Abstract
Background
The current phase 1, open-label, dose escalation study was conducted to establish the safety, tolerability, pharmacokinetic profile, and preliminary antitumor activity of the novel mitochondrial inhibitor ME-344 in patients with refractory solid tumors.
Methods
Patients with refractory solid tumors were treated in a 3 + 3 dose escalation design. ME-344 was administered via intravenous infusion on days 1, 8, and 15 of the first 28-day cycle and weekly thereafter. Pharmacokinetics was assessed on days 1 and 15 of the first cycle.
Results
A total of 30 patients (median age, 65 years; 67% of whom were female) received ME-344. There were 5 dose-limiting toxicities reported. Four patients developed grade 3 neuropathy (2 patients each at doses of 15 mg/kg and 20 mg/kg) and 1 patient treated at a dose of 10 mg/kg developed a grade 3 acute myocardial infarction (toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events [version 4.03]). The maximum tolerated dose (MTD) was defined as 10 mg/kg weekly. The most common adverse events were nausea, dizziness, and fatigue. At the MTD of 10 mg/kg, the maximal plasma concentration (Cmax) was 25.8 µg/mL and the area under the concentration curve from time zero to infinity was 25.9 hour*µg/mL. One patient with small cell lung cancer achieved a partial response for ≥52 weeks. Four patients had prolonged stable disease (1 patient each with urothelial carcinoma [47 weeks], carcinoid tumor [≥40 weeks], cervical leiomyosarcoma [39 weeks], and cervical cancer [≥31 weeks]).
Conclusions
The once-weekly administration of ME-344 was generally well tolerated in the current study, a first-in-human study; dose-limiting neuropathy was noted, but not at the MTD. Exposures at the 10-mg/kg dose level suggest a sufficient therapeutic index. The preliminary clinical activity as a monotherapy supports the further clinical development of ME-344 in combination with chemotherapy.
The current phase 1, open-label, dose escalating, first-in human study of ME-344 in patients with refractory solid tumors found that the maximum tolerated dose of once-weekly 10-mg/kg administration of the drug was generally well tolerated. The preliminary clinical activity as a monotherapy supports the further clinical development of ME-344 in combination with chemotherapy.
Keywords: ME-344, first-in-human, mitochondrial inhibitor, phase 1, refractory solid tumors, maximum tolerated dose (MTD)
Introduction
ME-344 is a synthetic small molecule based on the isoflavan ring structure. It is the demethylated metabolite of NV-128, an agent that disrupts tumor cell mitochondrial integrity, which results in translocation of endonuclease G to the nucleus, subsequent DNA degradation, and caspase-independent cell death.1,2 ME-344 has demonstrated single-agent activity greater than that of NV-128 in preclinical studies.1,2 NV-128 and ME-344 have also been shown to disrupt both mammalian target of rapamycin complex 1 and complex 2 (mTORC1 and mTORC2) upstream and downstream signal transduction. In addition, cells treated with NV-128 express markers of autophagy, including the presence of autolysosomes, confirming that NV-128 induces autophagic cell death.2 Of particular interest are the published reports that NV-128 inhibits growth of a lineage of paclitaxel-resistant and carboplatin-resistant human ovarian cancer tumor cells with the phenotype of ovarian cancer stem cells.1,2 ME-344 inhibits the growth of these cell lines as well, but at concentrations well below those necessary for NV-128,2 indicating that ME-344 is more potent than NV-128. ME-344 has also been reported to reduce tumor burden in an in vivo model of recurrent epithelial ovarian cancer.3
To our knowledge, the specific target for NV-128 and ME-344 binding is unknown; however, the biochemical effects after tumor cell treatment with ME-344 are similar to those observed with NV-128, including inhibition of the mTORC1-dependent and mTORC2-dependent pathways, the appearance of autophagic vacuoles, and activation of the mitochondrial pERK pathway.
The current first-in-human phase 1, open-label, multicenter, nonrandomized, dose escalation study was conducted with the primary objectives of determining the tolerability, adverse event (AE) profile, maximum tolerated dose (MTD), and dose-limiting toxicities (DLTs) of ME-344 in patients with refractory solid tumors. Secondary objectives were to characterize the pharmacokinetic (PK) profile and to describe any clinical antitumor activity observed in patients treated with ME-344.
