Abstract
Human immunodeficiency virus type 1 (HIV-1) infection decreases the production of interleukin-2 (IL-2) from CD4+ and CD8+ T cells. Recombinant IL-2 (rIl-2) has been given to HIV-infected individuals to generate significant increases in CD4+ T-cell counts. There are limited data regarding the effects of pregnancy and HIV infection on IL-2 production in humans. To investigate the effects of human pregnancy, HIV infection, and HIV therapy on IL-2 production, we evaluated 61 women. Intracellular IL-2 production by CD4+ T cells from nonpregnant HIV-infected women was significantly lower than in that in uninfected women (45% ± 8% versus 52% ± 8%, P = 0.04). In contrast, there was no difference in levels of intracellular IL-2 production between HIV-infected and uninfected pregnant women. These observations suggest that pregnancy may down-regulate IL-2 production regardless of HIV infection status. Future studies should evaluate IL-2 production patterns in larger cohorts of women so that the physiological significance of IL-2 down-regulation in pregnancy can be further evaluated. This information is essential to assess the possible use of IL-2 supplementation therapy as a means of enhancing immune responses among HIV-infected pregnant women.
Interleukin-2 (IL-2), a cytokine primarily produced by CD4+ T lymphocytes, is considered a T-cell differentiation factor, because it promotes proliferation of T and B lymphocytes, as well as thymocytes (23, 26). IL-2 also enhances natural killer cell activity and immune response and induces the secretion of other cytokines such as gamma interferon (INF-γ), IL-4, and tumor necrosis factor-alpha (TNF-α). IL-2 production is considered part of the pattern of cytokine secretion associated with a T-helper 1 (Th1) immune response (12, 26, 31).
The decrease in IL-2 production caused by HIV-1 infection in nonpregnant individuals is consistent with the progressive and profound impairment of CD4+ T lymphocytes caused by infection with HIV (8, 15). IL-2 deficiency is associated with antigen-specific anergy. This immune suppression increases the risk for opportunistic infections, morbidity, and mortality in HIV-infected subjects (4, 10, 33). Some studies to date have shown that intermittent doses of IL-2 in HIV-infected patients resulted in increases in CD4+ lymphocyte counts (1, 4, 10, 14, 20, 21, 28, 33) and decreased morbidity, (14, 28) without causing a significant increase in viral replication from dormant HIV-infected lymphocytes (1). These observations have led some HIV experts to advocate the use of this immune modulator in addition to antiretroviral therapy in selective cases to increase CD4+ lymphocyte counts and improve immune response to infections (1, 20, 28, 33).
Data to support the use of IL-2 supplementation therapy in HIV-infected pregnant women are lacking, yet HIV-1 seroprevalence among women of childbearing age remains high. Within the United States, a national prevalence of 1.7 per 1,000 live births has been reported, with even higher rates among women with no prenatal care (11). Advanced immune suppression has been observed among some pregnant women, requiring initiation of antiretroviral therapy to avert maternal morbidity and mortality or to decrease the risk of perinatal HIV transmission (9, 24, 29, 30; Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents and Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions To Reduce Perinatal HIV-1 Transmission in the United States, HIV/AIDS Treatment Information Service [ATIS] website at http://www.hivatis.org). Although IL-2 has been used for immune reconstitution of HIV-infected individuals, its clinical usefulness during pregnancy is uncertain. The risk/benefit ratio of such a therapy would depend on the degree of toxicity related to its use and the expectation that IL-2 therapy would not interfere with the normal physiological response of pregnancy.
There is not yet a full understanding of IL-2 production and its' role during pregnancy and postpartum. However, some studies of IL-2 production in normal human pregnancies suggest a decrease in IL-2 expression and Th1 cytotoxic responses and a concurrent increase in Th2 cytokine responses. These factors may play a role in maternal tolerance of the fetal allograft (7, 17, 27, 32). In addition, at least one murine model suggests that cytokine production during pregnancy favors antibody production over cytotoxic responses (13). Although this pattern is likely to reflect the need to protect the animal model fetal allograft during gestation, it disrupts the normal capacity of the pregnant mice to resist infections that require cytotoxic T-cell responses. If the same pattern were true of all human pregnancies, we would expect a decrease in IL-2 production, associated with an increase in IL-4 and IL-6, during pregnancy. Therefore, coexistence of HIV infection and pregnancy could further compromise IL-2 production and the strength of the immune response in women, making recombinant IL-2 supplementation a very intriguing treatment strategy for this population.
