufficiency in the fetal guinea pig affects neurochemical and neuroglial development but not neuronal numbers in the brainstem: a new method for combined stereology and immunohistochemistry. J Comp Neurol. 1997;379:99–112. doi: 10.1002/(sici)1096-9861(19970303)379:1<99::aid-cne7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 39.Nitsos I, Rees S. The effects of intrauterine growth retardation on the development of neuroglia in fetal guinea pigs. An immunohistochemical and an ultrastructural study. Int J Dev Neurosci. 1990;8:233–44. doi: 10.1016/0736-5748(90)90029-2. [DOI] [PubMed] [Google Scholar]
- 40.Garcia YJ, Rodríguez-Malaver AJ, Peñaloza N. Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J Neurosci Methods. 2005;144:127–35. doi: 10.1016/j.jneumeth.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 41.French HM, Reid M, Mamontov P, Simmons RA, Grinspan JB. Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res. 2009;87:3076–87. doi: 10.1002/jnr.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tatli M, Guzel A, Kizil G, Kavak V, Yavuz M, Kizil M. Comparison of the effects of maternal protein malnutrition and intrauterine growth restriction on redox state of central nervous system in offspring rats. Brain Res. 2007;1156:21–30. doi: 10.1016/j.brainres.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 43.Lodygensky GA, Seghier ML, Warfield SK, et al. Intrauterine growth restriction affects the preterm infant's hippocampus. Pediatr Res. 2008;63:438–43. doi: 10.1203/PDR.0b013e318165c005. [DOI] [PubMed] [Google Scholar]
- 44.Van Wassenaer A. Neurodevelopmental consequences of being born SGA. Pediatr Endocrinol Rev. 2005;2:372–7. [PubMed] [Google Scholar]
- 45.Dieni S, Rees S. Dendritic morphology is altered in hippocampal neurons following prenatal compromise. J Neurobiol. 2003;55:41–52. doi: 10.1002/neu.10194. [DOI] [PubMed] [Google Scholar]
- 46.Nishigori H, Mazzuca DM, Nygard KL, Han VK, Richardson BS. BDNF and TrkB in the preterm and near-term ovine fetal brain and the effect of intermittent umbilical cord occlusion. Reprod Sci. 2008;15:895–905. doi: 10.1177/1933719108324135. [DOI] [PubMed] [Google Scholar]
- 47.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro ML, O'Connor C. N-methyl-D-aspartate receptor antagonist MK-801 and spatial memory representation: working memory is impaired in an unfamiliar environment but not in a familiar environment. Behav Neurosci. 1992;106:604–12. doi: 10.1037//0735-7044.106.4.604. [DOI] [PubMed] [Google Scholar]
- 49.Schober ME, McKnight RA, Yu X, Callaway CW, Ke X, Lane RH. Intrauterine growth restriction due to uteroplacental insufficiency decreased white matter and altered NMDAR subunit composition in juvenile rat hippocampi. Am J Physiol Regul Integr Comp Physiol. 2009;296:R681–92. doi: 10.1152/ajpregu.90396.2008. [DOI] [PubMed] [Google Scholar]
- 50.Lebel D, Sidhu N, Barkai E, Quinlan EM. Learning in the absence of experience-dependent regulation of NMDAR composition. Learn Mem. 2006;13:566–70. doi: 10.1101/lm.276606. [DOI] [PubMed] [Google Scholar]
- 51.Haynes RL, Folkerth RD, Keefe RJ, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–50. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 52.Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–8. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- 53.Fern R, Möller T. Rapid ischemic cell death in immature oligodendrocytes: a fatal glutamate release feedback loop. J Neurosci. 2000;20:34–42. doi: 10.1523/JNEUROSCI.20-01-00034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–63. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segovia KN, McClure M, Moravec M, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63:520–30. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.See J, Zhang X, Eraydin N, et al. Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol Cell Neurosci. 2004;26:481–92. doi: 10.1016/j.mcn.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Feigenson K, Reid M, See J, Crenshaw EB, 3rd, Grinspan JB. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci. 2009;42:255–65. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 58.Miller RH, Dinsio K, Wang R, Geertman R, Maier CE, Hall AK. Patterning of spinal cord oligodendrocyte development by dorsally derived BMP4. J Neurosci Res. 2004;76:9–19. doi: 10.1002/jnr.20047. [DOI] [PubMed] [Google Scholar]