Materials and Methods
The current study (http://ClinicalTrials.gov identifier 01544322) was conducted in accordance with applicable regulatory guidelines, the International Conference on Harmonisation Guidelines for Good Clinical Practice, and the Declaration of Helsinki. The study was approved by the Institutional Review Boards at participating sites (Tennessee Oncology, Nashville, Tenn; The University of Oklahoma Stephenson Cancer Center, Oklahoma City, Okla; and the Florida Cancer Specialists, Sarasota, Fla), and patients were enrolled after providing written informed consent.
Patient Selection
Eligible patients were aged ≥18 years with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and histologically confirmed locally advanced or metastatic cancer that had no standard therapeutic alternatives with a minimum life expectancy of 12 weeks. Patients with tumor involvement of the central nervous system were required to receive approval from the medical monitor. Eligible patients could not have undergone any major surgery or received radiotherapy or immunotherapy within 21 days of initiating study treatment, or received limited palliative radiotherapy within 14 days. Patients with chemotherapy regimens with delayed toxicity within 4 weeks of the study initiation (or within 6 weeks for prior nitrosourea or mitomycin C) or chemotherapy regimens given continuously or on a weekly basis with limited potential for delayed toxicity within 2 weeks of the study initiation were also excluded.
Eligible patients had adequate bone marrow, hepatic, and renal function, defined as an absolute neutrophil count >1.5×109/L, a platelet count >100×109/L, hemoglobin >9 g/dL, aspartate aminotransferase and alanine aminotransferase ≤2.5 times the upper limit of normal (ULN) or <5 times the ULN for patients with liver metastases, and serum creatinine ≤1.5 times the ULN. Adequate cardiac function (as evidenced by creatine kinase [CK] and CK-MB below the ULN, troponin I levels within normal limits at baseline, an average QTc Fridericia interval from triplicate screening electrocardiograms of <470 milliseconds, and a left ventricular ejection fraction greater than the lower limit of the institutional normal value) was also required.
Study Design
Before the initiation of treatment, all patients underwent a physical examination, ECOG performance status, 12-lead electrocardiogram, echocardiogram, and tumor imaging. Fasting baseline laboratory assessments included a complete blood count, including differentials and platelets; blood chemistry, including sodium, potassium, calcium, creatinine, total protein, albumin, bilirubin (total and direct), aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and glucose; urinalysis (dipstick); and troponin I, CK, and CK-MB.
Patients were administered ME-344 intravenously (iv) over 30 minutes in 28-day cycles. The first treatment cycle for all cohorts consisted of iv infusions on days 1, 8, and 15, with an evaluation and 1 week of rest beginning on day 22. Beginning in cycle 2 for all cohorts, ME-344 was administered on days 1, 8, 15, and 22 of each 28-day cycle. Patients were allowed to continue weekly dosing at their assigned dose provided they were receiving clinical benefit as assessed by the investigator. Prophylactic antiemetics were not allowed, but supportive care measures and treatment for symptom control or drug-related toxicity were permitted.
Tumor assessments were performed at a minimum of every 12 weeks, and were classified according to the Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1).4 All AEs were graded according to the National Cancer Institute Common Terminology for Adverse Events (version 4.03),5 and were followed for 30 days after the last dose of study drug was administered or until they resolved (whichever occurred sooner).
Definition of MTD and DLT
The MTD was defined as the dose level immediately below the dose level at which 2 of the first 3 patients per cohort (or ≥2 of 6 patients) experienced a DLT during the first treatment cycle (28 days).
A DLT was defined as any of the following occurring during the first cycle of treatment and assessed as being related to the study drug: ≥grade 3 neutropenia lasting ≥5 days or febrile neutropenia (single oral temperature measurement of ≥38.3°C or a temperature of ≥38°C sustained over a 1-hour period); grade 4 thrombocytopenia or grade 3 thrombocytopenia associated with bleeding; ≥grade 3 abnormal laboratory values (except neutropenia and thrombocytopenia) that were assessed as being clinically significant; and ≥grade 3 nonlaboratory toxicity (excluding rash, nausea, diarrhea, and vomiting, if controlled with standard supportive therapy).