The primary objective of this study was to evaluate and describe IL-2 production patterns in pregnant, nonpregnant, HIV-infected, and uninfected women. Our study was designed to reach this objective by (i) evaluating intracellular IL-2 production in HIV-infected pregnant women and seronegative counterparts; (ii) evaluating the effect of immunological and virological factors on intracellular IL-2 production in pregnant women compared with nonpregnant women; (iii) assess the association between intracellular IL-2 production and other Th1 or Th2 cytokines, such as TNF-α, IFN-γ, and IL-4; and (iv) ascertain the effect of pregnancy trimesters on intracellular IL-2 production patterns.
MATERIALS AND METHODS
This study received Institutional Review Board approval and was conducted at University Hospital, Newark, N.J., between September 1996 and January 1998. Women giving written informed consent were enrolled to one of the following study groups: (i) HIV-positive pregnant women, (ii) HIV-negative pregnant women, (iii) HIV-positive nonpregnant women, or (iv) HIV-negative nonpregnant women. Enzyme-linked immunosorbent assay (ELISA) and Western blot serology determined the HIV status for study participants. All HIV-positive patients were assigned to a clinical category based on the 1993 Centers for Disease Control and Prevention Revised Classification for HIV-Infection in Adolescents and Adults (6). At each study visit, in addition to their routine clinical care, participants had peripheral whole blood drawn for measurement of intracellular cytokine levels and lymphocyte profiles. Demographic and clinical information, including age, race/ethnicity, gestational age, last menstrual period, history of drug use, and toxicology screen, was abstracted from the medical record. A substance user was defined as a patient who acknowledged using drugs or had a positive toxicology screen during the pregnancy. All HIV-related clinical information, including medications and HIV viral load determined within a week of study visit, was also abstracted from the medical record. Pregnant patients were approached for enrollment, regardless of gestational age, as soon as their HIV status was known. Those who consented to participate in the study then completed their study entry visit. Subsequent visits were scheduled every trimester thereafter, with the last evaluation occurring 4 to 6 weeks postpartum. Pregnant patients had at least two evaluations. However, for purposes of analysis, samples were grouped based on gestational age into the following study time points: 0, <20 weeks; 1, 26 ± 2 weeks; 2, 36 ± 2 weeks; and 3, 6 ± 2 weeks postpartum. Nonpregnant women were evaluated only once at a clinical visit, and their enrollment required a history of normal menstrual periods and no use of hormonal contraception or immune-suppressive therapies at the time of enrollment. All virologic and immunologic assays, including lymphocyte phenotyping and plasma HIV RNA quantitation by PCR, were done in an AIDS Clinical Trial Group-certified laboratory. A detailed description of the methodology to complete these assays has been published previously (2, 5).
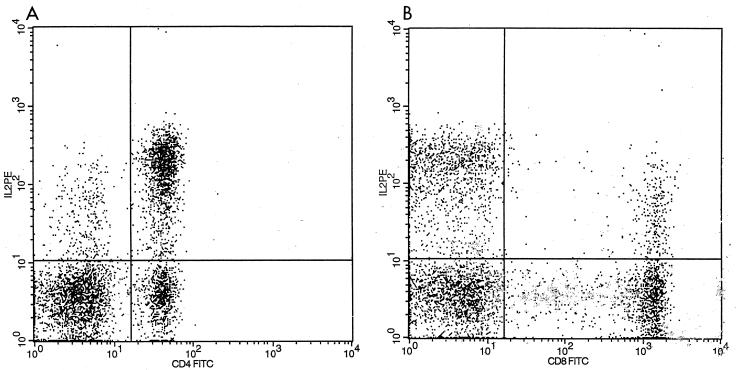
A modification of previously published methodology was used to evaluate all intracellular cytokine production of IL-2, INF-γ, IL-4, and TNF-α, and that procedure is briefly described here (16, 19, 25). Heparinized whole blood (0.5 ml) was diluted 1:1 in RPMI 1640 with l-glutamine. A mixture containing 20-μg/ml 12-phorbol myristate 13-acetate (PMA), 1-μg/ml ionomycin, and 10-μg/ml brefeldin A (BFA) was added to each whole-blood sample and vortexed lightly. The samples were incubated for 4 h at 37°C in 5% CO2 to activate. One hundred microliters of the activated blood was then aliquoted into small tubes for surface staining. Appropriate monoclonal antibodies (MAbs; 10 μl of fluorescein isothiocyanate [FITC]-conjugated CD4 and CD8 PerCP) from Pharmingen (San Diego, Calif.) were added to each tube, and these mixtures were vortexed and incubated for 15 min at room temperature. Samples were then lysed with 2 ml of prewarmed NH4Cl for 5 min, spun at 1,130 × g for 5 min, and then washed with 2 ml of 0.5% fetal bovine serum (FBS) in phosphate-buffered saline (PBS). Permeabilization buffer (500 μl) was added to each of the tubes, which were then vortexed and incubated for 15 min at room temperature. Samples were then washed with 2 ml of 0.5% FBS-PBS. MABs were added for intracellular staining (10 μl of phycoerythrin [PE]-conjugated immunoglobulin G2a [IgG2a] and 2.5 μl of IL-2-PE). Specimens were then incubated for 30 min at room temperature, after which 2 ml of 0.5% FBS-PBS was spun at 1,130 rpm for 5 min. Finally, the specimens were fixed with 500 μl of 1% formaldehyde before being acquired and analyzed by flow cytometry with a FACScan (Becton-Dickinson, Paramus, N.J.) (Fig. 1).
