Abstract
Aim
This study aims to describe the microbiology of middle ear fluid (MEF) in a cohort of children vaccinated with Streptococcus pneumoniae conjugate vaccine (PCV7) having ventilation tube insertion. Nasopharyngeal (NP) carriage of otopathogens in these children is compared with children without history of otitis media.
Methods
Between May and November 2011, MEF and NP samples from 325 children aged <3 years were collected in three major centres in New Zealand at the time of ventilation tube insertion. An age-matched non-otitis-prone comparison group of 137 children had NP samples taken. A questionnaire was completed by both groups.
Results
Immunisation coverage with at least one dose of PCV7 was 97%. Haemophilus influenzae was cultured in 19.4% of MEF and was polymerase chain reaction (PCR) positive in 43.4%. S. pneumoniae and Moraxella catarrhalis were cultured in <10% of MEF samples but were PCR positive for 23.1% and 38.7%, respectively. H. influenzae was the most common organism isolated from NP samples (60%) in the grommet group, while M. catarrhalis (56%) was the most common in the non-otitis prone group. S. pneumoniae was more commonly found in the nasopharynx of children with ear disease (41% vs. 29%). 19F was the most prominent S. pneumoniae serotype in NP samples of both groups, but no serotype dominated in MEF. Ninety-five per cent of H. influenzae isolates were confirmed to be non-typeable H. influenzae.
Conclusion
In this cohort of children with established ear disease requiring surgical intervention, non-typeable H. influenzae is the dominant pathogen in both the nasopharynx and MEF.
Keywords: Haemophilus influenzae, middle ear, otitis media, pneumococcal vaccination, Streptococcus pneumoniae
What is already known on this topic
Otitis media, both acute and chronic, is a large burden of disease for young children.
Conjugate pneumococcal vaccines impact on invasive pneumococcal disease and pneumonia; however, ear disease impacts are less clear.
The nasopharyngeal and middle ear bacterial pathogens are closely linked in children.
What this paper adds
Non-typeable Haemophilus influenzae is the most common bacterial cause of recurrent acute otitis media and otitis media with effusion in New Zealand children.
Nasopharyngeal carriage of otopathogens is more common in children with established ear disease in our population.
Despite conjugate vaccination, vaccine-associated pneumococcal serotype 19F remains a common pneumococcal serotype in the nasopharynx of young children and also most likely to carry resistance to antibiotics.
Otitis media (OM) is one of the most common disorders for which medical care is sought for children. This includes both acute OM (AOM) and OM with effusion (OME). In line with other countries, AOM and OME are a significant burden on the New Zealand (NZ) health-care system and are a common reason for antibiotic prescriptions for young children.1 Maori and Pacific children in NZ are disproportionately affected with OM, with medical admissions rates for OM-related conditions being twice those of European or other ethnic groups.1,2 Recurrent AOM (rAOM) and persistent OME are the most common indications for inserting ventilation tubes or ‘grommets’.3
Streptococcus pneumoniae, non-typeable Haemophilus influenzae and Moraxella catarrhalis are regarded as the main pathogens responsible for middle ear disease.4–9 The pathogenesis of OM is intricately related to the presence of bacteria colonising the nasopharynx, which provides the reservoir for respiratory pathogens. There is a relationship between nasopharyngeal (NP)-colonising organisms and rAOM.10–13 The co-colonisation of certain bacteria may also be associated with a higher risk of rAOM.14 However, the relationship of bacteria in the pathogenesis of OME is not so well established.12
Pneumococcal conjugate vaccines (PCVs) significantly decrease the burden of invasive pneumococcal disease such as meningitis but also impact on mucosal disease such as pneumonia and AOM caused by vaccine serotypes.15 While the efficacy of PCV7 against AOM in early clinical trials was reportedly less than 10%,16 the efficacy and effectiveness against rAOM and surgical interventions such as ventilation tubes have subsequently been shown to be much greater.17–19 The impacts of PCV are likely through reduction of NP colonisation by vaccine serotypes which may subsequently reduce OM due to these same vaccine serotypes.20
PCV7 was introduced into the NZ national immunisation schedule in September 2008 with catch-up vaccination offered to all infants born after January 2008. In late 2011 (after the completion of this study), the immunisation schedule was revised with replacement of PCV7 with the new 10 valent PCV (PHiD-CV). This change provided a window of opportunity to assess the infectious aetiology of OM following implementation of PCV7 and prior to the change in pneumococcal vaccination to PHiD-CV.
Our study was known at the “OMIVI” (Otitis Media Infectious aetiology & Vaccination Impact) Study. Our aims were to determine the bacteriological causes of rAOM and OME in NZ children. In addition, we wanted to document the NP carriage of organisms known to cause AOM in children with and without a history of rAOM or OME in the context of our changing pneumococcal vaccine schedule.