PK Assessments
Nonfasting serial blood samples were collected for PK analysis before infusion; just before the end of infusion; and 10 minutes, 20 minutes, 30 minutes, 1 hour, 1.5 hours, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours, and 24 hours after ME-344 dosing on days 1 and 15 of cycle 1, and were analyzed for levels of ME-344.
Statistical Analysis
Statistical analyses were descriptive in nature and statistical hypothesis testing was not performed. Estimation of the PK parameters was performed by noncompartmental methods. All patients who received at least 1 dose of the study drug were included in the evaluation of safety.
Results
Patient Characteristics
Between May 2012 and March 2013, 31 patients were screened and 30 patients were enrolled on the study. Baseline characteristics of the enrolled patients are found in Table1. The majority of patients were white (87%) and female (67%). The median age of the patients was 65 years (range, 19-85 years). Approximately 53% of the patients had an ECOG performance status of 0, and 47% had an ECOG performance status of 1. Nearly two-thirds of the patients had received ≥3 prior therapies. Colorectal cancer and non-small cell lung cancer were the most common tumor types (5 patients each [17%]).
Table 1.
Patient Characteristics (N=30)
| Median age (range), y | 65 (19-85) |
| Age 18-64 y, no. (%) | 15 (50%) |
| Age ≥65 y, no. (%) | 15 (50%) |
| Sex, no. (%) | |
| Female | 20 (67%) |
| Male | 10 (33%) |
| ECOG PS, no. (%) | |
| 0 | 16 (53%) |
| 1 | 14 (47%) |
| Median no. of prior therapies (range) | 3 (1-14) |
| 0-1 prior therapies, no. (%) | 3 (10%) |
| 2 prior therapies, no. (%) | 5 (17%) |
| 3 prior therapies, no. (%) | 8 (27%) |
| ≥4 prior therapies, no. (%) | 14 (46%) |
| Cancer type, no. (%) | |
| Colorectal | 5 (17%) |
| Non-small cell lung | 5 (17%) |
| Endometrial | 3 (10%) |
| Ovarian | 2 (7%) |
| Squamous cell carcinomaa | 2 (7%) |
| Urothelial carcinoma | 2 (7%) |
| Otherb | 11 (35%) |
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
Squamous cell carcinoma of the vagina and not otherwise specified.
Other includes bladder, breast, carcinoid of the ileum, cervical, cervical leiomyosarcoma, small cell lung, melanoma, pancreatic, peritoneal, sarcoma, and unknown primary (one patient each).
Treatment
Three to 8 patients were enrolled into each of 6 cohorts (Table2). A total of 21 patients (70%) completed at least 2 cycles of ME-344. Five patients completed ≥9 cycles, and 28 patients (93%) had discontinued treatment at the time of last follow-up. Treatment with ME-344 was ongoing for 2 patients at the time of data cutoff for the current study. As of July 30, 2014, one patient being treated at dose level 3 was on cycle 26 (101 weeks). A second patient was originally treated at dose level 5 (20 mg/kg), but had their dose reduced to 5 mg/kg, and was on cycle 20 (80 weeks) at the time of last follow-up. Disease progression was the most common reason for discontinuation of study treatment (16 patients; 52%). Five patients (16%) who had a DLT were required as per protocol to be discontinued from study treatment.
Table 2.
Dose Escalation and DLTs (N=30)
| Dose Level | Dose | No. of Patients Treated | No. of Patients With DLT | DLT Description |
|---|---|---|---|---|
| 1 | 1.25 mg/kg | 4a | 0 | |
| 2 | 2.5 mg/kg | 3 | 0 | |
| 3 | 5 mg/kg | 5b | 0 | |
| 4 | 10 mg/kg | 6 | 1 | Non-ST segment elevation myocardial infarction (grade 3c) |
| 5 | 20 mg/kg | 8d | 2 | Peripheral neuropathy (grade 3), peripheral sensory neuropathy (grade 3) |
| 6 | 15 mg/kg | 4 | 2 | Peripheral neuropathy (grade 3), peripheral motor neuropathy (grade 3) |
Abbreviation: DLTs, dose-limiting toxicities.
The last 2 patients were screened at the same time and were both allowed to be treated at this dose level.