FIG. 1.
Flow cytometric dot plots of subset-specific single-cell cytokine expression following stimulation with PMA-ionomycin in the presence of BFA. (A) IL-2 distribution on CD4+ cells. Cells in the upper quadrants show cytokine expression (upper right, CD4+ IL-2 positive, 65%). (B) IL-2 distribution on CD8+ cells. The rate of CD8+ IL-2 positive in the upper right quadrant is 21%. The lower quadrants of each figure show cells expressing the lineage marker without cytokine expression. TC, T cells.
Statistical Analysis System, version 6.09, software (SAS, Cary, N.C.) was used for statistical analysis. For data analysis, the samples of pregnant women were grouped into the following study time points: 0, <20 weeks; 1, 26 ± 2 weeks; 2, 36 ± 2 weeks; or 3, 6 ± 2 weeks postpartum. All pregnant women had a minimum of two samples available for analysis. The IL-2 measurement for nonpregnant women was included under time point 3, which physiologically coincided with the postpartum visit for women who were enrolled while pregnant. A Student's t test was used to compare demographic characteristics of HIV-positive and -negative women. We used nonparametric analyses to compare the differences in IL-2 production among four groups of patients within our study: (i) HIV-infected and uninfected women regardless of pregnancy status, (ii) pregnant and nonpregnant women, (iii) HIV-infected pregnant and nonpregnant women, and (iv) substance users and nonusers. We used Wilcoxon scores (rank sums) to evaluate changes in IL-2 production within the same patient during pregnancy and postpartum. A chi-square analysis was used to assess the association between HIV serostatus, substance use, viral load range, and IL-2 production. Spearman's correlation coefficient was used to assess the correlation of IL-2 intracellular production and that of other cytokines, specifically TNF-α, INF-γ, and IL-4. Spearman's correlation coefficient was also used to assess the correlation between IL-2 production and CD4 or CD8 lymphocyte counts and between IL-2 production and HIV-1 viral load. Except for the chi-square analysis, all tests were two tailed. Finally, to assess the relative importance of individual variables in predicting intracellular CD4 IL-2 production, a linear regression analysis was done, which included HIV status, pregnancy status, CD4 count of 350 cells/mm3, viral load of 1,000 copies/ml, and drug use as pertinent variables. A P value of < 0.05 was considered significant.
RESULTS
During the study period of 1996 through 1998, 61 women were enrolled in this study. Among these women, 39 were pregnant (20 HIV positive, 19 HIV negative) and 22 were nonpregnant (11 HIV positive and 11 HIV negative) at the time of enrollment. Thirty-one of the enrolled women were HIV infected. Table 1 illustrates other demographic characteristics of the study population. All participants belong to racial or ethnic minority groups, with self-identified blacks comprising 90% (55 of 61) of the study population, and self-identified Latinas comprising the remaining 10% (6 of 61).
TABLE 1.
Demographic characteristics for women enrolled in the study
| Parameter | HIV status
|
P value | |
|---|---|---|---|
| Seropositive | Seronegative | ||
| Mean age in yr (SD)a | 28 (7) | 24 (5) | 0.04 |
| Ethnicity (n) | |||
| Black | 29 | 26 | |
| Latina | 2 | 4 | |
| Parity | 2.5 ± 2.7 | 0.3 ± 0.7 | 0.0001 |
| Pregnant during study (n) | 20 | 19 | |
| Current or past substance use (n) | 12 | 0 | 0.00013 |
| Total enrolled (n) | 31 | 30 | |
SD, standard deviation.
