Dengue is an endemic viral disease affecting tropical and subtropical regions around the world, predominantly in urban and semiurban areas. Dengue fever (DF) and its more serious forms, dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), are becoming important public health problems and were formally included within the disease portfolio of the United Nations Development Programme/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases by the Joint Coordination Board in June 1999 (100). The global prevalence of dengue has grown dramatically in recent decades. The disease is now endemic in more than 100 countries in Africa, the Americas, the eastern Mediterranean, Southeast Asia, and the Western Pacific, threatening more than 2.5 billion people (33). The World Health Organization estimates that there may be 50 million to 100 million cases of dengue virus infections worldwide every year, which result in 250,000 to 500,000 cases of DHF and 24,000 deaths each year (25, 99)
Dengue virus is a mosquito-borne flavivirus and the most prevalent arbovirus in tropical and subtropical regions of the world (32). Dengue virus is a positive-stranded encapsulated RNA virus. The genomic RNA is approximately 11 kb in length and is composed of three structural protein genes that encode the nucleocapsid or core protein (C), a membrane-associated protein (M), an envelope protein (E), and seven nonstructural (NS) protein genes. The gene order is 5′-C-prM(M)-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5-3′, as for other flaviviruses (11, 18, 60, 74). The proteins are synthesized as a polyprotein of about 3,000 amino acids that is processed cotranslationally and posttranslationally by viral and host proteases. There are four distinct serotypes, serotypes 1 to 4. Infection induces a life-long protective immunity to the homologous serotype but confers only partial and transient protection against subsequent infections by the other three serotypes. Instead, it has generally been accepted that secondary infection or infection with secondary or multiple infections with various dengue virus serotypes is a major risk factor for DHF-DSS due to antibody-dependent enhancement (8, 34, 37, 38). Other factors have been postulated to be important in the pathogenesis of DHF, including viral virulence (29, 76), host genetic background (4), T-cell activation (26, 54), the viral burden (93), and autoantibodies (59, 61). As attempts to eradicate Aedes aegypti, the most efficient mosquito vector of dengue virus, are not successful in countries where dengue is endemic, the control of dengue will be possible only after an efficient vaccine has been developed. At present, no dengue vaccine has been licensed. The development of an efficient dengue vaccine is difficult because the vaccine must be tetravalent so that it includes all four serotypes. In addition, there is no acceptable animal model for DHF. Although several candidate vaccines are in clinical trials, an efficient, safe, low-cost vaccine will not be available in the near future.
Dengue virus causes a broad spectrum of illnesses, ranging from inapparent infection, flu-like mild undifferentiated fever, and classical DF to the more severe form, DHF-DSS, from which rates of morbidity and mortality are high (33, 36, 70, 99). DF is characterized by fever of 3 to 5 days' duration, headache, muscle and joint pain, and a rash, which is self-limited and from which patients usually recover completely. There is no specific treatment for DF, and most forms of therapy are supportive in nature. DHF-DSS is characterized by the same signs and symptoms as classic DF, but it is followed by increased vascular permeability and hemorrhage, which may lead to vascular collapse and death. Careful clinical management by experienced medical professionals is important in saving the lives of DHF patients. Diagnosis of dengue virus infection on the basis of clinical syndromes is not reliable, and the diagnosis should be confirmed by laboratory studies, because more than half of infected individuals either are asymptomatic or have a mild undifferentiated fever (8, 20). Therefore, there is a great demand for the rapid detection and differentiation of dengue virus infection in the acute phase of illness in order to provide timely clinical treatment and etiologic investigation and disease control.
LABORATORY DIAGNOSIS OF DENGUE VIRUS INFECTION
Laboratory diagnosis of dengue virus infection can be made by the detection of specific virus, viral antigen, genomic sequence, and/or antibodies (33, 35, 36, 94, 99). At present, the three basic methods used by most laboratories for the diagnosis of dengue virus infection are viral isolation and characterization, detection of the genomic sequence by a nucleic acid amplification technology assay, and detection of dengue virus-specific antibodies. After the onset of illness, the virus is found in serum or plasma, circulating blood cells, and selected tissues, especially those of the immune system, for approximately 2 to 7 days, roughly corresponding to the period of fever (99). Molecular diagnosis based on reverse transcription (RT)-PCR, such as one-step or nested RT-PCR, nucleic acid sequence-based amplification (NASBA), or real-time RT-PCR, has gradually replaced the virus isolation method as the new standard for the detection of dengue virus in acute-phase serum samples.