Two patients did not complete a full cycle due to non-DLTs (1 sinus bradycardia and 1 subarachnoid hemorrhage attributed to the patient's meningeal carcinomatosis).
Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).
Two patients did not complete a full cycle due to non-DLTs (1 infusion reaction and 1 case of intractable vomiting attributed to the patient's bladder cancer).
Dose Escalation and MTD
Dose escalation and DLTs are shown in Table2. Dose levels 1 (1.25 mg/kg), 2 (2.5 mg/kg), and 3 (5 mg/kg) each enrolled at least 3 evaluable patients without occurrence of a DLT. At the fourth dose level (10 mg/kg), no patients experienced a DLT, and therefore the ME-344 dose was escalated to 20 mg/kg (dose level 5). Two of the 8 patients treated at this dose level developed dose-limiting peripheral neuropathy. One patient experienced grade 3 peripheral neuropathy after the second dose of ME-344. The neuropathy resolved to a new baseline of grade 1 after 2 weeks. The second patient developed grade 3 peripheral sensory neuropathy at the time of the first infusion of ME-344. This neuropathy did not resolve after 10 weeks.
Because the dose of 20 mg/kg was not tolerable, an intermediate dose of 15 mg/kg was then studied (dose level 6). Two of the 4 patients treated at this dose level developed peripheral neuropathy DLTs. One patient had baseline grade 1 peripheral neuropathy from previous chemotherapy and developed grade 3 peripheral neuropathy after the second dose of ME-344. The neuropathy resolved back to baseline with discontinuation of the study drug. The second patient developed grade 3 peripheral neuropathy of the right arm on day 2 of cycle 1, which resolved within 6 days and study treatment was therefore continued. However, the grade 3 peripheral neuropathy of the right arm recurred after the day 15 infusion on cycle 1 and the patient was removed from study for DLT. The neuropathy did resolve to baseline. Because dose level 6 resulted in 2 DLTs, an additional 3 patients were dosed at dose level 4 (10 mg/kg). One patient experienced a DLT of non-ST segment elevation myocardial infarction that occurred at the end of the first infusion of ME-344 and was accompanied by hypertension, nausea, and vomiting. It should be noted that the patient had a history of hyperlipidemia and was receiving nitroglycerin and isosorbide mononitrate, both of which were used to treat angina, as well as the hypertension drug metoprolol when she was enrolled to the study. No further cardiac workup was performed by the treating physicians due to the patient having advanced lung cancer, although the patient did recover from the event. This patient subsequently came off study. No additional DLTs were observed, and the MTD was therefore declared to be 10 mg/kg administered iv weekly.
Safety
Every patient experienced at least 1 AE of any grade, and 63% of patients (19 patients) experienced a treatment-related AE, primarily grade 1/2. The most frequent AEs considered to be related to treatment with ME-344 and reported by ≥10% of patients were peripheral neuropathy (20%), nausea (20%), dizziness (20%), fatigue (17%), vomiting (13%), diarrhea (10%), and asthenia (10%) (Table3).
Table 3.
Treatment-Related Adverse Events Occurring in ≥10% of Patients (N= 30)a
| Toxicityb | Grade 1 | Grade 2 | Grade 3 | Total |
|---|---|---|---|---|
| Neuropathyc | 1 (3%) | 1 (3%) | 4 (14%) | 6 (20%) |
| Nausea | 4 (13%) | 2 (7%) | 0 | 6 (20%) |
| Dizziness | 3 (10%) | 1 (3%) | 2 (7%) | 6 (20%) |
| Fatigue | 2 (7%) | 3 (10%) | 0 | 5 (17%) |
| Vomiting | 2 (7%) | 2 (7%) | 0 | 4 (13%) |
| Diarrhea | 1 (3%) | 2 (7%) | 0 | 3 (10%) |
| Asthenia | 1 (3%) | 1 (3%) | 1 (3%) | 3 (10%) |
There were no grade 4 treatment-related adverse events reported.
Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).
Includes peripheral neuropathy, peripheral motor neuropathy, and peripheral sensory neuropathy.
Importantly, of the 6 total patients who experienced peripheral neuropathy, 5 had baseline neuropathy before initiation of the study. There was 1 treatment-related AE noted, but no DLTs were reported due to central nervous system dysfunction.