Methods
Children less than 36 months of age undergoing ventilation tube (grommet) surgery were recruited from three major centres: Starship Children's Hospital (Auckland District Health Board), KidzFirst (Counties Manukau District Health Board) and Christchurch Hospital (Canterbury District Health Board) between May and November 2011 (Fig. 1). These sites represent diverse ethnicities and are the three major referral centres for children requiring surgical intervention for rAOM or OME in NZ. In these centres, the criteria for grommet insertion is >6 episodes of AOM in 12 months or persistent bilateral middle ear effusions for >3 months. Diagnosis at referral was made by trained practitioners, together with micro-otoscopy and tympanometry prior to booking for surgery.
Fig 1.
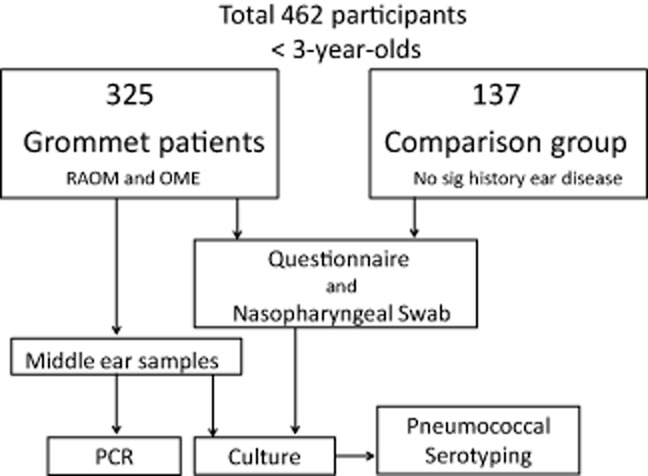
Otitis Media Infectious aetiology & Vaccination Impact study design.
In each study centre, NP carriage data were also collected on a non-otitis-prone group of children of same age, ethnicity and vaccination status to form our comparison group. On parental history, they had no significant previous ear disease (less than three episodes of AOM in 12 months, no history of OME). These children were having a general anaesthetic for non-ear-related procedures (such as CT/MRI scans, general or non-ear-related surgery). Children with known immune deficiency, cystic fibrosis or craniofacial malformation were excluded from both groups.
Informed consent was obtained by a member of our research team prior to their procedure. Both groups had risk factors for ear disease and epidemiological data collected via parental/carer questionnaire.
All children enrolled were eligible for PCV7 and Hib vaccinations as part of the NZ immunisation schedule. Receipt of vaccinations was confirmed for each child using the National Immunisation Register.
Laboratory method for NP and middle ear samples
NP swabs from both groups were collected as per previously published protocols.21 In the grommet group, middle ear fluid (MEF) was collected by sterile suction through the myringotomy prior to grommet placement.
Samples were transferred to laboratory within 4 h. MEF were vortexed and 1 mL aliquots stored at −70°C with remaining MEF immediately cultured for bacterial pathogens by standard methods including extended 5-day culture on blood agar to detect Alliococcus otiditis.22
S. pneumoniae, H. influenzae and M. catarrhalis isolates were tested for susceptibility to standard antimicrobials by agar disc diffusion.22 Conservative breakpoints for S. pneumoniae penicillin susceptibility were used (≤0.06 μg/mL susceptible; 0.12–1 μg/mL intermediate; ≥2 μg/mL resistant). All S. pneumoniae had capsular serotyping performed via the Quellung reaction at the National reference laboratory.23
For nucleic acid extraction and polymerase chain reactions (PCRs), 200 μL of sample in broth and 5.0 μL of internal control DNA were extracted with EasyMag (BioMerieux, Auckland, New Zealand) generic 2.0.1 protocol. Nucleic acid was recovered in 60 μL of elution buffer. PCRs were based on the Real-time TaqMan (Applied Biosystems Inc., Foster City, CA, USA) PCR format with S. pneumoniae24 performed as a duplex with an internal control (unpublished assay), while H. influenzae25 and M. catarrhalis26 were detected using singleplex PCR assay. Primer and probe sequences are available on request.
Differentiation between H. influenzae and H. haemolyticus was performed using absence or presence of hpd#3 PCR.27 Samples identified as H. influenzae by culture or PCR were also tested for presence of capsule using bexB PCR.28
Ethical approval was obtained from the New Zealand Northern Regional Ethics Committee (NTX/11/04/029).
