Abstract
Recently, noninvasive diagnostic tests for Helicobacter pylori infection have gained in significance. We have developed a sensitive and specific noninvasive immunoassay based on the detection of an H. pylori circulating antigen (HpCA) in sera from H. pylori-infected individuals. Monospecific antibody and Western blot analyses were used to demonstrate the presence of the target antigen in H. pylori cell lysate and serum samples. A novel enzyme-linked immunosorbent assay (ELISA) was developed for the detection of HpCA in serum. Endoscopic biopsy specimens from the gastric antra of 221 individuals (143 males and 78 females) with dyspeptic symptoms were evaluated for H. pylori infection, with culture used as a “gold standard” for diagnosis. The target H. pylori antigen was identified at 58 kDa. HpCA has been detected by ELISA with high degrees of sensitivity, specificity, and efficiency (>90%), and ELISA results show no significant difference (P > 0.05) from results of H. pylori culture of gastric biopsy specimens. The test's positive and negative predictive values were also high (95 and 86%, respectively). In conclusion, a sensitive and specific immunoassay was developed for the detection of HpCA in human serum. This test can be applied for noninvasive laboratory and field diagnoses of H. pylori infection.
Helicobacter pylori is a common bacterial infection in humans that is responsible for a variety of gastroduodenal pathologies, peptic and gastric ulcers, mucosa-associated lymphoid tissue lymphoma, and gastric carcinoma (7, 7a, 14, 25). Several tests can be used to diagnose H. pylori infection; the selection of the appropriate test depends on the clinical setting (12, 18). H. pylori infection can be diagnosed by tests requiring upper gastrointestinal endoscopy for the retrieval of a gastric biopsy specimen (microbiological culture, histological examination, and rapid urease tests). These methods have high sensitivities and specificities (10), yet the invasiveness and expense of direct observation of the organism have led to a search for valid and reliable noninvasive alternatives (33). During recent years, noninvasive diagnostic tests for H. pylori infection have gained in significance (29). Although PCR, a powerful method known for its high sensitivity, can detect low numbers of H. pylori and has been used to follow up eradication therapy, PCR requires specialized laboratory facilities and is not generally available as a routine diagnostic test (13). The urea breath test has been the most widely used accurate noninvasive test, both in the pretreatment examination of infected individuals and for early-posttreatment follow-up, and meets the requirements for such a test (9). However, the performance of the test has been associated with some disadvantages. Although it is less costly than endoscopy, the urea breath test requires a specialized technician and expensive instrumentation that is not available in routine clinical laboratories (a scintillation counter or a mass spectrometer) for analyzing and handling the radioactive isotope in a specific way; also, patients may be hesitant to ingest radioactive test material (31). Enzyme immunoassays have been used to detect H. pylori infection in human secretions, such as feces, urine, and saliva (14). Such assays are attractive in comparison with other noninvasive methods because they are simple, inexpensive, and less of a burden for the patient (1, 15). Here, we have identified an H. pylori antigen in the sera from infected individuals and described the development of an antigen detection enzyme-linked immunosorbent assay (ELISA) suitable for the laboratory diagnosis of and the screening of large populations for H. pylori infection.
MATERIALS AND METHODS
Clinical specimens.
A total of 221 individual serum samples were collected in the Gastro-Enterology and Surgery Center, Mansoura University, Mansoura, Egypt. The individuals were 143 males aged 14 to 74 years (mean, 40.83 ± 11.72 years; median, 42 years) and 78 females aged 16 to 75 years (mean, 41.69 ± 13.81 years; median, 39.5 years). All sera were stored at −20°C until used. All patients underwent upper gastroduodenal endoscopy, and multiple gastric biopsy specimens were taken from the antrum and then processed for microbiological culture of H. pylori. The endoscopy showed 69 gastritis cases (31.2%), 21 gastric erosion cases (9.5%), 8 gastric ulcer cases (3.6%), 17 duodenitis cases (7.7%), 4 duodenal erosion cases (1.8%), 32 duodenal ulcer cases (14.5%), and 70 normal endoscopic mucosa cases (31.7%). The Ethical Committee of the Gastro-Enterology and Surgery Center, Mansoura University, approved the present study. Informedconsent was obtained from all participants, and they were fully informed of the diagnostic procedures involved and nature of the disease. Most of these subjects had received no antimicrobial therapies during the previous 3 months.