Six patients experienced serious AEs that led to treatment discontinuation. These included 3 events of peripheral neuropathy and 1 event each of sinus bradycardia, non-ST segment elevation myocardial infarction, and an infusion reaction (hypertension and bradycardia). As described above, 5 of these AEs/serious AEs were considered to be DLTs.
Targeted safety evaluations, based on preclinical toxicology findings, including both cardiac function (cardiac enzyme evaluation) and QTc prolongation, were performed in all patients. One patient treated at the dose of 10 mg/kg experienced grade 2 prolonged QTc Fridericia intervals assessed as being related to study treatment. No other clinically significant cardiac function findings were noted in the current study.
Two deaths occurred within 30 days of treatment discontinuation: 1 patient treated at a dose of 5 mg/kg died after a subarachnoid hemorrhage attributed to the patient's known meningeal carcinomatosis, and a second patient treated at a dose of 20 mg/kg died after an event of intractable vomiting and a general deterioration in health attributed to his progressive bladder cancer. There were no deaths reported while patients were receiving the study treatment.
Pharmacokinetics
Samples from 28 patients were available for PK analysis. All 17 patients in the first 4 cohorts (1.25, 2.5, 5, and 10) had PK sampling on days 1 and 15. In the cohort of patients treated at a dose of 15 mg/kg, 4 patients had PK data from day 1, one of whom also completed day 15 PK sampling. In the cohort of patients treated with 20 mg/kg, 7 patients had day 1 PK data, 4 of whom also completed day 15 PK sampling.
At the time of visual inspection of the mean area under the concentration curve (AUC) curves, ME-344 plasma levels declined in a biexponential or triexponential manner on both day 1 and day 15. The AUC and the maximal plasma concentrations (Cmax) were essentially identical when comparing day 1 and day 15 across the dose levels (Fig. 1). To assess accumulation, the ratio between the day 1 and day 15 dose-adjusted Cmax and the AUC was evaluated. The geometric mean ratio of the day 1/day 15 Cmax was 1.99 µg/mL/mg (95% confidence interval [95% CI], −0.29 to 2.80) and the day 1/day 15 dose-adjusted AUC was 0.008 hour* µg/mL/mg (95% CI, −1.01 to 0.04), also supporting no accumulation at day 15 of cycle 1. The Cmax was observed at the end of the 30-minute infusion, and demonstrated an increase with increasing dose, as did the AUC.
Figure 1.
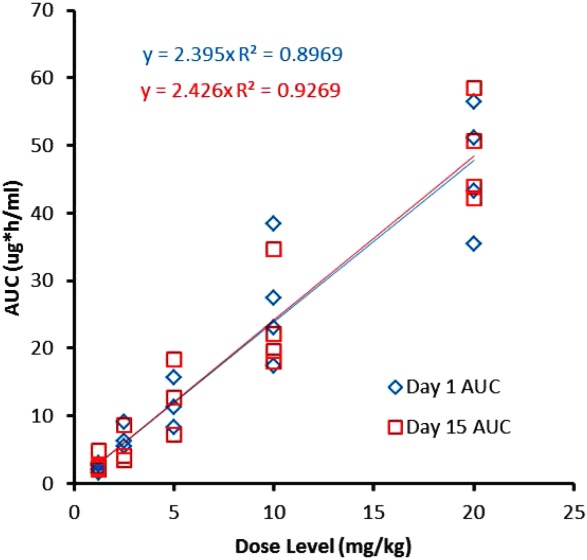
Relationship between ME-344 dose versus day 1 and day 15 area under the concentration curve (AUC) is shown.
Regression analysis of plots of Cmax and AUC by dose level on days 1 and 15 suggest dose linearity with increasing dose. For AUC, the correlation coefficient was 0.9883 on day 1 and 0.7111 on day 15 (Fig. 2). Similarly, the correlation for Cmax by dose level on day 1 was 0.9827 and was 0.7701 on day 15. The decrease in correlation on day 15 was likely driven by the 15-mg/kg dose level, on which only 1 patient was treated.
Figure 2.
