Abstract
A bispecific monoclonal antibody (bsMAb) that detects Bordetella pertussis, the causative agent of whooping cough, and horseradish peroxidase (HRPO) has been developed by use of the quadroma technology. A quadroma, P123, was produced by fusing two well-characterized hybridomas against the bacterium and the enzyme and was subcloned to obtain a stable bsMAb-secreting cell line. The quadroma was theoretically expected to produce up to 10 different molecular species of immunoglobulins, so secreted bispecific antibody was complexed with excess HRPO and the HRPO-bsMAb complex was purified in one step by benzhydroxamic acid-agarose affinity cochromatography. An ultrasensitive homosandwich molecular “velcro” enzyme-linked immunosorbent assay for the detection of B. pertussis whole bacteria with HRPO-bsMAb was established in both microplate and nasopharyngeal swab formats. This assay demonstrates a high sensitivity that approaches the theoretical limit of detection of one bacterium. This new nanoprobe can be used to develop a new generation of assays that are simple, inexpensive alternatives to quantitative PCR and that can be used by clinical laboratories. This strategy of homosandwich assays with solid-phase monospecific antibodies and solution-phase bsMAb with specificity for the same repeating surface determinants can be applied to generate ultrasensitive immunodiagnostic assays for viruses and bacteria.
Whooping cough (pertussis) is a vaccine-preventable disease caused by the gram-negative bacterium Bordetella pertussis. Despite required vaccination in most countries, the incidence of whooping cough is still unacceptably high and has recently emerged in the adolescent age group (2, 10). Accurate diagnosis of B. pertussis is essential to proper surveillance of whooping cough, and improved diagnostic methods are desirable.
One of the present methods for the diagnosis of B. pertussis infection is a direct fluorescent-antibody assay that uses a fluorescence-labeled monoclonal antibody (MAb) directed against the lipopolysaccharide (LPS) in the outer membrane of the bacterium (14). Unlike many other bacteria, B. pertussis produces one predominant antigenic type of LPS molecule (17), making it a good specific target for immunodiagnosis. Like other endotoxins, B. pertussis LPS is a durable molecule that can easily withstand the conditions encountered during handling and transport. The detection of antibody to LPS is a good strategy for the diagnosis of B. pertussis infection, but the method could be improved to make it easier to use and a more sensitive reagent is needed (20).
Here we report on the development and characterization of a bispecific MAb (bsMAb) against horseradish peroxidase (HRPO) and B. pertussis LPS that could be used for enzyme-based detection of soluble LPS antigen and whole B. pertussis bacteria in clinical samples, immunochemical structural studies, and serological characterization of B. pertussis LPS, with some potential advantage over present MAb- or polyclonal antibody-labeled immunoassays. In particular, we observed the ultrasensitive detection of B. pertussis by use of the molecular “velcro” assay concept.
MATERIALS AND METHODS
Bacterial strains and extraction of LPS fractions.
B. pertussis strain BP347 was obtained from Alison Weiss, University of Cincinnati. Cultivation of bacteria, extraction of LPS with hot phenol-water from heat-killed bacteria at 90°C for 30 min, and purification by ultracentrifugation were carried out as described previously (17).
Cell lines.
Murine hybridoma cell line 1H2, which recognizes the terminal trisaccharide of B. pertussis LPS, was developed by two of us (M.S.P. and R.T.I.) to produce a commercial diagnostic assay (AccuMab; Altachem Pharma Ltd., Edmonton, Alberta, Canada). The 1H2 hybridoma was subcloned to obtain 1H2P4, a mouse hybridoma secreting an immunoglobulin G1 (IgG1) anti-B. pertussis LPS MAb.
YP4, a rat hybridoma producing an IgG2a anti-HRPO MAb, was a gift of the late C. Milstein, Laboratory of Molecular Biology, Medical Research Council, Cambridge, United Kingdom.
Quadroma generation. (i) Cell labeling.