Statistical analyses
Demographic and response data were compared across centres and study cohorts using odds ratios and t-tests. Detection of MEF organisms was analysed with respect to NP organisms using McNemar change test with continuity correction (to allow for matched nature of data). Confidence intervals generated were at 95% level, with P value of 0.05 as cut-off. Analysis used R software package version 2.15.2.29
Results
Study population
Four hundred and sixty-two children were recruited with 325 in the grommet group and 137 in non-otitis prone group. Our grommet cohort captured 78% of all grommets performed in children in this age group in the three centres over the study period; non-participation was due to missed recruitment and 5% refusing participation. Of the 325 children having grommets, 29% had rAOM, 32% had OME and 37% had rAOM with persistent MEF between acute episodes as indication for surgery. One hundred and thirty-seven children (42%) were estimated by parental history to have had 5–10 episodes of AOM in the last 12 months, with 41 children (13%) estimated to have had >10 episodes. Less than 2% had tympanic membrane retraction as main indication for surgery.
Baseline demographic and clinical data are shown in Table 1. There were no significant differences in age, ethnicity, birthweight, history of breastfeeding, reflux symptoms or number of household occupants between the two groups. Overall, 97% of all participants had received ≥1 dose of PCV7.
Table 1.
Questionnaire data: demographics and otitis media risk factors
| Grommet | Non-otitis prone | P values | |
|---|---|---|---|
| Total number | 325 | 137 | – |
| Mean age in months (range) | 22 (6–36) | 21 (3–25) | NS |
| Male (%) | 203 (62%) | 98 (71.5%) | NS |
| Birthweight average: kg (range) | 3.39 (0.7–5.2 kg) | 3.36 (1.4–4.89) | NS |
| Gestational age <38 weeks | 52/325 (16%) | 32/137 (23%) | NS |
| Child's history of atopy | 61/323 (19%) | 19/137 (14%) | NS |
| Family's history of ear disease | 155 (48%) | 29 (12%) | P < 0.01 |
| Ethnicity | |||
| European | 58% | 45% | P < 0.05 |
| Maori | 22% | 18% | NS |
| Pacific Island | 12% | 15% | NS |
| Other | 8% | 23% | P < 0.01 |
| Vaccination status (%) | |||
| One or more of age-appropriate vaccinations | 315 (97%) | 134 (97%) | NS |
| Fully vaccinated | 247 (76%) | 87 (64%) | P < 0.01 |
| Full and ‘on time’ vaccination† | 140 (43%) | 41 (30%) | P < 0.01 |
| Environmental factors | |||
| Breastfed | 281/324 (87%) | 116/137 (85%) | NS |
| Exposure to cigarette smoke | 101/324 (31%) | 43/136 (31%) | NS |
| Day care attendance (>4 h/week) | 205/322 (64%) | 54/134 (40%) | P < 0.01 |
| Having any siblings | 226/324 (70%) | 88/136 (65%) | NS |
| More than five people in home | 59/325 (18%) | 23/137 (17%) | NS |
| Antibiotic use | |||
| Antibiotic use in last month | 198/321 (62%) | 51/133 (38%) | P < 0.01 |
‘On time’ defined as all vaccinations being given within 4 weeks of intended age: 6 weeks, 3 months, 5 months and 15 months. NS, not statistically significant.
Microbiology: MEF
Of the 325 grommet children, 70 children had no MEF bilaterally (21%), 71 had unilateral effusions (22%) and 184 had bilateral effusions (57%). Thus, 255 children had fluid in one or both ears. Of the 441 MEF samples collected, 13 were lost or unable to be processed, giving results from a total of 428 middle ear samples.
One hundred and one children (31%) had a positive culture in one or both ears for one or more of the three main otopathogens. Sixty-three children (19%) had MEF culture positive for H. influenzae, 26 (8%) were positive for S. pneumoniae and 26 (8%) for M. catarrhalis. The majority was culture positive for a single organism (53 grew H. influenzae alone, 20 M. catarrhalis, 18 S. pneumoniae). Ten children (3%) had MEF positive for ≥2 organisms, all having H. influenzae as one of the organisms.
The MEF of 66 children (19%) had a positive culture for another organism, of which 23 (7%) were also positive for one of the three main pathogens. Thirty-three children (10%) grew Alloiococcus otitidis (11 bilaterally), and five children had Pseudomonas aeruginosa cultured from their MEF. Twenty-two children grew presumed skin flora (7%), and six children grew Staphylococcus aureus (one of which was methicillin resistant S. aureus).