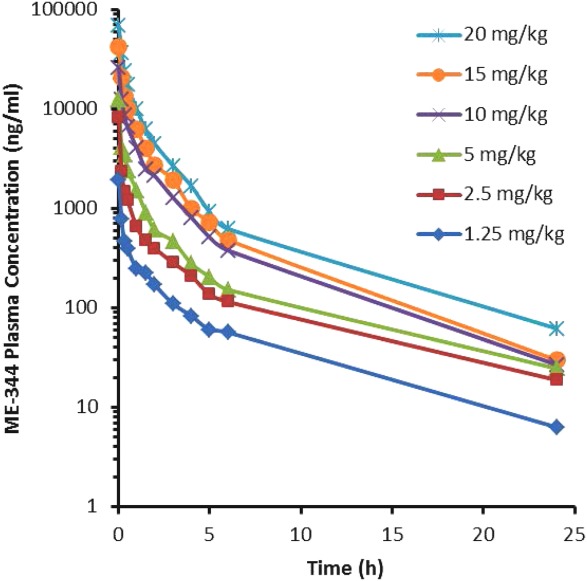
ME-344 plasma concentration on day 1 versus time is shown.
The results of the PK analyses demonstrated that ME-344 plasma concentrations decline in a multiexponential fashion with a mean half-life of approximately 6 hours. A linear relationship is suggested by plotting the ME-344 dose (as mg/kg) and the day 1 Cmax levels and exposure (AUC) levels, with good correlation noted between day 1 and day 15.
ME-344 values for volume of distribution at steady state (Vdss), half-life (t1/2), and drug clearance (Cl) appear to be dose-independent. In addition, there were no statistical differences with regard to drug clearance and Vdss noted between day 1 and day 15.
Efficacy
Of the 30 patients enrolled on the current study, 21 (70%) received at least 3 cycles of treatment and were evaluated for response at 12 weeks. One patient, a 54-year-old man with small cell lung cancer (SCLC) treated at a dose of 5 mg/kg, had achieved a partial response at his cycle 11 assessment. The patient had developed disease progression on 3 prior regimens and the treatment duration for the preceding regimen was 11 weeks. He was continuing treatment and on his 11th cycle of therapy (≥52 weeks) with an ongoing partial response noted at the time of data cutoff for the current study. Computed tomography images of this patient at week 52 demonstrated a marked decrease in tumor size from baseline (Fig. 3). In addition, 10 patients (33%) experienced stable disease during the study. The 4 most prolonged cases included 1 patient each with urothelial carcinoma (47 weeks), carcinoid of the ileum on cycle 10 of treatment (≥40 weeks), cervical leiomyosarcoma (39 weeks), and cervical cancer on cycle 8 of treatment (31 weeks) at the time of data cutoff. Ten patients (33%) had developed disease progression at the time of the first evaluation. For 7 of 11 patients experiencing a partial response or stable disease, the duration of ME-344 therapy was greater than the duration of the previous treatment regimens (Fig. 4).
Figure 3.
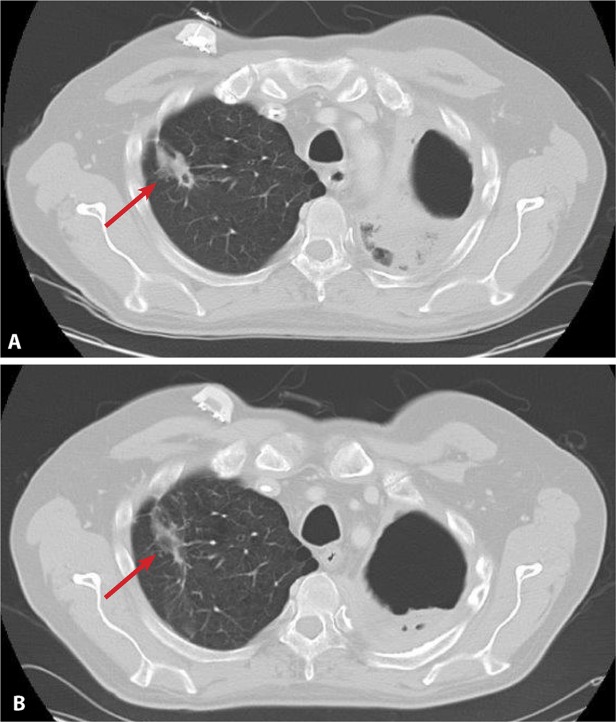
Computed tomography images from a patient with small cell lung cancer at (A) baseline and (B) week 52 are shown.