- 59.Ara J, See J, Mamontov P, et al. Bone morphogenetic proteins 4, 6, and 7 are up-regulated in mouse spinal cord during experimental autoimmune encephalomyelitis. J Neurosci Res. 2008;86:125–35. doi: 10.1002/jnr.21462. [DOI] [PubMed] [Google Scholar]
- 60.Cate HS, Sabo JK, Merlo D, et al. Modulation of bone morphogenic protein signalling alters numbers of astrocytes and oligodendroglia in the subventricular zone during cuprizone-induced demyelination. J Neurochem. 2010;115:11–22. doi: 10.1111/j.1471-4159.2010.06660.x. [DOI] [PubMed] [Google Scholar]
- 61.O'Grady SP, Caprau D, Ke X-R, et al. Intrauterine growth restriction alters hippocampal expression and chromatin structure of Cyp19a1 variants. Syst Biol Reprod Med. 2010;56:292–302. doi: 10.3109/19396368.2010.490871. [DOI] [PubMed] [Google Scholar]
- 62.Dell'Anna ME, Calzolari S, Molinari M, Iuvone L, Calimici R. Neonatal anoxia induces transitory hyperactivity, permanent spatial memory deficits and CA1 cell density reduction in developing rats. Behav Brain Res. 1991;45:125–34. doi: 10.1016/s0166-4328(05)80078-6. [DOI] [PubMed] [Google Scholar]
- 63.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571–6. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 64.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 65.Yager JY. Animal models of hypoxic-ischemic brain damage in the newborn. Semin Pediatr Neurol. 2004;11:31–46. doi: 10.1016/j.spen.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Johnston MV, Ferriero DM, Vannucci SJ, Hagberg H. Models of cerebral palsy: which ones are best? J Child Neurol. 2005;20:984–7. doi: 10.1177/08830738050200121001. [DOI] [PubMed] [Google Scholar]
- 67.Nelson KB, Ellenberg JH. Antecedents of cerebral palsy. I. Univariate analysis of risks. Am J Dis Child. 1985;139:1031–8. doi: 10.1001/archpedi.1985.02140120077032. [DOI] [PubMed] [Google Scholar]
- 68.Nelson KB, Ellenberg JH. Antecedents of cerebral palsy. Multivariate analysis of risk. N Engl J Med. 1986;315:81–6. doi: 10.1056/NEJM198607103150202. [DOI] [PubMed] [Google Scholar]
- 69.Lahti J, Räikkönen K, Kajantie E, et al. Small body size at birth and behavioural symptoms of ADHD in children aged five to six years. J Child Psychol Psychiatry. 2006;47:1167–74. doi: 10.1111/j.1469-7610.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- 70.Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007;49:74–8. doi: 10.1017/s0012162207000199.x. [DOI] [PubMed] [Google Scholar]
- 71.Hurn PD, Vannucci SJ, Hagberg H. Adult or perinatal brain injury: does sex matter? Stroke. 2005;36:193–5. doi: 10.1161/01.STR.0000153064.41332.f6. [DOI] [PubMed] [Google Scholar]
- 72.Corrigan J, Armstrong E, Faulkner S, Ruff C, Fehlings M, Yager JY. Placental insufficiency causing fetal growth restriction and cerebral palsy. Anim Models Dev Disabil. In: Humana Press (Springer): (in press) [Google Scholar]
- 73.Badawi N, Kurinczuk JJ, Keogh JM, et al. Antepartum risk factors for newborn encephalopathy: the Western Australian case–control study. BMJ. 1998;317:1549–53. doi: 10.1136/bmj.317.7172.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jarvis S, Glinianaia SV, Arnaud C, et al. Case gender and severity in cerebral palsy varies with intrauterine growth. Arch Dis Child. 2005;90:474–9. doi: 10.1136/adc.2004.052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jarvis S, Glinianaia SV, Blair E. Cerebral palsy and intrauterine growth. Clin Perinatol. 2006;33:285–300. doi: 10.1016/j.clp.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 76.Chase HP, Welch NN, Dabiere CS, Vasan NS, Butterfield LJ. Alterations in human brain biochemistry following intrauterine growth retardation. Pediatrics. 1972;50:403–11. [PubMed] [Google Scholar]
- 77.Tolsa CB, Zimine S, Warfield SK, et al. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res. 2004;56:132–8. doi: 10.1203/01.PDR.0000128983.54614.7E. [DOI] [PubMed] [Google Scholar]