PCR increased identification of presence for the three main otopathogens from 19% (63/325 culture positive of one or more of the three main otopathogens in one or both ears) to 65% (210/325 PCR positive) (Fig. 2). Detection of H. influenzae increased from 19% (63/325 children) by culture to 43% (141/324) by PCR. M. catarrhalis detection increased from 8% (26/325) to 39% (182/441) and S. pneumoniae from 8% to 23%. Two MEF samples had positive culture for H. influenzae without concordant positive PCR, and similarly, one sample for M. catarrhalis and two samples for S. pneumoniae. Sixty-eight children (21%) had two otopathogens detected in their MEF by PCR, and 32 children (10%) had all three otopathogens present. Of the 100 children with more than two organisms detected by PCR present in MEF, 82 had H. influenzae present.
Fig 2.
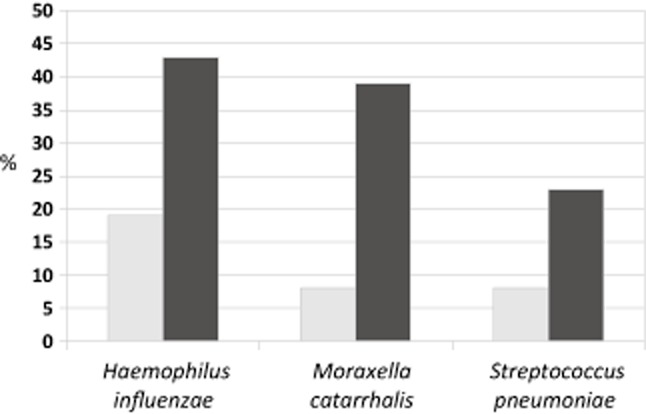
Culture versus polymerase chain reaction detection of pathogens in Grommet group (n = 325): middle ear effusions.  , culture;
, culture;  , PCR.
, PCR.
Microbiology: NP carriage
There were 137 NP specimens collected from 137 children in the non-otitis prone group and 316 NP specimens collected from the 325 grommet participants. The rates of carriage of otopathogens in the two groups are summarised in Table 2. Culture rates were not statistically different for the three major organisms when compared between ethnic groups.
Table 2.
Bacterial nasopharyngeal carriage in grommet versus comparison groups
| Bacteria | Grommet group n = 316 (%) | Comparison group n = 137 (%) | P value |
|---|---|---|---|
| NP specimen culture positive for one or more pathogens | 275 (87) | 105 (77) | P < 0.01 |
| Haemophilus influenzae: total (%) | 195 (62) | 58 (42) | P < 0.05 |
| Streptococcus pneumoniae: total (%) | 135 (43) | 40 (29) | P < 0.05 |
| VT (PCV7) | 36 (11) | 11 (8) | NS |
| NVT | 98 (31) | 29 (21) | P < 0.05 |
| Moraxella catarrhalis: total (%) | 181 (57) | 68 (50) | NS |
| NP specimen culture positive for Single pathogen only | 102 (32) | 58 (42) | P < 0.05 |
| NTHi | 57 (18) | 21 (15) | NS |
| S. pneumoniae | 13 (4) | 5 (4) | NS |
| M. catarrhalis | 32 (10) | 32 (23) | P < 0.01 |
| NP specimen culture positive for Two pathogens | 128 (41) | 33 (24) | P < 0.01 |
| S. pneumoniae + NTHi | 30 (9) | 11 (8) | NS |
| S. pneumoniae + M. catarrhalis | 41 (13) | 10 (7) | NS |
| M. catarrhalis + NTHi | 57 (18) | 12 (9) | P < 0.05 |
| NP specimen culture positive for all Three pathogens: NTHi + S. pneumoniae + M. catarrhalis | 51 (16) | 14 (10) | NS |
NP, nasopharyngeal; NS, not susceptible; NTHi, non-typeable H. influenzae; NVT, S. pneumoniae serotypes not included in PCV7; VT, S. pneumoniae serotypes included in PCV7.
Correlating NP culture and MEF culture or PCR results
The likelihood that a cultured otopathogen from NP was indicative of the same pathogen in the MEF by culture or by PCR was highly correlated for all three organisms. If culture positive for H. influenzae in the NP, a child was >10 times more likely to be culture positive for the same organism in MEF than a child who was culture negative. Similarly, if culture positive for S. pneumoniae or M. catarrhalis in NP, the child was 15 times more likely to be culture positive for those respective organisms in MEF. The negative predictive value of NP culture for culture negativity of MEF was 96%, 97% and 99%, respectively, for H. influenzae, M. catarrhalis and S. pneumoniae, while the positive predictive values (PPV) were poor (30%, 12% and 18%, respectively). If these otopathogens were cultured from the nasopharynx, the likelihood of the same organism being detected by PCR in MEF had an improved but still poor PPV (61%, 50% and 40%, respectively).