Figure 4.
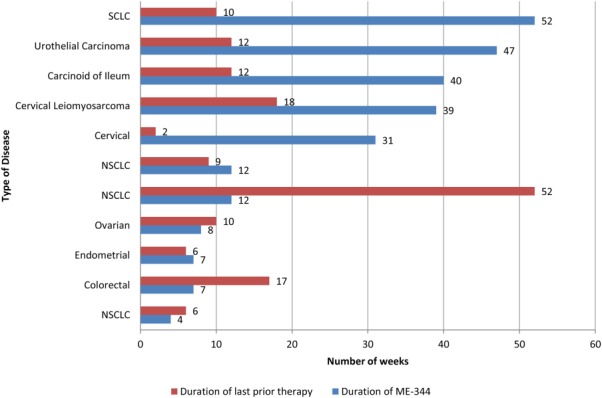
Duration of prior therapy compared with ME-344 dosing in patients achieving a partial response or stable disease is shown. SCLC indicates small cell lung cancer; NSCLC, non-small cell lung cancer.
The median progression-free survival was 6.9 weeks (95% CI, 1.6-7.1 weeks), with a range of 0 to ≥52 weeks.
Since the time of database lock, the 3 patients who were still receiving study treatment continued to be monitored. As of July 30, 2014, the patient with SCLC with a partial response remained on treatment and was on his 26th cycle of therapy at the time of last follow-up (≥101weeks). One patient with carcinoid tumor continued to maintain stable disease after 20 cycles of treatment (≥80 weeks), whereas 1 patient with cervical cancer developed disease progression at week 42 and discontinued treatment.
Discussion
Once-weekly administrations of ME-344 were generally well tolerated in this first-in-human study of patients with refractory solid tumors. There were 5 DLTs observed, 4 of which were peripheral neuropathy. The MTD was determined to be 10 mg/kg weekly. The most common AEs related to ME-344 across all dose levels were neuropathy, nausea, dizziness, and fatigue.
PK analysis revealed that ME-344 plasma concentrations decline in a multiexponential fashion with a mean half-life of approximately 6 hours. A linear relationship was suggested by plotting the ME-344 dose (mg/kg) and day 1 Cmax levels and exposure (AUC) levels, with good correlation noted at day 1 and day 15. Volume of distribution values, terminal half-life, and drug clearance values appeared to be independent of ME-344 dose. There were no statistical differences noted in the volume of distribution and clearance values between days 1 and 15.
A preliminary assessment suggests that neurotoxicity is dose-related, with all neuropathy occurring in the 2 highest dose levels (15 mg/kg and 20 mg/kg, respectively), with higher AUCs and Cmax. It should be noted that neuropathy was noted only at dose levels higher than the determined MTD and the majority of patients with neuropathic toxicity (5 of 6 patients) had experienced it during previous treatment regimens.
The increased neuropathy observed in the current study would be expected based on ME-344's disruption of mitochondrial integrity. Mitochondrial dysfunction has been implicated in chemotherapy-induced peripheral neuropathy,6–8 and patients with congenital mitochondrial syndromes such as ataxia telangiectasia,9 Alpers-Huttenlocher syndrome,10 and ataxia with oculomotor apraxia11 often have a prominent neurological dysfunction.
ME-344 has a mechanism of action that is distinct from ME-143, a related drug candidate currently under clinical evaluation.12 The overall safety profile of ME-344 was similar to that described in the first-in-human trial of ME-143, with the exception of the AE of peripheral neuropathy observed in the current trial. Infusion reactions were noted in 2 patients who were treated at the maximal administered dose studied (20 mg/kg) in the ME-143 trial, whereas only 1 patient treated with ME-344 (15 mg/kg) had an infusion reaction that resulted in treatment discontinuation. Although there were no objective responses observed with ME-143, 1 patient with SCLC who was being treated with ME-344 on the current trial (at a dose of 5 mg/kg) achieved a partial response, and 10 others were noted to have stable disease. Based on in vitro results with NV-128, future studies with ME-344 will include biomarker analysis of targets downstream of mTOR, such as pAKT or pS6K.1
The preliminary efficacy observed in the current study appears promising and merits further study of the drug. Approximately 75% of patients with stable disease or better had more durable disease control on ME-344 than on the treatment regimen immediately preceding ME-344.