The first parental hybridoma, 1H2P4 (anti-B. pertussis LPS), was labeled with tetramethyl rhodamine isocyanate (TRITC [red fluorescence]; Sigma, St. Louis, Mo.); and the second hybridoma, YP4 (anti-HRPO), was labeled with fluorescein isothiocyanate (FITC [green fluorescence]; Sigma). The cell-labeling protocol was similar to one described previously (12), with some modifications. Approximately 2 × 107 cells (viability, >90%) of the 1H2P4 hybridoma and 2 × 107 cells (viability, >95%) of the YP4 hybridoma were washed three times with serum-free Dulbecco modified Eagle medium (DMEM; Gibco BRL, Gaithersburg, Md.) (SFDMEM). 1H2P4 cells were labeled with freshly prepared TRITC solution (2 μg/ml in SFDMEM [pH 7.4]). YP4 cells were labeled with FITC solution (1 μg/ml in SFDMEM [pH 6.8]). The cells were incubated with their respective fluorescence dyes for 15 min at 37°C to label surface NH2 groups and were washed three times with SFDMEM.
(ii) PEG fusion.
Approximately 2 × 106 cells from the FITC-labeled YP4 hybridoma were mixed with the same number of cells from the TRITC-labeled 1H2P4 hybridoma, and 250 μl of polyethylene glycol (PEG) solution (Sigma) was added to the cell suspension. Following a 2-min incubation at 37°C, the cells were pelleted by centrifugation (500 × g for 5 min at 37°C), the supernatant was decanted, and the cells were resuspended in 15 ml of DMEM with 20% fetal bovine serum (FBS). After the mixture was washed three times, the fused cells were resuspended in 5 ml of DMEM with 20% FBS, transferred to a 25-cm2 tissue culture flask, and incubated for 1 h at 37°C in an atmosphere containing 5% CO2.
(iii) Fluorescence-activated cell sorter (FACS) analysis.
Flow cytometric experiments were performed with an Epics Elite cell sorter from the Coulter Corporation (Hialeah, Fla.). Cells with dual fluorescence were sorted at two cells per well in a 96-well tissue culture plate with DMEM-20% FBS. The plate was then incubated at 37°C with 5% CO2. Screening of the quadroma fusion supernatants was performed after 10 days of culture by a direct enzyme-linked immunosorbent assay (ELISA), as described below. The stronger positive clones were then selected and subcloned by the limiting dilution method three successive times to obtain the best quadroma, termed P123.
Screening method to detect quadromas secreting bsMAb (bridge ELISA).
A direct ELISA was used for all screening procedures. Microtiter plates (Nunc, Roskilde, Denmark) were coated with purified B. pertussis LPS at 3 μg/well or whole bacteria at 108 CFU per well for 4 h at 37°C and overnight at 22°C, respectively. The remaining binding sites were blocked with 200 μl of 1% (wt/vol) bovine serum albumin (BSA) in 10 mM phosphate-buffered saline (PBS; pH 7.2) (PBS-BSA) at 37°C for 1 h, and the plates were washed three times with PBS-0.05% Tween 20 (PBS-T). A 100-μl aliquot of various quadroma cell culture supernatants was serially diluted with PBS-BSA, 50 μl of 10 μg of HRPO per ml was added to each well, and the plate was incubated for 1 h at 37°C. The plates were washed three times with PBS-T. Finally, 100 μl of tetramethylbenzidine (TMB; Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) substrate was added to the wells. Positive quadromas secreting bsMAb were selected after 15 to 45 min of color development, measured at 650 nm. This bridge ELISA measures only the activities of bsMAbs that bind to the solid phase with one paratope and to HRPO in solution with the other paratope. Monospecific antibodies are not detected by this assay since they do not cross-link the two different antigens.
Purification of monospecific MAbs.
The anti-B. pertussis LPS MAb present in culture supernatants was purified by using the Affi-prep protein A MAPS preparative cartridge (Bio-Rad, Hercules, Calif.), according to the instructions of the manufacturer. The bound immunoglobulins were eluted at pH 4.5 and dialyzed with PBS (pH 7.2) with three changes of PBS.
Affinity cochromatography of HRPO-labeled bsMAbs.
The affinity purification protocol was similar to one described previously (9, 11), with some slight modifications. The bsMAb supernatants from the quadroma cell lines were precipitated with solid ammonium sulfate to 50% saturation and then dialyzed against phosphate buffer (pH 7.2). Approximately 150 mg of crude bsMAb along with 10 mg of HRPO (dissolved in phosphate buffer [pH 7.0]) was incubated for 20 min at 37°C and loaded onto a column (1.25 by 30 cm) containing 15 ml of benzhydroxamic acid-agarose (BHA) matrix. The column flow rate was 20 ml/h. The column was washed with approximately six times the column volume of phosphate buffer, and the bound proteins were eluted as a preformed HRPO-bsMAb complex in 2-ml fractions with 0.1 M borate buffer [pH 9.0]. The bsMAb fractions were tested for activity by ELISA on an LPS-coated plate. Active fractions were pooled and dialyzed against PBS (pH 7.2).