Microbiological culture.
H. pylori was cultured by rubbing the gastric biopsy specimens of Egyptian patients onto Columbia agar plates supplemented with lysed horse blood (5%) and Skirrow's supplement containing vancomycin, trimethoprim lactate, cefsulodin, and amphotericin B (Oxoid, Basingstoke, United Kingdom). Agar plates were incubated at 37°C for 4 to 7 days in a microaerophilic atmosphere (5% O2, 10% CO2, 85% N2, and 99% relative humidity) provided by a CO2 incubator (Heraeus Instruments, Berlin, Germany). The microorganism was identified as H. pylori by the standard methods, on the basis of colony morphology, Gram staining, and the production of urease, catalase, and oxidase enzymes (11).
Preparation of H. pylori cell lysate.
Bacterial cells were harvested, washed three times in phosphate-buffered saline (PBS; pH 7.2), and disrupted by sonication three times at 4°C for 15 s each time at 47 kHz with a Bransonic ultracleaner (B-1200 E-1; Branson Ultrasonic Corporation, Danbury, Conn.). After centrifugation at 10,000 rpm (J2-HS; Beckman Instruments, Inc., Fullerton, Calif.) for 10 min at 4°C, the protein content of the supernatant solution was determined with the use of bovine serum albumin as a standard (17). The supernatant was split into aliquots and stored at −20°C until used.
Production of anti-H. pylori antibody.
A group of three New Zealand rabbits were immunized subcutaneously at three different inoculation sites with 500 μg of H. pylori cell lysate diluted (by volume) with Freund's complete adjuvant. Another group of three New Zealand rabbits were immunized with 500 μg of the purified 58-kDa H. pylori antigen diluted (by volume) with Freund's complete adjuvant. On day 15, the rabbits were immunized again with the same dose of antigen emulsified with incomplete Freund's adjuvant. On day 28, rabbits were immunized with one more dose of antigen with incomplete adjuvant, and they were sacrificed 4 days later. Blood samples were collected from all rabbits on day 0 and at 28 and 32 days after immunization, and sera were separated. The reactivity of the collected sera was tested against serial concentrations of H. pylori lysate and the purified 58-kDa antigen by using an indirect ELISA and anti-rabbit immunoglobulin G (IgG) alkaline phosphatase conjugate (Sigma). Incubation with the enzyme substrate produced a color in proportion to the amount of anti-H. pylori IgG antibodies present.
SDS-PAGE and gel electroelution.
Various samples (see Results) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), at 50 μg/lane, using vertical slabs of 12 or 16% polyacrylamide (16). Molecular weight standards (Sigma) were run in parallel. After staining with Coomassie blue R-250, the band of interest (58 kDa) was cut, and the antigen was electroeluted from the polyacrylamide gel (2). The protein content of the electroeluted antigen was determined, and the samples were stored at −20°C until used. Initially, the purity of the electroeluted antigen was assessed using SDS-PAGE and Coomassie blue staining. Then, serum samples (diluted 1:100 in 0.05 M Tris buffer containing 200 mM NaCl and Tris-buffered saline [TBS; pH 7.4]) of 10 infected and 5 noninfected individuals, as proven by microbiological culture, were used to confirm the immunogenicity of the purified antigen by using anti-human IgG alkaline phosphatase conjugate (Sigma) and Western blotting.
Western blots.
Samples separated by SDS-PAGE (as described above) were electrotransferred onto a nitrocellulose membrane (0.45-μm pore size; Sigma) in a protein transfer unit (28). The nitrocellulose filter was blocked by 5% (wt/vol) nonfat dry milk dissolved in 0.05 M TBS (pH 7.4), rinsed in TBS, and incubated with rabbit anti-H. pylori lysate antibodies or anti-58-kDa-antigen antibodies diluted in blocking buffer with constant shaking. The blots were then washed, followed by incubation for 2 h with goat anti-rabbit IgG alkaline phosphatase conjugate (Sigma) diluted 1:350 in TBS. After being washed, the blots were soaked in substrate (premixed 5-bromo-4-chloro-3-indolylphosphate [BCIP] and Nitro Blue Tetrazolium in 0.1 M Tris buffer, pH 9.6 [ABC Diagnostics, New Damietta, Egypt]). The color reaction was observed within 10 min, and dipping the blots in distilled water then stopped the reaction.