Among enrolled women who were HIV infected, most (23 of 31 [74%]) were asymptomatic, and only 8 of 31 (16%) met the Centers for Disease Control and Prevention criteria for advanced HIV disease (6). At study time point 0 (<20 weeks of gestation), only 28% (4 of 14) of the HIV-infected pregnant women were on antiretroviral therapy. Two of these patients were on two nucleoside reverse transcriptase inhibitors (NRTIs), and the others were on monotherapy or highly active antiretroviral therapy (HAART), respectively. However, by study point 3 (the postpartum visit), 88% (14 of 16) were receiving combination antiretroviral therapy. Eleven of these patients were on two NRTIs, and the remainder of them were on HAART. Among the patients who had viral load determined for clinical evaluation purposes, only 1 of 7 (14%) had an HIV load of less than 1,000 copies/ml at the initial visit (study time point 0) compared to 5 of 6 (83%) who had viral loads of less than 1,000 copies/ml by the postpartum visit. HIV-infected women were more likely to have a current or past history of substance use (P = 0.00013), be older (28 ± 7 years [n = 31] versus 24 ± 5 years [n = 30], P = 0.04), and of higher parity (P = 0.0001) than their HIV-negative counterparts (Table 1). HIV-infected women also had significantly lower CD4 lymphocyte counts (421 ± 210 cells/mm3) than the uninfected women (892 ± 262 cells/mm3, P = 0.0006). Among enrolled women, the mean CD8 counts were significantly different for HIV-infected and HIV-uninfected women, with mean counts of 1,186 ± 1,143 cells/mm3 and 618 ± 236 cells/mm3 (P = 0.02), respectively. As expected, HIV-infected women had lower mean absolute CD4 lymphocyte counts and higher CD8 lymphocyte counts than noninfected women.
Among healthy nonpregnant HIV-negative women, the mean intracellular IL-2 production levels in CD4 and CD8 were 52% ± 8% and 15.6% ± 3%, respectively. The HIV-infected nonpregnant women in our cohort produced significantly less intracellular CD4− IL-2 (45% ± 8%) than their HIV-uninfected counterparts (52% ± 8%, P = 0.04). On the other hand, we observed no significant differences in intracellular CD4 IL-2 or CD8 IL-2 production between HIV-infected and uninfected pregnant women in our cohort when measured cross-sectionally (Tables 2 and 3). Furthermore, there were no temporal variations in intracellular IL-2 measurements within each group at various study time points during the pregnancy (Tables 2 and 3).
TABLE 2.
Intracellular CD4 IL-2 production in HIV-infected and uninfected women during pregnancy and postpartum
| Group | % CD4 IL-2 production (n) at time pointa:
|
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| HIV− | 49.9 ± 7.2 (8) | 54.3 ± 5.2 (9) | 49.1 ± 7.5 (10) | 52.3 ± 7.9 (12) |
| HIV+ | 47.5 ± 7.1 (13) | 48.8 ± 10.9 (13) | 51.4 ± 9.4 (15) | 47.7 ± 7.3 (27) |
| P value | 0.586 | 0.056 | 0.9557 | 0.1603 |
Shown are the mean ± standard deviation percentage of cells expressing IL-2 for n observations. Study time points: 0, < 20 weeks gestation; 1, 24 to 28 weeks gestation; 2, 34 to 38 weeks gestation; 3, 6 ± 2 weeks postpartum and nonpregnant women.
TABLE 3.
Intracellular CD8 IL-2 production in HIV-infected and uninfected women during pregnancy and postpartum
| Group | % CD8 IL-2 production (n) at time pointa:
|
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| HIV− | 15.7 ± 3.2 (12) | 19.2 ± 6.9 (9) | 14.5 ± 3.9 (10) | 16.3 ± 5.3 (12) |
| HIV+ | 14.2 ± 5.2 (13) | 14.4 ± 5.6 (13) | 15.8 ± 4.9 (15) | 14.5 ± 6.4 (27) |
| P value | 0.3391 | 0.06 | 0.4167 | 0.3126 |
Shown are the mean ± standard deviation percentages of cells expressing IL-2 for n observations. Study time points: 0, <20 weeks gestation; 1, 24 to 28 weeks gestation; 2, 34 to 38 weeks gestation; 3, 6 ± 2 weeks postpartum and nonpregnant women.