S. pneumoniae serotyping
Of S. pneumoniae cultured from NP (n = 174), 19F was the most common serotype followed by non-typeable and 19A. Serotyping of the S. pneumoniae from MEF (n = 32) showed a wide spread of serotypes (Fig. 3). Only one patient had an incongruous serotype in MEF and NP (serotype 35 in NP and 23B in MEF).
Fig 3.
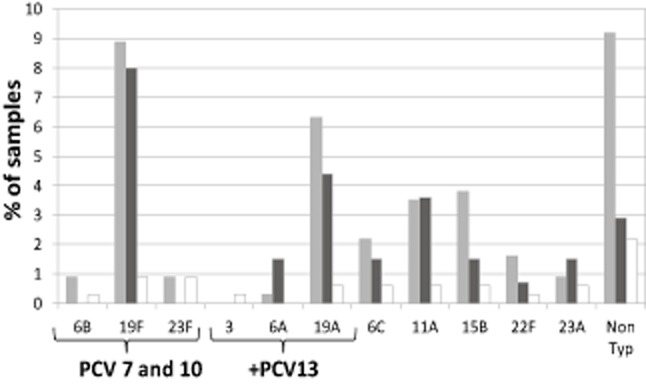
Streptococcus pneumoniae serotypes: culture of nasopharynx and middle ear in grommet group and nasopharynx of comparison group. Only one incongruous result: Ear 23B Nasopharynx 35NT.  , grommet nasopharynx (n = 134);
, grommet nasopharynx (n = 134);  , comparison nasopharynx (n = 40);
, comparison nasopharynx (n = 40);  , grommet middle ear (n = 32).
, grommet middle ear (n = 32).
Antibiotic susceptibilities
H. influenzae isolates from both MEF and NP were amoxycillin resistant in 20% (16/57 NP comparison group, 31/194 NP grommet group and 20/94 MEF) predominantly due to β-lactamase presence (60/67). Cotrimoxazole resistance was found in 30%, and other antibiotic resistance was uncommon.
Of M. catarrhalis isolates, 87% had detectable β-lactamase present, all were sensitive to co-amoxycillin clavulanate, and other resistance was uncommon.
Antibiotic susceptibility data were available in 206 S. pneumoniae isolates (Table 3). Reduced susceptibility to penicillin and resistance to cotrimoxazole were most commonly found. Among those with penicillin resistance (minimum inhibitory concentration ≥ 2 μg/mL), 19/21 (90%) were multi-resistant (resistant to ≥3 additional antibiotic classes).
Table 3.
Antimicrobial resistance and non-susceptibility among Streptococcus pneumoniae isolates from grommet and comparison children aged <3 years
| Penicillin* | Cotrimoxazole R (%) | Clindamycin R (%) | Erythromycin R (%) | Tetracycline R (%) | ||
|---|---|---|---|---|---|---|
| R (%) | NS (%) | |||||
| MEF n = 31 | 2 (6) | 7 (23) | 6 (19) | 6 (19) | 5 (16) | 4 (13) |
| NP n = 175 | 19 (11) | 57 (33) | 56 (32) | 32 (18) | 41 (23) | 40 (23) |
| Total n = 206 | 21 (10) | 64 (31) | 62 (30) | 38 (19) | 46 (22) | 44 (21) |
For penicillin: Resistance represents: Minimum Inhibitory Concentration (MIC) > 2. NS represents intermediate plus resistant isolates: MIC > 0.06. R, resistant; NS, not susceptible; MEF, middle ear fluid; NP, nasopharyngeal.
The vast majority of penicillin resistant and multi-resistant isolates were serotype 19F (18/19 isolates) with the remaining one being serotype 19A. Of those with intermediate penicillin susceptibility and multi-resistance, 12/19 were serotype 19F.
Molecular differentiation of Haemophilus species
Among the archived H. influenzae isolates from NP and MEF, 281 isolates (82% of all H. influenzae detected) had extractable nucleic acid available for species differentiation.
Twelve isolates (4%) were identified as Haemophilus haemolyticus with negative hpd#3 PCR. One of 12 was from MEF, and the rest was from NP. Of the remaining confirmed H. influenzae (269), two were positive for bexB PCR; therefore, likely capsulated Hi and the remaining 99.3% were non-typeable H. influenzae (NTHi) (capsule absent). Therefore, of all suspected H. influenzae, 95% were NTHi, 4% were H. haemolyticus and <1% were capsulated.
Discussion
Recurrent AOM and OME have a significant disease burden in children younger than 3 years of age in NZ. We describe for the first time microbiology of rAOM and OME in a cohort of NZ children who have received pneumococcal and Hib vaccination. The major strength of this study is that almost 80% of children referred for surgical intervention for OM were captured from the two major NZ cities.