ELISA for H. pylori circulating antigen (HpCA) in serum.
After optimization of ELISA conditions, each well of polystyrene microtiter plates was coated with 50 μl of a tested human serum sample diluted in carbonate-bicarbonate buffer (pH 9.6). The plates were incubated overnight at room temperature and washed three times with 0.05% (vol/vol) PBS-T20 (pH 7.2), and then free active sites were blocked with 0.2% (wt/vol) nonfat milk in carbonate-bicarbonate buffer. After washing of the plates, 50 μl of the specific antisera/well was added to the 58-kDa antigen diluted 1:100 in PBS-T20, and the mixture was incubated at 37°C for 2 h. After the plates were washed, 50 μl of anti-rabbit IgG alkaline phosphatase conjugate (Sigma)/well diluted in 0.2% (wt/vol) nonfat milk in PBS-T20 was added, and the mixture was incubated at 37°C for 1 h. The amount of coupled conjugate was determined by incubation with 50 μl of p-nitrophenyl phosphate substrate (Sigma)/well for 30 min at 37°C. The reaction was stopped by using 3 M NaOH, and absorbance was read at 405 nm. The cutoff level of the ELISA, above or below which the tested sample is considered positive or negative, was calculated as the mean ELISA optical densities (range, 0.135 to 0.377) of a group of 24 serum samples from noninfected healthy individuals ± 3 standard deviations [i.e., 0.257 ± (3 × 0.047) = 0.398]. The mean absorbance value of a group of 32 H. pylori-positive individuals was 0.751 (range, 0.411 to 1.250).
Characterization of the purified H. pylori antigen.
To determine some of the target antigen's chemical characteristics, samples of the antigen were treated with protease and other chemical reagents and then tested by ELISA to see if these treatments affected the active epitope as described by Attallah et al. (2). Lysate and bovine serum albumin were tested in parallel, as positive and negative controls, respectively.
Statistical analysis.
Standard methods were used to calculate sensitivity, specificity, efficiency, and positive and negative predictive values. All parameters were transferred to an IBM PC-AT-compatible computer for analysis using statistical analysis program package Instate Software for Science, version 2.3 (Graphpad Software, Inc., San Diego, Calif.). The Mann-Whitney U test was used to compare the means of two distributions. Fisher's exact test was used to compare the differences between two proportions. P values (two-tailed test) of <0.05 were considered significant.
RESULTS
Reactivity of the developed anti-H. pylori antibodies.
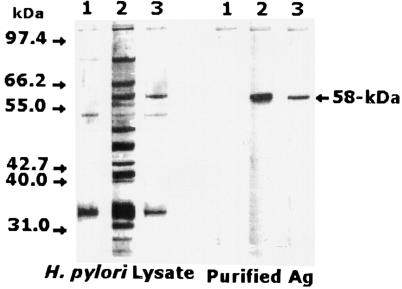
Sera collected from the rabbits immunized with the H. pylori lysate reacted strongly with several bands of various molecular weights on Western blots. However, a broad band at 58 kDa in H. pylori lysate showed particularly high reactivity. The 58-kDa H. pylori antigen was isolated and purified from H. pylori lysate by using a gel electroelution technique. Antibodies specific to the 58-kDa H. pylori antigen were produced in rabbits immunized with the purified H. pylori antigen. In a Western blot, the developed IgG antibodies appeared to react only with an antigen of 58 kDa in whole H. pylori cell lysate and purified antigen (Fig. 1). Sera collected from these rabbits at zero time as control sera were not specifically reactive with that band (Fig. 1). In addition, sera collected from immunized rabbits showed increased levels of specific IgG antibodies (P < 0.05) towards H. pylori lysate and the purified antigen in comparison with levels in the control sera as determined by ELISA. No significant batch-to-batch variation (P > 0.05) was shown by ELISA in the reactivities of various blood specimens from the various rabbits.
FIG. 1.