To examine the effect of pregnancy among HIV-infected women, we analyzed data for HIV-infected women seen postpartum (16/20 [80%] of those enrolled during pregnancy completed a postpartum visit) compared to HIV-infected women who were never pregnant (n = 11). No significant difference in intracellular CD4 IL-2 production was observed between these two groups (49% ± 6% versus 45% ± 8%, P = 0.12). To evaluate the effect of pregnancy on IL-2 production among HIV-infected women, we did paired analyses among women who had both an early pregnancy visit (prior to 20 weeks gestation) and a postpartum evaluation (n = 11). In these analyses, we observed no significant difference in intracellular IL-2 production between the two study time points (48% ± 7% versus 47% ± 5%, P = 0.53).
All drug users in our cohort were HIV-infected women. To evaluate the effect of drug use on IL-2 production, we compared IL-2 production among HIV-infected women with (n = 4) and without (n = 9) drug use at baseline. We observed no significance difference in IL-2 production among HIV-infected women with or without drug use (44% ± 6% versus 48% ± 7%, P = 0.24), although this may be due to the small sample size.
There was a statistically significant positive correlation between intracellular CD4 IL-2 production and older age (r = −0.4782, P = 0.01), earlier stage of disease (r = 0.5685, P = 0.0072), and antiretroviral use (r = 0.6770, P = 0.03) among HIV-infected women at study point 3. We also observed a statistically significant correlation between intracellular CD4 IL-2 production and production of TNF-α (r = 0.7245, P = 0.0002) and INF γ (r = 0.4599, P = 0.03) by the CD4 cell population. There was no significant correlation between intracellular CD4 IL-2 production and CD4 IL-4 production among HIV-infected patients (r = −0.1120, P = 0.7), but this correlation was significant among HIV-negative patients (r = 0.6363, P = 0.02).
An intriguing finding from the regression analysis was that among HIV-infected pregnant women, CD4+ T lymphocytes accounted for 42% of the CD4 IL-2 variability, although this was not statistically significant—likely due to the small sample size (P = 0.113).
DISCUSSION
Our data confirmed that intracellular IL-2 production is higher in HIV-negative nonpregnant women than their HIV-positive nonpregnant counterparts. However, this HIV-related effect was not seen among pregnant women in our cohort. The fact that we did not observe the same difference among pregnant women suggests that pregnancy may down-regulate intracellular IL-2 production regardless of HIV status. These observations are compatible with at least one study that demonstrated that the production of IL-2 by in vitro mitogen-stimulated peripheral blood mononuclear cells is decreased in successful human pregnancies and that antigen-stimulated IL-2 production is decreased in all trimesters of pregnancy compared that in nonpregnant controls (22). However, since we observed no significant change in IL-2 production throughout pregnancy or postpartum, our study findings do differ with the Marzi observation of a type 1-to-type 2 shift in the cytokine profile only during the third trimester of pregnancy. This discrepancy could be explained by the fact that different methodologies were used to assess cytokine production (intracellular versus stimulation of peripheral blood mononuclear cells), or it could reflect the unique effect of HIV infection on the immune system (18).
Also, although most HIV-infected women in our study group were receiving combination antiretroviral therapy at the final study evaluation (postpartum visit), there were no significant increases in production of IL-2 despite well-documented viral responses. This could be because the length of antiviral therapy was short, not allowing for adequate immune reconstitution or because antiretroviral therapy does not always confer complete immune reconstitution. This concept is consistent with prior observations that IL-2 supplementation could further increase CD4 counts by decreasing apoptosis (4, 33).
The hormonal secretion characteristics of normal pregnancy are likely to influence cytokine production by activated T lymphocytes. Dudley and collaborators have shown that, in a murine model, cytokine production during pregnancy favors antibody production over cytotoxic responses; they found a decrease in IL-2 production, associated with an increase in IL-4 and IL-6 as gestation advanced (13). This pattern is likely to reflect the need to protect the fetal allograft during gestation. However, it disrupts the normal capacity of the pregnant mice to resist infections that require cytotoxic T-cell responses. If the same pattern occurs in humans, we can anticipate that the physiological responses of pregnancy will alter the capacity to resist HIV infection since the cytotoxic response will be compromised. The fact that we did not observe a decrease in IL-2 production in HIV-infected pregnant women when compared to their seronegative counterparts, which was seen among the nonpregnant women, highlights the potential role that pregnancy can exert on cytokine regulation. It is therefore important to ascertain the effects of pregnancy and pathological states, including infections, in cytokine modulation, since these agents have been proposed as potential treatment adjuvants for HIV-infected patients.