Homosandwich molecular velcro ELISA.
The two-step homosandwich ELISA was performed with 96-well microtiter plates (Nunc). The wells were coated overnight at 4°C with purified anti-B. pertussis LPS monospecific MAb (1.5 μg/ml, 100 μl/well) in 50 mM carbonate buffer (pH 9.6). The remaining binding sites were blocked with 200 μl of 1% BSA by incubation for 30 min at 37°C. After the plate was washed with 0.05% (vol/vol) PBS-T (pH 7.2), 100 μl of heat-killed B. pertussis whole bacteria serially diluted in PBS was added, and the mixture was incubated for 1 h at 37°C. The plates were washed and incubated with 100 μl of HRPO-labeled bsMAb in 1% BSA for 1 h at 37°C. Even though two structurally different MAbs were used, we consider this a homosandwich ELISA because, functionally, the same LPS paratope is involved in the formation of the sandwich. The molecular velcro effect represents the almost irreversible binding of the ternary complex to the solid phase, as described in the Results section. TMB substrate was added after the final wash, and readings were taken at 650 nm. In order to observe the molecular velcro effect of the two steps of the molecular velcro ELISA, we initially coated the bivalent MAb on the microtiter plate and added B. pertussis, incubated the mixture for 1 h, and then washed the mixture 1 to 16 times with PBS-T. The bound bacteria were detected directly by the bsMAbs following three subsequent washes with PBS-T. Similarly, bsMAb binding to the B. pertussis organisms directly coated on the polystyrene microtiter plate (instead of an MAb-coated plate) at 107 CFU/well was studied. Again, the plate was washed 1 to 16 times before measurement of the ELISA activity.
Immunoswab assays.
A nasopharyngeal type 1 Dacroswab and a Calgiswab (Spectrum Labs, Houston, Tex.) on a flexible aluminum shaft were used in a simulated immunoswab assay. The swabs were spiked with different amounts of B. pertussis in 20 μl of PBS, dried at room temperature for 5 min, fixed with 20 μl of 95% ethanol for 5 min, and finally blocked with 25 μl of 5% BSA in PBS for 5 min. Control swabs were processed in a similar fashion, but without the addition of the bacteria. The control and test swabs were incubated with 50 μl of the HRPO-bsMAb complex for 15 min in an Eppendorf tube. The swabs were finally washed extensively with 0.05% BSA-PBS eight times by use of simple fill-and-aspiration steps. The final wash included a centrifugation step to remove any residual fluid. The TMB substrate (100 μl) was added, and the development of the blue color on the swab tip was monitored.
A modification of the direct detection procedure described above was tested, in which the fresh swabs were coated with 10 μl of the 1H2 MAb per ml to capture the bacteria in the first step, as in a homosandwich assay. Following a blocking step with 5% BSA-PBS, the rest of the steps were the same as described above.
RESULTS
Quadromas secreting bsMAb.
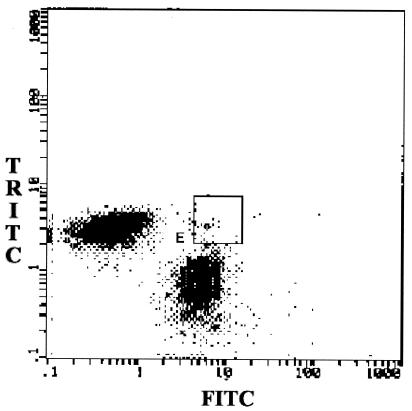
Approximately 80% of the 1H2P4 hybridoma cells and 85% of the YP4 hybridoma cells were labeled with TRITC and FITC, respectively. By PEG-mediated fusion of the two hybridomas, we evaluated the effect of PEG fusion on cell viability by using different amounts of PEG (0.3 and 0.5 ml) and different incubation times (2 and 5 min). Compared with unfused cells (trypan blue exclusion method), 0.25 ml of PEG and 2 min of incubation decreased the cell viability, on average, by 12.6% (range, 15 to 6%). Increasing the time of incubation with PEG to 5 min decreased the cell viability by 20.8%. On the other hand, 0.5 ml of PEG and 2 min of incubation decreased the cell viability by 16.5 to 24.4%. Hence, on the basis of the cytotoxicity data, the two partner cells in our study (2 × 106 each) were fused by 0.25 ml of PEG with the shorter 2-min incubation. Dot plot FACS analysis was carried out after PEG fusion (Fig. 1). The hybrid hybridomas or quadromas exhibiting dual fluorescence in area E in Fig. 1 were sorted and seeded into a 96-well tissue culture plate at two cells per well in 20% FBS-DMEM. The 108 quadroma clones that appeared after 10 days were screened for bsMAb positivity by the bridge ELISA. The best four clones were recloned twice by the limiting dilution method (Table 1) to select the final quadroma. It is important to mention that the culture supernatants of quadroma cultures contained bsMAb as well as monospecific parental MAbs. Hence, for undiluted supernatants, the ELISA signal was weak, and this was magnified for the 1:10 and 1:100 dilutions upon recloning. The likely reason for this effect is the presence of the competing monospecific anti-LPS antibodies during measurement of bsMAb by the bridge ELISA. This dilution effect is not seen with affinity-purified bsMAb.