Western blot analysis of H. pylori cell lysate and the 58-kDa purified antigen. Lane 1, immunostaining with nonimmunized-rabbit serum; lane 2, immunostaining with polyclonal rabbit IgG to H. pylori cell lysate; lane 3, immunostaining with rabbit IgG-monospecific antibody to the 58-kDa purified antigen. A highly reactive band was identified at 58 kDa by using rabbit IgG anti-H. pylori lysate and the anti-58-kDa antigen. Molecular mass markers (bands are not shown but are indicated by arrows) include phosphorylase B (97.4 kDa), bovine serum albumin (66.2 kDa), glutamate dehydrogenase (55.0 kDa), ovalbumin (42.7 kDa), aldolase (40 kDa), carbonic anhydrase (31 kDa), and soybean trypsin inhibitor (21.5 kDa).
Partial biochemical characteristics of the H. pylori antigen.
The purified H. pylori antigen was analyzed by SDS-PAGE and was Coomassie blue stained at 58 kDa. The reactivity of the purified H. pylori antigen by ELISA was lost after treatment with a solution containing 0.2 M HCl or 0.2 M NaOH or 180 mM β-mercaptoethanol and was maintained after sodium meta-periodate oxidation. Trichloroacetic acid (40%)-precipitated antigen showed high reactivity against the specific anti-H. pylori antibody, and the trichloroacetic acid supernatant did not show reactivity. Also, the antigen was treated with α-chymotrypsin enzyme, and the enzymatic reactions were stopped at different time intervals. The reactivity of the H. pylori antigen was decreased by increasing the incubation time of the enzyme but was completely lost after 20 min.
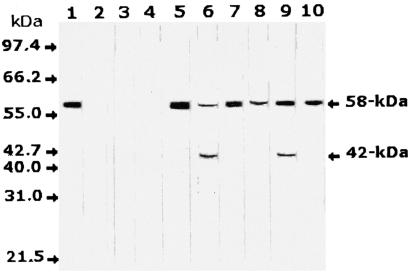
Identification of the H. pylori antigen in human serum by Western blot analysis.
Anti-H. pylori-monospecific antibodies in Western blot analysis of serum samples from 24 H. pylori-infected individuals recognized the 58-kDa band in the sera of all infected patients. In addition, a possible degradation product with a molecular mass of 42 kDa was identified in some of these serum samples. No specific reaction was observed with the sera from eight uninfected individuals as a control group. The detection of the H. pylori target antigen in selected serum samples from infected and noninfected individuals by Western blotting is shown in Fig. 2.
FIG. 2.
Western blot analysis of serum samples from H. pylori-infected and noninfected individuals with antisera monospecific to the 58-kDa antigen. A single band was identified at 58 kDa in serum samples from infected individuals only, in addition to a degradation product of 42 kDa in some of these samples. Lane 1, H. pylori cell lysate as a positive control; lanes 2 to 4, serum samples from three noninfected healthy individuals; lanes 5 to 10, serum samples from six individuals infected with H. pylori. Molecular mass bands are not shown but are indicated by arrows.
Detection of the 58-kDa HpCA in human serum by ELISA.
A novel ELISA based on the detection of the target HpCA in serum was developed for the diagnosis of H. pylori infection. The mean absorbance values of the serum samples were 0.751 (range, 0.411 to 1.250; all positive) for 32 H. pylori-positive subjects and 0.271 (range, 0.135 to 0.377; all negative) for 24 H. pylori-negative subjects (P < 0.001, Mann-Whitney U test). To evaluate the clinical application of the test, endoscopic biopsy specimens from the gastric antra of 221 individuals were evaluated for H. pylori infection by using culture as the gold standard for diagnosis. A total of 144 individuals were diagnosed as H. pylori infected and 77 were diagnosed as noninfected by microbiological culture. No significant differences (P > 0.05, Fisher's exact test) were shown between the results of circulating-antigen detection by ELISA and H. pylori culture of gastric biopsy samples. The sensitivity, specificity, efficiency, and predictive values of positive and a negative results for the newly developed ELISA in comparison to those of microbiological culture are shown in Table 1.
TABLE 1.
ELISA detection of a 58-kDa HpCA in 221 serum samples of individuals who were confirmed by culture to be infected or not infected with H. pylori
| Result of microbiological culture of gastric biopsy specimena | No. of samples | No. of samples with indicated HpCA ELISA resultb
|
% Positive | |
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | 144 | 133 (TP) | 11 (FN) | 92 |
| Negative | 77 | 7 (FP) | 70 (TN) | 9 |
The gold standard for the diagnosis of H. pylori infection.