This pilot study contributes to the limited information available regarding cytokine activation in normal and abnormal pregnancies. We observed that some of the anticipated correlations of IL-2 production with other TH-1 cytokines are absent in HIV-infected women but present in HIV-uninfected women. This finding highlights the fact that HIV infection alters the expected physiological immune responses. Our data also support the correlation of IL-2 production with other cytokines, like TNF and IFN, as demonstrated in previous studies (13, 22, 27).
In addition, our study demonstrates that, although CD4 accounted for 42% of the CD4 IL-2 variability, there was no significant difference in IL-2 production for patients above or below that threshold of 350 cells/mm3, which is the value used to initiate antiretroviral therapy. So, there is no CD4 cutoff at which the IL-2 production is worse or better, at least using 350 as a cutoff.
The fact that we did not find a significant alteration in IL-2 production among women who admitted to current or past drug use within our study group is also an interesting finding. However, our sample size was very small, and as drug use is often a documented exposure among HIV-infected women in this country, it will remain important to include this group in future studies to better understand the impact of drug use on immune function and possible IL-2 supplementation therapies.
Our study is limited by several factors. First, an ideal comparison of IL-2 levels between the same patients in their pregnant and nonpregnant states would provide the best information regarding the effect of pregnancy on the IL-2 levels. Unfortunately, only a few patients were available for a complete postpartum evaluation in our group of HIV-negative pregnant women, making this type of comparison not possible. Second, we only evaluated IL-2 production 6 weeks after delivery, although some physiological changes of pregnancy can last up to 12 weeks after delivery. To further evaluate and clarify the lack of IL-2 production change among HIV-infected compared to uninfected pregnant women, future studies should either look at later postpartum (12 weeks) data or a larger sample size.
Our study adds to the limited body of data regarding the immune mechanisms which develop or may shift during pregnancy (3). Although, IL-2 supplementation therapy used in conjunction with appropriate antiretroviral therapy has been advocated to increase immune reconstitution in selective cases, the use of this agent during pregnancy should be carefully evaluated. If larger studies confirm our observations of a decreased IL-2 level among pregnant women, it is likely that this cytokine could play a role in the required physiological response to maintain the fetal allograft. Thus, the risks may outweigh the benefits of IL-2 supplementation therapy as a mechanism to enhance the immune response among pregnant women, and therefore such IL-2 supplementation may not be favorable. In other words, we need to better understand the implications of such supplementation to the survival of the fetus in utero among both HIV-infected and -uninfected women.
Acknowledgments
This work was supported in part by the Flow Cytometry Multi-Parameter Analysis/Sorting grant no. 1S10RR13008-01 from the National Center for Research Resources, National Institutes of Health.
We thank all of the participating patients, research nurses, residents, and staff for their selfless contributions toward the completion of this study.
REFERENCES
- 1.Abrams, D. I., J. D. Bebchuk, E. T. Denning, R. T. Davey, L. Fox, H. C. Lane, J. Sampson, R. Verheggen, D. Zeh, and N. P. Markowitz. 2002. Randomized, open-label study of the impact of two doses of subcutaneous recombinant interleukin-2 on viral burden in patients with HIV-1 infection and CD4+ cell counts of > or = 300/mm3: CPCRA 059. J. Acquir. Immune Defic. Syndr. 29:221-231. [DOI] [PubMed] [Google Scholar]
- 2.AIDS Clinical Trials Group. 1993. Virology manual for laboratories. Division of AIDS, National Institutes of Allergy and Infectious Diseases, Bethesda, Md.