FIG. 1.
FACS analysis after PEG fusion of the two fluorescent hybridomas. The dot plot analysis performed after PEG fusion shows the number of double-fluorescent cells (area E). The double-positive cells from area E were initially sorted at two cells per well.
TABLE 1.
Quadroma cell lines secreting bsMAb against B. pertussis and HRPO screening
| Quadroma cell line | bsMAb titer in cell culture supernatant at the following dilution:
|
||||
|---|---|---|---|---|---|
| 1:10 | 1:102 | 1:103 | 1:104 | Blank | |
| P123-1a | 1.023 | 0.212 | 0.111 | 0.093 | 0.076 |
| P123-2a | 1.282 | 0.243 | 0.115 | 0.103 | |
| P123-3a | 0.986 | 0.205 | 0.107 | 0.098 | |
| P123-4a | 0.99 | 0.225 | 0.111 | 0.09 | |
| IC9b | 0.196 | 0.078 | 0.060 | 0.056 | |
| 6C7b | 0.284 | 0.086 | 0.078 | 0.066 | |
| IG8b | 0.217 | 0.099 | 0.080 | 0.066 | |
Quadroma cell lines cloned and subcloned twice.
Original quadroma cell lines.
Affinity cochromatography of bsMAb-HRPO immune complex.
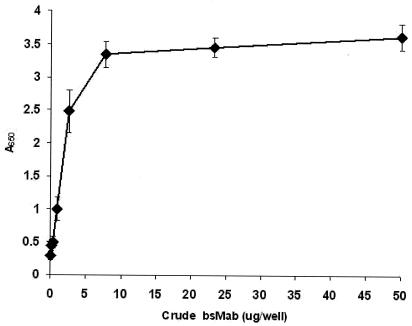
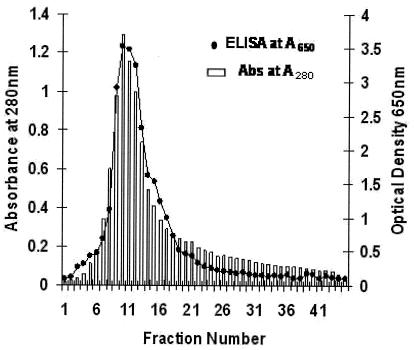
Before affinity copurification, crude bsMAb samples were allowed to incubate with excess HRPO. Direct ELISA was used to measure the optimum concentration of HRPO needed to saturate crude bsMAb. Different concentrations of crude bsMAb samples and a fixed amount of HRPO (0.5 μg/well) were added to each well of the bacterium-coated plates, and the plates were incubated for 1 h at 37°C. The plate was washed three times with PBS-T, TMB was added, and the absorbance was read at 650 nm. As shown in Fig. 2, saturation of crude bsMAb with HRPO occurred with ∼15 μg of crude bsMAb per ml. Affinity purification of bsMAb-HRPO as a preformed complex was accomplished with the BHA column matrix, which is known to bind to HRPO (9). The crude HRPO-bsMAb mixture was loaded, and unbound proteins (especially monospecific anti-LPS) were removed by washing. The MAb-HRPO complex was eluted with borate buffer and was effectively resolved from the competing anti-LPS antibodies and contaminating proteins which were in the unbound fractions. The chromatographic profile of the bsMAb in the eluted fractions shows that the A280 peak was seen to correspond to the peak of bsMAb-HRPO complex activity (Fig. 3). The ELISA data show the copurification of the bsMAb- and HRPO enzyme-related activities in the eluted peak. Since the preformed bsMAb-HRPO complex has one free paratope, it can be conveniently detected on a B. pertussis-coated plate.