The once-weekly administration of ME-344 was generally well tolerated in this first-in-human study of patients with refractory solid tumors. ME-344 demonstrated the DLT of neuropathy and the MTD was found to be 10 mg/kg. In vitro studies of ME-344 in combination with topotecan have demonstrated that these drugs act synergistically (data on file). Because topotecan has a different toxicity profile from ME-344 in clinical trials, this was a rational combination for development.13 Therefore, a phase 1B study of ME-344 in combination with iv topotecan as treatment of patients with ovarian, cervical, and small cell lung cancers currently is ongoing (http://ClinicalTrials.gov identifier 02100007).
Funding Support
Funded by MEI Pharma Inc.
Conflict of Interest Disclosures
Dr. Ofir Moreno, Ms. Esquibel, and Dr. Levin are employed by MEI Pharma Inc. Dr. Moreno holds patents from MEI Pharma and owns stock options for work performed outside of the current study. Dr. Moore has acted as paid member of the advisory boards of Advaxis, ImmunoGen, and Genentech for work performed outside of the current study.
References
- 1.Alvero AB, Montagna MK, Chen R. NV-128, a novel isoflavone derivative, induces caspase-independent cell death through the Akt/mammalian target of rapamycin pathway. Cancer. 2009;115:3204–3216. doi: 10.1002/cncr.24397. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvero AB, Montagna MK, Holmberg JC, Craveiro V, Brown D, Mor G. Targeting the mitochondria activates two independent cell death pathways in the ovarian cancer stem cells. Mol Cancer Ther. 2011;10:1385–1393. doi: 10.1158/1535-7163.MCT-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvero AB, Sumi N, Craveiro V. ME-344 delays tumor kinetics in an ovarian cancer in vivo recurrence model [abstract] Cancer Res. 2013;73:Page. et al.. Abstract LB-286. [Google Scholar]
- 4.Eisenhauer EA, Therasse P, Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guidelines (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. et al. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) 2010. . Version 4.03. Washington, DC: US Department of Health and Human Services;
- 6.Xiao WH, Bennett GJ. Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain. 2012;153:704–709. doi: 10.1016/j.pain.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyama S, Shimoyama N, Ishida Y, Koyasu T, Szeto HH, Shimoyama M. Characterization of acute and chronic neuropathies induced by oxaliplatin in mice and differential effects of a novel mitochondria-targeted antioxidant on the neuropathies. Anesthesiology. 2014;120:459–473. doi: 10.1097/01.anes.0000435634.34709.65. [DOI] [PubMed] [Google Scholar]
- 8.Areti A, Yerra VG, Naidu V, Kumar A. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014;2:289–295. doi: 10.1016/j.redox.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valentin-Vega YA, MacLean KH, Tait-Mulder J. Mitochondrial dysfunction in ataxia-telangiectasia. Blood. 2012;119:1490–1500. doi: 10.1182/blood-2011-08-373639. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copeland WC. Defects in mitochondrial DNA replication and human disease. Crit Rev Biochem Mol Biol. 2012;47:64–74. doi: 10.3109/10409238.2011.632763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sykora P, Croteau DL, Bohr VA, Wilson DM., 3rd Aprataxin localizes to mitochondria and preserves mitochondrial function. Proc Natl Acad Sci USA. 2011;108:7437–7442. doi: 10.1073/pnas.1100084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pant S, Burris H, 3rd, Moore K. A first-in-human dose-escalation study of ME-143, a second generation NADH oxidase inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2014;32:87–93. doi: 10.1007/s10637-013-9949-4. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. GlaxoSmithKline. Topotecan UP Package Insert. Brentford, UK: GlaxoSmithKline; 2014.gsksource.com/gskprm/en/US/adirect/gskprm?cmd=ProductDetailPage&product_id=1342013985525&featureKey=603583#nlmhighlightsD. Accessed on October 29, 2014.