NTHi was the organism most commonly identified from both the NP and MEF in our cohort of children with rAOM or OME. Our findings are consistent by both culture and PCR detection methods and are also in keeping with studies from several other countries where H. influenzae has been found to be the most common pathogen isolated in the context of widespread conjugate pneumococcal vaccination.21,30
Haemophilus haemolyticus, a non-pathogenic commensal organism, is indistinguishable from H. influenzae by conventional microbiology.27 A further strength in our study was the accurate molecular characterisation27 to demonstrate over 95% of H. influenzae isolates from both NP and MEF samples were truly NTHi. This established an important baseline for monitoring changes rates of H. influenzae carriage which could otherwise be masked by H. haemolyticus replacing H. influenzae in the nasopharynx.
S. pneumoniae was the least commonly detected otopathogen by culture or PCR in MEF or by culture of NP. A wide range of S. pneumoniae serotypes were demonstrated in MEF samples with no single serotype that dominated.
In all but one patient, there was a concordance of the S. pneumoniae serotype identified in MEF and NP samples, supporting the theory that invasion of the MEF is preceded by colonisation of the NP. Similarly, NP culture positivity for other otopathogens (H. influenzae or M. catarrhalis) showed a child to be much more likely to be culture positive in MEF than a child not carrying those organisms. The high negative predictive values for all three organisms may enable a negative NP culture in OME or rAOM cases to help rule out the likelihood of organisms in the MEF.31
The pneumococcal serotypes most frequently found on NP samples in both the grommet and comparison groups were 19F (PCV7 serotype) and 19A (non-PCV7 serotype). Persisting carriage of vaccine 19F serotype in our vaccinated population (>70% fully vaccinated) may be due to several factors. Although PCV7 was introduced in NZ 3 years prior to our study, improved immunisation rates and timely (age-appropriate) vaccine administration have been more gradually achieved as a priority national health target; this may have slowed the herd immunity impact of PCV7.32
Serotype 19F was most associated with multi-resistance and penicillin resistance. Reduced penicillin susceptibility was seen to a much lesser extent with serotype 19A. This is in contrast to other countries where 19A is commonly multi-drug resistant in PCV7-vaccinated and unvaccinated populations.33,34 Both the grommet and non-otitis prone groups had high rates of antibiotic pre-treatment (50% received antibiotics in last month). This high antibiotic exposure in our cohort may have selected for persistence of NP carriage of drug-resistant 19F serotype despite vaccination.
Despite relatively high NP carriage of 19F and 19A serotypes, these serotypes were infrequently isolated from MEF. A global review of studies following introduction of PCV7 has suggested that serotypes 3, 6 and 19A may be increasingly important in AOM.35 In our study, high prevalence of 19F and 19A in the NP was not reflected by higher rates of these serotypes in the MEF, suggesting these serotypes showed NP colonisation with a low rate of invasion into the middle ear cleft in child with established ear disease. However, small numbers of culture-positive S. pneumoniae from MEF limit conclusions around dominant serotypes in our cohort.
Evaluation of otopathogens and their antimicrobial patterns may have implications for empiric treatment of recurrent AOM. In our study, a significant proportion of M. catarrhalis, and to a lesser extent H. Influenzae, produced beta-lactamase and was resistant to amoxycillin. However, both comparison and cases were likely to have received antibiotics in the prior month (40% and 60%, respectively) which along with vaccination status will impact upon NP organisms and resistance profiles.
In our cohort, NP carriage of one or more otopathogens was more likely in the grommet children. Studies have described the carriage of multiple otopathogens in the NP to be significantly associated with presence of bacteria in MEF.36 We have shown when H. influenzae and S. pneumoniae co-colonise the NP, H. influenzae is more likely to be identified in MEF.14
Conclusion
This study represents the most comprehensive national data collection on the microbiology of middle ear disease in NZ children and demonstrates that NTHi is the most commonly isolated organism in established ear disease.
The 10 valent PCV (PHiD-CV) was included in the NZ national immunisation schedule in 201137,38 and has the potential to reduce the impact of NTHi infections including OM. This is due to the conjugation of pneumococcal polysaccharide antigens to the Protein D component of H. influenzae. We aim to repeat data collection in 2014 with a cohort of children who have been vaccinated with PHiD-CV. This will enable us to compare the infectious aetiology of OM and NP carriage of OM pathogens in both the PCV7- and PHiD-CV-vaccinated cohorts.
Acknowledgments
Many thanks to all of the participating families across NZ and the research nurses and theatre staff at each of the DHBs. Funding support was received from GlaxoSmithKline and the A+ Research Committee.