FIG. 2.
Optimum concentrations of crude bsMAb to HRPO (n = 3). B. pertussis bacterium-coated plates were incubated with crude bsMAb to measure the optimum concentration of HRPO needed to saturate the HRPO paratopes. The enzyme activity following three washes was measured at 650 nm.
FIG. 3.
Purification of HRPO-labeled bsMAb from a cell culture supernatant on a BHA column. The absorbance at 280 nm (Abs at A280) of each 2 ml of eluted fraction was measured. B. pertussis bacterium-coated plates were used to measure specifically only the HRPO-bsMAb activity (optical density, 650 nm). Diluted fractions (1:20) were used in the HRPO-bsMAb assay.
Homosandwich molecular velcro ELISA.
The traditional forward two-step sandwich assay procedure was performed to demonstrate the molecular velcro effect. This involves the progressive steps of incubation of bacteria with a MAb-coated solid phase, washing, incubation with bsMAb-HRPO, washing, and addition of substrate to measure the signal intensity. Interestingly, we observed that the binding of B. pertussis to the bivalent monospecific MAb-coated solid phase in the first step was irreversible after the first three washes conducted to remove nonspecific binding. We believe that this is due to the molecular velcro effect, analogous to the effects of macrovelcros used in several applications. The molecular velcro effect allowed ultrasensitive detection of this pathogenic organism because of its ability to avidly capture the multiple, exposed LPS molecules on the bacterial surface. Resistance to leaching of B. pertussis in the ELISA indicated that B. pertussis both was almost irreversibly bound to the MAb-coated solid phase and resisted even 16 washings. Similarly, bsMAb also resisted leaching after 16 washes when it was added to the plate containing the bacteria attached to the coated anti-B. pertussis MAbs. The latter experimental observation of the functionally monovalent bsMAb in the solution phase is more difficult to explain, and we speculate that slow antibody dissociation rates and some secondary lateral interactions could stabilize the ternary complex.
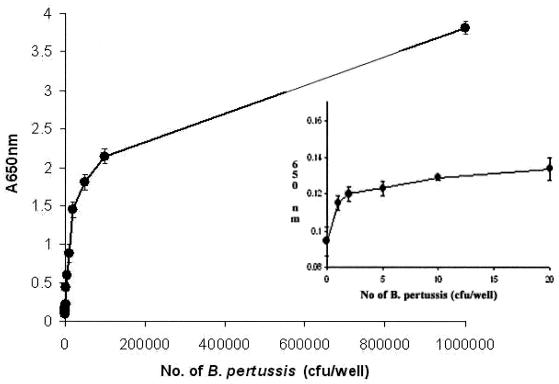
B. pertussis BP347 cultivated overnight in Stainer-Scholte medium was heat killed at 90°C for 30 min prior to assay. Bivalent monospecific MAb 1H2P4 was used as the capture antibody. Tenfold dilutions of B. pertussis bacteria at concentrations ranging from 1 to 107/well were tested to measure the sensitivity of the assay. HRPO-bsMAb (P123-2) was used as the tracer antibody at 1.5 μg/well. The incubation period with antigen was 1 h at 37°C. The homosandwich ELISA showed a high sensitivity, with practical detection of B. pertussis occurring at ∼5 CFU (Fig. 4). The extrapolated theoretical lower limit of detection was one bacterium by using the mean + 2 standard deviations (SDs) of 20 control assays without B. pertussis.
FIG. 4.
Detection of B. pertussis by the microplate molecular velcro assay with the homosandwich ELISA format. The plates were coated with 1H2P4 and detected with HRPO bsMAb P123-2. The points are the means of pentuplicate measurements of each point and SDs. (Inset) Detection of B. pertussis in the low range. These are essentially the same data shown outside the inset but more clearly show the counts from 0 to 20 CFU. All points for the bacteria are significantly different from the control values by Student's t test (P < 0.001).