- 3.Burns, D. N., P. Nourjah, D. J. Wright, H. Minkoff, S. Landesman, A Rubinstein, J. J. Goedert, and R. P. Nugent. 1999. Changes in immune activation markers during pregnancy and postpartum. J. Reprod. Immunol. 42:147-165. [DOI] [PubMed] [Google Scholar]
- 4.Caggiari, L., S. Zanussi, M. T. Bortolin, M. D'andrea, G. Nasti, C. Simonelli, U. Tirelli, and P. De Paoli. 2000. Effects of therapy with highly active anti-retroviral therapy (HAART) and Il-2 on CD4 and CD8 lymphocyte apoptosis in HIV+ patients. Clin. Exp. Immunol. 120:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvelli, T., T. N. Denny, H. Paxton, R. Gelman, and J. Kagan. 1993. Guidelines for flow cytometry immunophenotyping. A report from the National Institute of Allergy and Infectious Diseases/ Division of AIDS. J. Cytom. 14:702-715. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1992. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morb. Mortal. Wkly. Rep. 41:RR-17. [PubMed]
- 7.Chao, K. H., M. Y. Wu, J. H. Yang, S. U. Chen, Y. S. Yang, and H. N. Ho. 2002. Expression of the interleukin-2 receptor alpha (CD25) is selectively decreased on decidual CD4(+) and CD8(+) T lymphocytes in normal pregnancies. Mol. Hum. Reprod. 8:667-673. [DOI] [PubMed] [Google Scholar]
- 8.Clerici, M., F. T. Hakim, D. J. Venzon, S. Blatt, C. W. Hendrix, T. A. Wynn, and G. M. Shearer. 1993. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J. Clin. Investig. 91:759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clerici, M., A. V. Sison, J. A. Berzofsky, T. A. Rakusan, C. D. Brandt, M. Ellaurie, M. Villa, C. Colie, D. J. Venzon, J. L. Sever et al. 1993. Cellular immune factors associated with mother-to-infant transmission of HIV. AIDS 7:1427-1433. [DOI] [PubMed] [Google Scholar]
- 10.Davey, R. T., R. L. Murphy, F. M. Graziano, S. L. Boswell, A. T. Pavia, M. Cancio, J. P. Nadler, D. G. Chaitt, R. L. Dewar, D. K. Sahner, A. M. Duliege, W. B. Capra, W. P. Leong, M. A. Giedlin, H. C. Lane, and J. O. Kahn. 2000. Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy. A randomized controlled trial. JAMA 284:183-189. [DOI] [PubMed] [Google Scholar]
- 11.Davis, S. F., D. H. Rosen, S. Steinberg, P. M. Wortley, J. M. Karon, and M. Gwinn. 1998. Trends in HIV prevalence among childbearing women in the United States, 1989-1994. J. Acquir. Immune Defic. Syndr. 19:158-164. [DOI] [PubMed] [Google Scholar]
- 12.Denny, T. N. 2001. Cytokines: a common signaling system for cell growth inflammation, immunity, and differentiation, p. 29-78. In I. Cohen and L. Segal (ed.), Design principles for the immune systems and other distributed autonomous systems. Oxford University Press, Santa Fe, N.Mex.
- 13.Dudley, D. J., C. Chen, M. D. Mitchell, R. A. Daynes, and B. A. Araneo. 1993. Adaptive immune responses during murine pregnancy: pregnancy-induced regulation of lymphokine production by activated T lymphocytes. Am. J. Obstet. Gynecol. 168:1155-1163. [DOI] [PubMed] [Google Scholar]
- 14.Emery, S., W. B. Capra, D. A. Cooper, R. T. Mitsuyasu, J. A. Kovacs, P. Vig, M. Smolskis, L. D. Saravolatz, H. C. Lane, G. A. Fyfe, and P. T. Curtin. 2000. Pooled analysis of 3 randomized, controlled trials of interleukin-2 therapy in adult human immunodeficiency virus type 1 disease. J. Infect. Dis. 182:428-434. [DOI] [PubMed] [Google Scholar]
- 15.Fauci, A. S. 1993. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science 262:1011-1018. [DOI] [PubMed] [Google Scholar]
- 16.Gruber, R., C. Reiter, and G. Riethmuller. 1993. Triple immunofluorescence flow cytometry, using whole blood, of CD4+ and CD8+ lymphocytes expressing CD45RO and CD45RA. J. Immunol. Methods 163:173-179. [DOI] [PubMed] [Google Scholar]
- 17.Gucer, F., P. Balkanli-Kaplan, M. Yuksel, N. C. Sayin, M. A. Yuce, and T. Yardim. 2001. Maternal serum levels of tumor necrosis factor-alpha and interleukin-2 receptor in threatened abortion: a comparison with normal and pathologic pregnancies. Fertil. Steril. 76:707-711. [DOI] [PubMed] [Google Scholar]