References
- 1.Milne R, Vander-Hoorn S. Burden and cost of hospital admissions for vaccine-preventable paediatric pneumococcal disease and non-typeable Haemophilic influenzae otitis media in New Zealand. Appl. Health Econ. Health Policy. 2010;8:281–290. doi: 10.2165/11535710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Craig E, Adams J, Oben G, Reddington A, Wicken A, Simpson J, NZCYES . The Health Status of Children and Young People in New Zealand. Dunedin: Ministry of Health; 2011. Available from: http://dnmeds.otago.ac.nz/departments/womens/paediatrics/research/nzcyes/pdf/Rpt2011_NZReport.pdf [accessed July 2014] [Google Scholar]
- 3.Mandell E, Cassellbrant M. Recent developments in treatment of otitis media. Drugs. 2006;66:1565–1576. doi: 10.2165/00003495-200666120-00003. [DOI] [PubMed] [Google Scholar]
- 4.Arguedas A, Dagan R, Soley C, et al. Microbiology of otitis media in Costa Rican children, 1999 through 2001. Pediatr. Infect. Dis. J. 2003;22:1063–1068. doi: 10.1097/01.inf.0000101189.81501.e9. [DOI] [PubMed] [Google Scholar]
- 5.Arguedas A, Dagan R, Guevara S, Porat N, Soley C. Middle ear fluid Streptococcus pneumoniae serotype distribution in Costa Rican children with otitis media. Pediatr. Infect. Dis. J. 2005;24:631–634. doi: 10.1097/01.inf.0000168748.92510.45. [DOI] [PubMed] [Google Scholar]
- 6.Kilpi T, Herva E, Kaijalainen T, Syjanen R, Takala A. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 2001;20:654–662. doi: 10.1097/00006454-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Leibovitz E, Jacobs M, Dagan R. Haemophilic influenzae: a significant pathogen in acute otitis media. Pediatr. Infect. Dis. J. 2004;23:1142–1152. [PubMed] [Google Scholar]
- 8.Leibovitz E, Satran R, Piglansky L, et al. Can acute otitis media caused by Haemophilic influenzae be distinguished from that caused by Streptococcus pneumoniae? Pediatr. Infect. Dis. J. 2003;22:509–514. doi: 10.1097/01.inf.0000069759.79176.e1. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblut A, Santolaya M, Gonzalez P, et al. Bacterial and viral etiology of acute otitis media in Chilean children. Pediatr. Infect. Dis. J. 2001;20:501–507. doi: 10.1097/00006454-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Revai K, Mamidi D, Chonmaitree T. Association of nasopharyngeal bacterial colonization during upper respiratory tract infection and the development of acute otitis media. Clin. Infect. Dis. 2008;46:e34–37. doi: 10.1086/525856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harabuchi Y, Kodama H, Faden H. Outcome of acute otitis media and its relation to clinical features and nasopharyngeal colonization at the time of diagnosis. Acta Otolaryngol. 2001;121:908–914. [PubMed] [Google Scholar]
- 12.Marchisio P, Claut L, Rognoni A, et al. Differences in nasopharyngeal bacterial flora in children with nonsevere recurrent acute otitis media and chronic otitis media with effusion: implications for management. Pediatr. Infect. Dis. J. 2003;22:262–268. doi: 10.1097/01.inf.0000055063.40314.da. [DOI] [PubMed] [Google Scholar]
- 13.Faden H. The microbiologic and immunologic basis for recurrent otitis media in children. Eur. J. Pediatr. 2001;160:407–413. doi: 10.1007/s004310100754. [DOI] [PubMed] [Google Scholar]
- 14.Xu Q, Casey J, Chang A, Pichichero M. When co-colonizing the nasopharynx Haemophilus influenzae predominates over Streptococcus pneumoniae except 19A strains to cause acute otitis media. Pediatr. Infect. Dis. J. 2012;31:638–640. doi: 10.1097/INF.0b013e31824ba6f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor S, Marchisio P, Vergison A, Harriague J, Hausdorff W, Haggard M. Impact of pneumococcal conjugate vaccination on otitis media: a systematic review. Clin. Infect. Dis. 2012;54:1765–1773. doi: 10.1093/cid/cis292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. NEJM. 2001;344:403–409. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 17.Zhou F, Shefer A, Kong Y, NuortiZhou P. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997–2004. Pediatrics. 2008;121:253–261. doi: 10.1542/peds.2007-0619. [DOI] [PubMed] [Google Scholar]
- 18.Poehling K, Szilagyi P, Grijalva C, et al. Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics. 2007;119:707–715. doi: 10.1542/peds.2006-2138. [DOI] [PubMed] [Google Scholar]
- 19.Jardine A, Mezies R, Deeks S, Patel M, McIntyre P. The impact of pneumococcal conjugate vaccine on rates of myringotomy with ventilation tube insertion in Australia. Pediatr. Infect. Dis. J. 2009;28:761–765. doi: 10.1097/INF.0b013e31819e9bc5. [DOI] [PubMed] [Google Scholar]