In order to correct and accurately estimate the titers of B. pertussis bacteria, epifluorescence microscopy was used to count and correlate the concentration estimated by turbidimetry by using gold nucleic acid gel stain (Molecular Probes Inc.). Gold nucleic acid gel stain binds to double-stranded DNA, which allows the measurement of the fluorescence counts to estimate the bacterial titers for correction of the titers. In our study a discrepancy in titers of ∼20% was exhibited between turbidimetry and epifluorescence (data not shown). In our studies the heat-killed bacteria did not exhibit clumping or aggregation when they were observed by epifluorescence microscopy. To investigate the influence of matrix effects, we spiked the heat-killed bacteria into nasopharyngeal aspirates obtained from the University of Alberta Hospital. These clinical samples were obtained for analysis for respiratory syncytial virus and were used after heat inactivation. No significant matrix-related interference was observed.
Immunoswab assays.
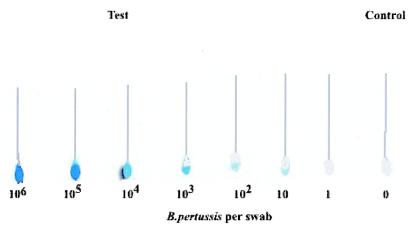
In order to investigate the utility of the bsMAb-HRPO immunoconjugate for detection of the bacterium in a primary health care setting, we conducted a simulated immunoswab assay. Dacroswab and Calgiswab were spiked with bacteria in a minimum volume of fluid and fixed to ensure adherence of the bacteria to the tip of the swab. The simulated capture of the bacteria was accomplished either directly with the fibers or following coating with the monospecific MAb to facilitate the capture step. Subsequently, the assay was conducted by using bsMAb-HRPO, as outlined in Materials and Methods. In tests with the two formats and the two different types of swabs, we observed that the best results were achieved with direct adsorption on the Calgiswab. The data in Fig. 5 show that as few as 10 bacteria can be detected when the results were compared with those achieved with the controls. While the sensitivity of detection by the alternative monospecific MAb-coated format was equally good, the level of background color development was slightly higher (data not shown). Optimized development of the direct assay could allow the B. pertussis screen to be done in a family physician's office setting to monitor outbreaks of whooping cough.
FIG. 5.
Direct detection of B. pertussis on nasopharyngeal swabs spiked with B. pertussis. Calgiswabs were allowed to adsorb 20 μl of sample containing various amounts of B. pertussis in an Eppendorf tube at room temperature for 5 min and dried. The swabs were fixed with 20 μl of 95% ethanol at room temperature for 5 min and blocked with 5% BSA in PBS at room temperature for 5 min. The swabs were incubated with 50 μl of bsMAb-HRPO complex at 3 μg/ml for 15 min, washed eight times with 0.05% BSA in PBS, and centrifuged at 14,000 rpm (MSE Mistral 2000) for 20 s. TMB substrate (100 μl) was added to observe color development.
DISCUSSION
Three different methods can be used to generate bsMAbs: traditional hybridoma fusions, chemical synthesis, and molecular biology techniques (1, 19). Karawajew and associates (11) described the generation of hybrid hybridomas by labeling the parental hybridomas with two distinct fluorescence markers before the fusion. After PEG fusion, cells with double fluorescence could be sorted and plated at one cell per well. Generally, the efficiency of quadroma production is low. In our experience with numerous fusions of two different hybridomas, the range of the proportion of cells with double fluorescence, as in area E in Fig. 1, varies from 0.5 to 3%. However, it must be emphasized that we need to sort out only one or two 96-well plates and screen for the best quadromas by the bridge assay. In the study described in this report, the labeled cells were fused with PEG and sorted by FACS analysis. The proportion of the double-positive population increased from 1 to 2.5% (Fig. 1) following PEG-mediated fusion, with 300 double-positive clones being obtained. An initial assay demonstrated that five clones produced a bsMAb, but only one clone displayed stable bsMAb production when one cell was seeded into each well. The results are similar to those obtained by Stratieva-Taneeva and coworkers (18). In our study, using PEG fusion and FACS analysis, we obtained 7 clones with double fluorescence from 400 cells when 2 cells were seeded into each well. Modifications such as the use of 5% dimethyl sulfoxide-PEG instead of PEG alone and the plating of two instead of one fused cells per well can account for the larger number of clones with stable bsMAb production by our protocol. Quadromas are polyploid cells and can exhibit considerable instability, and careful recloning is required to isolate a good, stable clone. An alternative approach is to generate bispecific antibodies from the same two hybridomas by genetic recombination techniques (1).