- 18.Hauser, G. J., A. Lidor, V. Zakuth, H. Rosenberg, T. Bino, M. P. David, and Z. Spirer. 1987. Immunocompetence in pregnancy: production of interleukin-2 by peripheral blood lymphocytes. Canc. Detect. Prev. 1987(Suppl. 1):39-42. [PubMed] [Google Scholar]
- 19.Jung, T., U. Schauer, C. Heusser, C. Neumann, and C. Rieger. 1993. Detection of intracellular cytokines by flow cytometry. J. Immunol. Methods 159:197-207. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs, J. A., S. Vogel, J. M. Albert, J. Falloon, R. T. Davey, Jr., R. E. Walker, M. A. Polis, K. Spooner, J. A. Metcalf, M. Baseler, G. Fyfe, and H. C. Lane. 1996. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N. Engl. J. Med. 335:1350-1356. [DOI] [PubMed] [Google Scholar]
- 21.Losso, M. H., W. H. Belloso, S. Emery, J. A. Benetucci, P. E. Cahn, M. C. Lasala, G. Lopardo, H. Salomon, M. Saracco, E. Nelson, M. G. Law, R. T. Davey, M. C. Allende, and H. C. Lane. 2000. A randomized, controlled, phase II trial comparing escalating doses of subcutaneous interleukin-2 plus antiretrovirals versus antiretrovirals alone in human immunodeficiency virus-infected patients with CD4+ cell counts >/=350/mm3. J. Infect. Dis. 181:1614-1621. [DOI] [PubMed] [Google Scholar]
- 22.Marzi, M., A. Vigano, D. Trabattoni, M. L. Villa, A. Salvaggio, E. Clerici, and M. Clerici. 1996. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin. Exp. Immunol. 106:127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul, W. E., and R. A. Seder. 1994. Lymphocyte responses and cytokines. Cell 76:241-251. [DOI] [PubMed] [Google Scholar]
- 24.Peckham, C., and D. Gibb. 1995. Mother-to-child transmission of the human immunodeficiency virus. N. Engl. J. Med. 333:298-302. [DOI] [PubMed] [Google Scholar]
- 25.Picker, L. J., M. K. Singh, Z. Zdraveski, J. R. Treer, S. L. Waldrop, P. R. Bergstresser, and V. C. Maino. 1995. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood 86:1408-1419. [PubMed] [Google Scholar]
- 26.Poli, G., and A. S. Fauci. 1992. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res. Hum. Retrovir. 2:191-197. [DOI] [PubMed] [Google Scholar]
- 27.Rich, K. C., J. N. Siegel, C. Jennings, R. J. Rydman, and A. L. Landay. 1995. CD4+ lymphocytes in perinatal human immunodeficiency virus (HIV) infection: evidence for pregnancy-induced immune depression in uninfected and HIV-infected women. J. Infect. Dis. 172:1221-1227. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz, D. H., G. Skowron, and T. C. Merigan. 1991. Safety and effects of interleukin-2 plus zidovudine in asymptomatic individuals infected with human immunodeficiency virus. J. Acquir. Immune Defic. Syndr. 4:11-23. [PubMed] [Google Scholar]
- 29.Thomas, P. A., J. Weedon, K. Krasinski, E. Abrams, N. Shaffer, P. Matheson, M. Bamji, A. Kaul, D. Hutson, K. T. Grimm, et al. 1994. Maternal predictors of perinatal human immunodeficiency virus transmission. Pediatr. Infect. Dis. J. 13:489-495. [DOI] [PubMed] [Google Scholar]
- 30.Thorne, C., M. L. Newell, D. Dunn, and C. Peckham. 1995. The European collaborative study: clinical and immunological characteristics of HIV 1-infected pregnant women. Br. J. Obstet. Gynecol. 102:869-875. [DOI] [PubMed] [Google Scholar]
- 31.Thorpe, R. 1998. Interleukin 2, p. 19-33. In A. Mire-Sluis and R. Thorpe (ed.), Cytokines. Academic Press, New York, N.Y.
- 32.Veenstra van Nieuwenhoven, A. L., A. Bouman, H. Moes, M. J. Heineman, L. F. de Leij, J. Santema, and M. M. Faas. 2002. Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertil. Steril. 77:1032-1037. [DOI] [PubMed] [Google Scholar]
- 33.Zanussi, S., C. Simonelli, M. Bortolin, M. D'Andrea, C. Crepaldi, E. Vaccher, G. Nasti, D. Politi, L. Barzan, U. Tirelli, and P. De Paoli. 1999. Immunological changes in peripheral blood and in lymphoid tissue after treatment of HIV-infected subjects with highly active anti-retroviral therapy (HAART) or HAART + IL-2. Clin. Exp. Immunol. 116:486-492. [DOI] [PMC free article] [PubMed] [Google Scholar]