- 78.Bjorgaas HM, Hysing M, Elgen I. Psychiatric disorders among children with cerebral palsy at school starting age. Res Dev Disabil. 2012;33:1287–93. doi: 10.1016/j.ridd.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 79.Brossard-Racine M, Hall N, Majnemer A, et al. Behavioural problems in school age children with cerebral palsy. Eur J Paediatr Neurol. 2012;16:35–41. doi: 10.1016/j.ejpn.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Surén P, Bakken IJ, Aase H, et al. Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy in Norwegian children. Pediatrics. 2012;130:e152–8. doi: 10.1542/peds.2011-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bjorgaas HM, Elgen I, Boe T, Hysing M. Mental health in children with cerebral palsy: does screening capture the complexity? ScientificWorldJournal. 2013;2013:468402. doi: 10.1155/2013/468402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brossard-Racine M, Waknin J, Shikako-Thomas K, et al. Behavioral difficulties in adolescents with cerebral palsy. J Child Neurol. 2013;28:27–33. doi: 10.1177/0883073812461942. [DOI] [PubMed] [Google Scholar]
- 83.Guellec I, Lapillonne A, Renolleau S, et al. Neurologic outcomes at school age in very preterm infants born with severe or mild growth restriction. Pediatrics. 2011;127:e883–91. doi: 10.1542/peds.2010-2442. [DOI] [PubMed] [Google Scholar]
- 84.Silbereis JC, Huang EJ, Back SA, Rowitch DH. Towards improved animal models of neonatal white matter injury associated with cerebral palsy. Dis Model Mech. 2010;3:678–88. doi: 10.1242/dmm.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Resić B, Tomasović M, Kuzmanić-Samija R, Lozić M, Resić J, Solak M. Neurodevelopmental outcome in children with periventricular leukomalacia. Coll Antropol. 2008;32(Suppl. 1):143–7. [PubMed] [Google Scholar]
- 86.Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724–30. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- 87.Burton H, Dixit S, Litkowski P, Wingert JR. Functional connectivity for somatosensory and motor cortex in spastic diplegia. Somatosens Mot Res. 2009;26:90–104. doi: 10.3109/08990220903335742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eixarch E, Batalle D, Illa M, et al. Neonatal neurobehavior and diffusion MRI changes in brain reorganization due to intrauterine growth restriction in a rabbit model. PLoS ONE. 2012;7:e31497. doi: 10.1371/journal.pone.0031497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wolff JJ, Gu H, Gerig G, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lawrence KE, Levitt JG, Loo SK, et al. White matter microstructure in subjects with attention-deficit/hyperactivity disorder and their siblings. J Am Acad Child Adolesc Psychiatry. 2013;52:431–40.e4. doi: 10.1016/j.jaac.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Langevin LM, Macmaster FP, Crawford S, Lebel C, Dewey D. Common white matter microstructure alterations in pediatric motor and attention disorders. J Pediatr. 2014;164:1157–64.e1. doi: 10.1016/j.jpeds.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 92.Delobel-Ayoub M, Arnaud C, White-Koning M, et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123:1485–92. doi: 10.1542/peds.2008-1216. [DOI] [PubMed] [Google Scholar]
- 93.Fazzi E, Bova S, Giovenzana A, Signorini S, Uggetti C, Bianchi P. Cognitive visual dysfunctions in preterm children with periventricular leukomalacia. Dev Med Child Neurol. 2009;51:974–81. doi: 10.1111/j.1469-8749.2009.03272.x. [DOI] [PubMed] [Google Scholar]
- 94.Counsell SJ, Edwards AD, Chew ATM, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–8. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- 95.Woodward LJ, Clark CAC, Pritchard VE, Anderson PJ, Inder TE. Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Dev Neuropsychol. 2011;36:22–41. doi: 10.1080/87565641.2011.540530. [DOI] [PubMed] [Google Scholar]
- 96.Wingert JR, Sinclair RJ, Dixit S, Damiano DL, Burton H. Somatosensory-evoked cortical activity in spastic diplegic cerebral palsy. Hum Brain Mapp. 2010;31:1772–85. doi: 10.1002/hbm.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]