TP, true-positive result; FP, false-positive result; TN, true-negative result; FN, false-negative result. Percent sensitivity = TP/(TP + FN) = 133/(133 + 11) × 100 = 92%; percent specificity = TN/(TN + FP) = 70/(70 + 7) × 100 = 91%; positive predictive value (percentage) = TP/(TP + FP) = 133/(133 + 7) × 100 = 95%; negative predictive value (percentage) = TN/(TN + FN) = 70/(70 + 11) × 100 = 86%; efficiency of the test (percentage) = (TP + TN)/total = (133 + 70)/221 × 100 = 92%.
DISCUSSION
Immunodiagnosis of H. pylori infection is attractive in comparison with other noninvasive diagnostic methods for the investigation of upper gastrointestinal symptoms. There are a number of different techniques for antibody detection (19, 30). However, antibody detection tests are less useful in children aged below 10 (21) and are not suitable for the follow-up examination of treated patients (15, 22). In addition, the accuracy of these tests is no longer adequate to justify their clinical use on clinical or economic grounds (29).
The H. pylori stool antigen enzyme immunoassay has been validated in various regions of the world with comparable sensitivities and specificities (13, 31). However, a potential problem with the H. pylori stool antigen test appears to be patient reluctance about stool handling, and this could prove to be a significant obstacle in patient compliance and the acceptability of the test in everyday clinical practice (5, 34). In addition, the accumulating data concerning the use of the test in evaluating treatment remain unconvincing (24).
Soluble bacterial antigens of H. pylori on the stomach mucosa can be passively absorbed by pinocytosis and can be transferred into the blood through injured tight junctions and by the absorption of intestinal mucosal epithelial cells (3, 4). In the present study, we were able to detect a specific 58-kDa antigen in H. pylori lysate and in serum samples from H. pylori-infected individuals. The molecular mass of the serum antigen is analogous to the 58-kDa fragment of the 87-kDa cytotoxin domain of the VacA protein (8, 23, 27), the subunit cellular antigen (59 kDa) of the native H. pylori catalase (26), and the H. pylori catalase gene product (20). However, further investigation of the structure of the target H. pylori serum antigen will be performed.
Based on these encouraging results, we have developed a direct ELISA based on the detection of HpCA in serum samples and suitable for the laboratory diagnosis and screening of large populations for H. pylori infection. The HpCA test has several potential advantages over other techniques for population studies. No expensive instrumentation or expertise is required to perform a standard ELISA. The advantages of the HpCA test include its ability to detect the presence of H. pylori and its usefulness with individuals for whom endoscopy is difficult to justify (8).
To evaluate the diagnostic performance of the HpCA diagnostic test, we included in the study 221 patients with dyspeptic symptoms who were examined by upper gastrointestinal endoscopy. The infection with H. pylori was judged to be present when a microbiological culture of gastric biopsy specimens was positive. The HpCA test detected the circulating antigen in 92% of sera from H. pylori-infected individuals, with high specificity (91%) among noninfected individuals.
The false-negative results (8%) of our HpCA ELISA may be explained as follows. The H. pylori antigen level among false-negative samples may be too low to be detected by the present assay conditions. The direct ELISA, with which the unknown elements are bound directly to the plate, may suffer from problems with uneven absorption and interference with absorption due to other serum components. Most commercial tests are of the antigen capture variety, which requires an additional antibody; however, with further work, the HpCA test can be improved. H. pylori antigens have been found as components of circulating immune complexes (34), so it may be necessary to dissociate the immune complexes to achieve a higher sensitivity in the immunoassay (6).
The false-positive results (9%) reported by the HpCA test may be due to biopsy sampling errors as a result of the patchy nature of the H. pylori infection, and additional biopsies from the corpus are required (32). The antigen detection method showed high efficiency (92%) and high positive (95%) and negative (86%) predictive values. The high positive predictive value of the HpCA test may enhance the applicability of this test to children.
Although the present study confirmed the sensitivity of the HpCA test in the diagnosis of H. pylori infection, exploring its use in posttreatment follow-up may have greater clinical implications, since endoscopy becomes less justifiable. In conclusion, the newly developed circulating antigen detection test, HpCA ELISA, is an alternative, easy-to-use, noninvasive test for the detection of H. pylori infection.