HRPO has been shown to bind to BHA at the HRPO active site (3), and the BHA agarose matrix that we used for affinity purification provided enhanced yields of antibodies complexed to HRPO, in which the active site is sterically available and remains functional (9). We have shown that this affinity cochromatography procedure can be adopted for HRPO and alkaline phosphatase, the most commonly used enzymes (6). The level of labeling of bsMAb antibodies with HRPO is potentially consistently one to one, which lessens batch-to-batch variation and increases overall reliability (13). Provided that the enzyme is available in excess, every molecule is uniformly labeled with HRPO. It is important that the concentration of crude bsMAb sample incubated with HRPO prior to the initiation of affinity cochromatography is optimal so that it also saturates and binds to the monospecific anti-HRPO MAb. Hence, this purification will also copurify monospecific antibodies directed toward HRPO. However, the copurified monospecific MAb and free excess HRPO should be of little concern, as they will not bind to the target antigen and will be eliminated in the subsequent washing steps.
Proper identification of bacteria in patients with suspected cases of whooping cough usually requires that clinical nasopharyngeal swab or nasopharyngeal aspirate samples be cultivated for 3 to 7 days (16). B. pertussis is a highly fastidious bacterium. The successful isolation of this organism depends on several factors, such as the proper collection of the sample from the respiratory tract, followed by transport of the specimens in freshly made transport medium or direct plating of the sample on freshly prepared Regan-Lowe or Bordet-Gengou agar plates (7). A sensitive and specific method for the rapid detection of B. pertussis infection is highly desirable. The molecular velcro sandwich ELISA presented in this study directly detected whole bacteria with a practical lower limit of detection of about five bacteria and a theoretical limit, obtained by extrapolation of the mean for 20 blanks and 2 SDs, of one bacterium. Translation of the diagnostic assay with bsMAb to spiked nasopharyngeal swab samples appears to show a lower limit of detection of 10 CFU, and the technique can easily be developed into a diagnostic assay for use at the point of care in the primary health care setting. However, it is recognized that interference by mucins and other agents is likely to reduce the sensitivity of detection shown with ideally spiked samples (Fig. 5). Direct examination of smears by the monoclonal fluorescent-antibody technique (14) is successful for the detection of B. pertussis, although rates of false-negative results as high as 50% have been reported (5, 20). Also, the use of UV microscopy is labor-intensive, and accuracy typically falters in times of large outbreaks, when it is needed the most (4). The use of a highly specific bsMAb lacking batch-to-batch variations in a simple ELISA may circumvent these problems. We emphasize that we merely derivatized well-established and widely used antibody 1H2, which is the main probe in the AccuMab kit. This kit is extensively used at present for clinical diagnostics. The bsMab retains the 1H2 binding arm and is expected to retain the specificity of the parental MAb. However, further studies with clinical isolates are needed to validate this.
Antibodies with enzyme tags are used extensively in biochemical and immunochemical applications. The enzyme tag is generally attached to an MAb or a polyclonal antibody by a covalent linker (8). This chemical cross-linking technique can be problematic because of variable and partial inactivation of either the enzyme or the antibody; competition from unreacted species, which results in a low specific activity; a decreased shelf life; and variations in the sizes and properties of the conjugate from one batch to the next (13, 15). Cross-linked large protein aggregates could also cause nonspecific binding, which would increase the noise in the assay. bsMAbs are bifunctionally engineered antibodies bearing two different antigen-binding sites in a single antibody molecule. The highest specific activity of the nanoprobe is, theoretically, one in which every molecule is uniformly bound to a signal moiety. With this in mind, use of our hybrid hybridoma (quadroma) has resulted in the production of a designer bsMAb nanoprobe that has one antigen-binding site capable of binding to B. pertussis LPS and the other capable of binding to an enzymatic marker (HRPO). The immunoprobe with an intrinsic enzyme marker-binding capability in every molecule can be used directly as a tracer in a simple immunoassay with a homosandwich molecular velcro assay format with a theoretical limit of detection that approaches one bacterium. A direct comparison of our new assay with quantitative PCR and the traditional culture method for B. pertussis diagnostics is in progress as a pilot clinical trial.
Acknowledgments
M.R.S. thanks CBDN-NCE for an operating grant and the CIHR Industry award for salary support.
Thanks are also due to Sujatha Guttikonda for help in revising the manuscript.
The term “velcro” is a registered trademark of Velcro Group Corporation.