- 20.Schuermann L, Borys D, Hoet B, Forsgren A, Prymula R. Prevention of otitis media: now a reality? Vaccine. 2009;27:5748–5754. doi: 10.1016/j.vaccine.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien K, Hanna N, The WHO Pneumococcal Vaccine Trials Carriage Working Group Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr. Infect. Dis. J. 2003;22:e1–11. doi: 10.1097/01.inf.0000049347.42983.77. [DOI] [PubMed] [Google Scholar]
- 22.Wayne P, editor. Clinical Laboratory Standards Institute. Performance and Standards for Antimicrobial Susceptibility Testing – Approved Standard. Pennsylvania: CLSI; 2009. [Google Scholar]
- 23.Heffernan H, Martin D. Serotypes and Antimicrobial Resistance among Non-Invasive Pneumococci in New Zealand, 2008. Wellington: Institute of Environmental Science and Research; 2008. Available from: https://surv.esr.cri.nz/antimicrobial/s_pneumoniae_non-invasive.php [accessed July 2014] [Google Scholar]
- 24.Carvalho M, Tondella M, McCaustland K, et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of Pneumococcal DNA. J. Clin. Microbiol. 2007;45:2460–2467. doi: 10.1128/JCM.02498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith-Vaughan H, Byun R, Nadkarni M, et al. Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord. 2006;6:1–9. doi: 10.1186/1472-6815-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greiner O, Day P, Altwegg M, Nadal D. Quantitative detection of Moraxella catarrhalis in nasopharyngeal secretions by real-time PCR. J. Clin. Microbiol. 2003;41:1386–1392. doi: 10.1128/JCM.41.4.1386-1390.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binks M. Molecular surveillance of true nontypeable Haemophilus influenzae: an evaluation of PCR screening assays. PLoS ONE. 2012;7:1–8. doi: 10.1371/journal.pone.0034083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis G, Sandstedt S, Patel M, Marrs C, Gilsdor J. The use of bexB to detect the capsule locus in Haemophilus influenzae. J. Clin. Microbiol. 2011;49:2594–2601. doi: 10.1128/JCM.02509-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. Available from: http://www.R-project.org/ [accessed 2014] [Google Scholar]
- 30.Wiertsema S, Kirkham L, Corscadden K, et al. Predominance of nontypeable Haemophilus influenzae in children with otitis media following introduction of a 3 + 0 pneumococcal conjugate vaccine schedule. Vaccine. 2011;29:5163–5170. doi: 10.1016/j.vaccine.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 31.VanDongen T, van der Heijden G, van Zon A, Bogaert D, Sanders E, Schilder A. Evaluation of concordance between the microorganisms detected in the nasopharynx and middle ear of children with otitis media. Pediatr. Infect. Dis. J. 2013;32:549–552. doi: 10.1097/INF.0b013e318280ab45. [DOI] [PubMed] [Google Scholar]
- 32.Ministry of Health. Targeting Immunisation: Increased Immunisation. Wellington: Ministry of Health; 2011. Available from: http://www.health.govt.nz/system/files/documents/publications/targeting-immunisation-health-target.pdf [accessed July 2014] [Google Scholar]
- 33.Dagan R, Givon-Lavi N, Leibovitz E, Greenberg D, Porat N. Introduction and proliferation of multidrug-resistant Streptococcus pneumoniae serotype 19A clones that cause acute otitis media in an unvaccinated population. J. Infect. Dis. 2009;199:776–785. doi: 10.1086/597044. [DOI] [PubMed] [Google Scholar]
- 34.Moore M, Gertz R, Woodbury R, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States. J. Infect. Dis. 2008;197:1016–1027. doi: 10.1086/528996. [DOI] [PubMed] [Google Scholar]
- 35.Rodgers G, Arguedas A, Cohen R, Dagan R. Global serotype distribution among Streptococcus pneumoniae isolates causing otitis media in children: potential implications for pneumococcal conjugate vaccines. Vaccine. 2009;27:3802–3810. doi: 10.1016/j.vaccine.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 36.Stol K, Verhaegh S, Graamans K, Engel J. Microbial profiling does not differentiate between childhood recurrent acute otitis media and chronic otitis media with effusion. Int. J. Pediatr. Otorhinolaryngol. 2013;77:488–495. doi: 10.1016/j.ijporl.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prymula R, Schuerman L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev. Vaccines. 2009;8:1479–1501. doi: 10.1586/erv.09.113. [DOI] [PubMed] [Google Scholar]
- 38.Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typeable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367:740–748. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]