REFERENCES
- 1.Archimandritis, A., A. Giontzis, A. Smilakou, M. Tzivras, and P. Davaris. 1999. Diagnosis of Helicobacter pylori infection by HpSA test. Lancet 354:1210-1211. [DOI] [PubMed] [Google Scholar]
- 2.Attallah, A. M., C. A. Abdel Malak, H. Ismail, A. El-Saggan, M. Omran, and A. Tabll. 2003. Rapid and simple detection of a Mycobacterium tuberculosis circulating antigen in serum using dot-ELISA for field diagnosis of pulmonary tuberculosis. J. Immunoass. Immunochem. 24(1):87-93. [DOI] [PubMed]
- 3.Cao, P., M. S. McClain, M. H. Forsyth, and T. L. Cover. 1998. Extracellular release of antigenic proteins by Helicobacter pylori. Infect. Immun. 66:2984-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crabtree, J. E. 1995. Immune and inflammatory response to Helicobacter pylori infection. Scand. J. Gastroenterol. 31(215):3-10. [PubMed] [Google Scholar]
- 5.Cullen, K. P., B. M. Broderick, J. Jayaram, B. Flynn, and H. J. O'Connor. 2002. Evaluation of the Helicobacter pylori stool antigen (HpSA) test in routine clinical practice—is it patient-friendly? Ir. Med. J. 95:305-306. [PubMed] [Google Scholar]
- 6.De Jonge, N., Y. E. Fillie, and A. M. Deelder. 1987. A simple and rapid treatment (trichloroacetic acid precipitation) of serum samples to prevent non-specific reactions in the immunoassay of a proteoglycan. J. Immunol. Methods 99:195-197. [DOI] [PubMed] [Google Scholar]
- 7.Eck, M., B. Schmausser, R. Haas, A. Greiner, S. Czub, and H. Muller-Hermelink. 1997. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology 112:1482-1486. [DOI] [PubMed] [Google Scholar]
- 7a.Forman, D. 1998. Helicobacter pylori: the gastric cancer problem. Gut 43:533-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garner, J. A., and T. L. Cover. 1996. Binding and internalization of the Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect. Immun. 64:4197-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatta, L., N. Vakil, C. Ricci, J. F. Osborn, A. Tampieri, F. Perna, M. Miglioli, and D. Vaira. 2003. A rapid, low-dose, 13C-urea tablet for the detection of Helicobacter pylori infection before and after treatment. Aliment. Pharmacol. Ther. 17:793-798. [DOI] [PubMed] [Google Scholar]
- 10.Graham, D. Y., and W. A. Qureshi. 2001. Markers of infection, p. 499-510. In H. L. T. Mobley, G. L. Mendz, and S. L. Hazell (ed.), Helicobacter pylori: physiology and genetics. ASM Press, Washington, D.C. [PubMed]
- 11.Hachamkin, I. 1995. Campylobacter and Acrobacter, p. 483-491. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. ASM Press, Washington, D.C.
- 12.Howden, C. W., and R. H. Hunt. 1998. Guidelines for the management of Helicobacter pylori infection. Am. J. Gastroenterol. 93:2330-2338. [DOI] [PubMed] [Google Scholar]
- 13.Kabir, S. 2001. Detection of Helicobacter pylori in feces by culture, PCR, and enzyme immunoassay. J. Med. Microbiol. 50:1021-1029. [DOI] [PubMed] [Google Scholar]
- 14.Kabir, S. 2003. Clinic-based testing for Helicobacter pylori infection by enzyme immunoassay of faeces, urine and saliva. Aliment. Pharmacol. Ther. 17:1345-1354. [DOI] [PubMed] [Google Scholar]
- 15.Kokkola, A., H. Rautelin, P. Puolakkainen, P. Sipponen, M. Farkkila, R. Haapiainen, and T. U. Kosunen. 2000. Diagnosis of H. pylori infection in patients with atrophic gastritis: comparison of histology, 13C-urea breath test, and serology. Scand. J. Gastroenterol. 35(2):138-141. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with Folin-phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 18.Megraud, F. 1997. How should Helicobacter pylori infection be diagnosed? Gastroenterology 113:S93-S98. [DOI] [PubMed] [Google Scholar]
- 19.Newell, D., and A. Stacet. 1993. Serology, p. 139-148. In T. C. Northfield, M. Mendall, and P. M. Goggin (ed.), Helicobacter pylori infection. Kluwer Academic Publishers, London, United Kingdom.