REFERENCES
- 1.Cao, Y., and M. R. Suresh. 1998. Bispecific antibodies as novel bioconjugates. Bioconj. Chem. 9:635-644. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2002. Pertussis 1997-2000. Morb. Mortal. Wkly. Rep. Surveill. Sum. 51:73-76. [Google Scholar]
- 3.De Ropp, J. S., P. K. Mandal, and G. N. La Mar. 1999. Solution 1H NMR investigation of the heme cavity and substrate binding site in cyanide-inhibited horseradish peroxidase. Biochemistry 38:1077-1086. [DOI] [PubMed] [Google Scholar]
- 4.Ewanowich, C. A, L. W. Chui, M. G. Paranchych, M. S. Peppler, R. G. Marusyk, and W. L. Albritton. 1993. Major outbreak of pertussis in northern Alberta, Canada: analysis of discrepant direct fluorescent-antibody and culture results by using polymerase chain reaction. J. Clin. Microbiol. 31:1715-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Field, L. H., and C. D. Parker. 1977. Pertussis outbreak in Austin and Travis County. J. Clin. Microbiol. 6:154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta, S., and M. R. Suresh. 2002. Affinity chromatography and co-chromatography of bispecific monoclonal antibody immunoconjugates. J. Biochem. Biophys. 51:203-216. [DOI] [PubMed] [Google Scholar]
- 7.Gustafsson, B., U. Lindquist, and M. Andersson. 1988. Production and characterization of monoclonal antibodies directed against Bordetella pertussis lipopolysaccharide. J. Clin. Microbiol. 26:188-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermanson, G. T. 1996. Bioconjugate techniques. Academic Press, Inc., San Diego, Calif.
- 9.Husereau, D. R., and M. R. Suresh. 2001. A general affinity method to purify peroxidase-tagged antibodies. J. Immunol. Methods 249:33-41. [DOI] [PubMed] [Google Scholar]
- 10.Joseph, J. E. 2000. Pertussis in the adolescent and adult: a primary care concern. Clin. Excell. Nurse Pract. 4:361-365. [PubMed] [Google Scholar]
- 11.Karawajew, L., O. Behrsing, G. Kaiser, and B. Micheel. 1988. Production and ELISA application of bispecific monoclonal antibodies against fluorescein isothiocyanate (FITC) and horseradish peroxidase (HRP). J. Immunol. Methods 111:95-99. [DOI] [PubMed] [Google Scholar]
- 12.Kreutz, F. T., D. Xu, and M. R. Suresh. 1998. A new method to generate quadromas by electrofusion and FACS sorting. Hybridoma 17:267-273. [DOI] [PubMed] [Google Scholar]
- 13.Kricka, L. J. 1994. Selected strategies for improving sensitivity and reliability of immunoassays. Clin. Chem. 40:347-357. [PubMed] [Google Scholar]
- 14.McNicol, P., S. M. Giercke, M. Gray, D. Martin, B. Brodeur, M. S. Peppler, T. Williams, and G. Hammond. 1995. Evaluation and validation of a monoclonal immunofluorescence regent for direct detection of Bordetella pertussis. J. Clin. Microbiol. 33:2868-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milstein, C., and A. C. Cuello. 1983. Hybrid hybridomas and their use in immunohistochemistry. Nature 305:537-540. [DOI] [PubMed] [Google Scholar]
- 16.Parker, C. D., and B. J. Payne. 1985. Bordetella pertussis, p. 394-399. In E. H. Lennette, A. Balows, W. J. Hausler, Jr., and H. J. Shadomy (ed.), Manual of clinical microbiology, 4th ed. American Society for Microbiology, Washington, D.C.
- 17.Peppler, M. S. 1984. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect. Immun. 43:224-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stratieva-Taneeva, P. A., S. V. Khaidukov, V. A. Kovalenko, I. V. Nazimov, L. V. Samokhvalova, and V. A. Nesmeyanov. 1993. Bispecific monoclonal antibodies to human interleukin 2 and horseradish peroxidase. Hybridoma 12:271-284. [DOI] [PubMed] [Google Scholar]
- 19.Suresh, M. R., A. C. Cuello, and C. Milstein. 1986. Advantages of bispecific hybridomas in one step immunocytochemistry and immunoassays. Proc. Natl. Acad. Sci. USA 83:7989-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tilley, P. A., M. V. Kanchana, I. Knight, J. Blondeau, N. Antonishyn, and H. Deneer. 2000. Detection of Bordetella pertussis in a clinical laboratory by culture, polymerase chain reaction and direct fluorescence antibody staining; accuracy, and cost. Diagn. Microbiol. Infect. Dis. 37:17-23. [DOI] [PubMed] [Google Scholar]