- 20.Odenbreit, S., B. Wieland, and R. Haas. 1996. Cloning and genetic characterization of Helicobacter pylori catalase and construction of a catalase-deficient mutant strain. J. Bacteriol. 178:6960-6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okuda, M., E. Miyashiro, M. Koike, T. Tanaka, M. Bouoka, S. Okuda, and N. Yoshikawa. 2002. Serodiagnosis of Helicobacter pylori infection is not accurate for children aged below 10. Pediatr. Int. 44:387-390. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Perez, G. I., A. F. Cutler, and M. J. Blaser. 1997. Value of serology as a noninvasive method for evaluating the efficacy of treatment of Helicobacter pylori infection. Clin. Infect. Dis. 25:1038-1043. [DOI] [PubMed] [Google Scholar]
- 23.Reyrat, J. M., S. Lanzavecchia, P. Lupetti, M. de Bernard, C. Pagliaccia, V. Pelicic, M. Charrel, C. Ulivieri, N. Norais, X. Ji, V. Cabiaux, E. Papini, R. Rappuoli, and J. L. Telford. 1999. 3D imaging of the 58 kDa cell binding subunit of the Helicobacter pylori cytotoxin. J. Mol. Biol. 290:459-470. [DOI] [PubMed] [Google Scholar]
- 24.Roth, D. E., D. N. Taylor, R. H. Gilman, R. Meza, U. Katz, C. Bautista, L. Cabrera, B. Velapatiño, C. Lebron, M. Razúri, J. Watanabe, T. Monath, and the Gastrointestinal Physiology Working Group. 2001. Posttreatment follow-up of Helicobacter pylori infection using a stool antigen immunoassay. Clin. Diagn. Lab. Immunol. 8:718-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suerbaum, S., and P. Michetti. 2002. Helicobacter pylori infection. N. Engl. J. Med. 347:1175-1186. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki, N., M. Wakasugi, S. Nakaya, N. Kokubo, M. Sato, H. Kajiyama, R. Takahashi, H. Hirata, Y. Ezure, Y. Fukuda, and T. Shimoyama. 2002. Catalase, a specific antigen in the feces of human subjects infected with Helicobacter pylori. Clin. Diagn. Lab. Immunol. 9:784-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Telford, J. L., P. Ghiara, M. Dell'Orco, M. Comanducci, D. Burroni, M. Bugnoli, M. F. Tecce, S. Censini, A. Covacci, Z. Xiang, et al. 1994. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J. Exp. Med. 179:1653-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaira, D., and N. Vakil. 2001. Blood, urine, stool, breath, money, and Helicobacter pylori. Gut 48:287-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaira, D., J. Holton, M. Menegatti, C. Ricci, F. Landi, A. Ali, L. Gatta, C. Acciardi, S. Farinelli, M. Crosatti, S. Berardi, and M. Miglioli. 1999. New immunological assays for the diagnosis of Helicobacter pylori infection. Gut 45(Suppl. 1):I23-I27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaira, D., J. Holton, M. Menegatti, C. Ricci, L. Gatta, A. Geminiani, and M. Miglioli. 2002. Invasive and non-invasive tests for Helicobacter pylori infection. Aliment. Pharmacol. Ther. 14(3):13-22. [DOI] [PubMed] [Google Scholar]
- 32.van Zwet, A. A., J. C. Thijs, R. Roosendaal, E. J. Kuipers, S. Pena, and J. de Graaff. 1996. Practical diagnosis of Helicobacter pylori infection. Eur. J. Gastroenterol. Hepatol. 8:501-507. [PubMed] [Google Scholar]
- 33.Westblom, T. U., and B. D. Bhatt. 1999. Diagnosis of Helicobacter pylori infection. Curr. Top. Microbiol. Immunol. 241:215-235. [DOI] [PubMed] [Google Scholar]
- 34.Zhu, Y., J. Lin, D. Li, Q. Du, K. Qian, Q. Wu, and S. Zheng. 2002. Helicobacter pylori antigen and its IgG, IgA-type specific immunocomplexes in sera from patients with Helicobacter pylori infection. Chin. Med. J. 115:381-383. [PubMed] [Google Scholar]