Two patterns of serological response can be observed in patients with dengue virus infection: primary and secondary antibody responses, depending on the immunological status of the infected individuals. A primary antibody response is seen in individuals who are not immune to flaviviruses. A secondary antibody response is seen in individuals who have had a previous flavivirus infection. For acute- and convalescent-phase sera, serological detection of antibodies based on capture immunoglobulin M (IgM) and IgG enzyme-linked immunosorbent assay (ELISA) has become the new standard for the detection and differentiation of primary and secondary dengue virus infections (31, 46, 99). This is important, since a sensitive and reliable assay for the detection and differentiation of primary versus secondary or multiple dengue virus infection is critical for the analysis of data for epidemiological, pathological, clinical, and immunological studies.
Virus isolation and characterization.
For virus detection, virus isolation by cell culture and from mosquitoes remains the “gold standard,” although it has gradually been replaced by the RT-PCR method for rapid diagnosis. This is mainly due to its lower sensitivity and the fact that a longer time for detection is required if indirect immunofluorescence is performed to identify the isolated virus with dengue- or serotype-specific monoclonal antibodies (24, 40, 99). However, the molecular method based on RT-PCR has been combined with the cell culture method to improve the sensitivity and reduce the time needed to identify the cultured viruses (71). The latter method was reported to detect the cultured virus at day 1 (versus day 4 by the indirect immunofluorescence method) if 104 viruses/ml were inoculated into the culture. It is obvious that viral isolation is indispensable for most laboratories interested in studies of the basic virology, molecular epidemiology and pathogenesis of dengue virus. The isolation of viruses from clinical samples can be conveniently carried out with cultured mosquito cells, such as the AP-61, Tra-284, C6/36, AP64, and CLA-1 cell lines, or mammalian cells, such as the LLCMK2, Vero, and BHK21 cell lines (35). Because of its higher sensitivity, the mosquito inoculation technique is still the method of choice for attempting dengue virus isolation from deceased patients with fatal cases or patients with severe hemorrhagic disease (55, 75). Aedes albopictus (28, 50) and Toxorhynchites spendens (97) have been shown to be useful for dengue virus recovery. At present, virus isolation with the C6/36 cell line with acute-phase serum or plasma from patients is the method of choice for routine dengue virus isolation.
Molecular diagnosis.
The field of molecular diagnosis has changed significantly over the past decade, leading to assays that are much more reliable for the detection and characterization of various pathogens. Previously, rapid laboratory diagnosis did not contribute significantly to clinical treatment, etiologic investigation, or control of dengue virus infection due to the lack of a reliable and sensitive assay system for the detection of virus in acute-phase serum. However, several laboratories have published various RT-PCR protocols for dengue virus identification (35, 39, 41, 53, 57, 68, 79, 87). Among these, the two-step nested RT-PCR protocols originally reported by Lanciotti et al. (57) and later modified to a single-step multiplex RT-PCR for the detection and typing of dengue virus by Harris et al. (39) are well known. These assays used the dengue virus core to premembrane gene regions as the target sequence for dengue virus detection. They had the advantage of detecting and differentiating the four dengue virus serotypes by analyzing the unique sizes of the amplicons in the agarose gel. Alternatively, the NASBA assay, an isothermal RNA-specific amplification assay, has been developed for the detection of viral and bacterial RNA in clinical samples. Wu and coworkers (101) reported on the detection of dengue viruses by the NASBA assay, which had high degrees of sensitivity and specificity. Since the amplification procedure used with the NASBA assay is entirely isothermal and is conducted at 41°C, it would be suitable for epidemiological studies in the field.
More recently, several investigators have reported on fully automatic real-time RT-PCR assays for the detection of dengue virus in acute-phase serum samples (9, 13, 19, 42, 58, 84, 95, 96). The real-time PCR or RT-PCR assay has many advantages over conventional PCR or RT-PCR methods, including rapidity, the ability to provide quantitative measurements, a lower contamination rate, a higher sensitivity, a higher specificity, and easy standardization. Therefore, real-time PCR has gradually replaced conventional PCR as the new gold standard for the rapid diagnosis of dengue virus infection with acute-phase serum samples. Five main chemical formats (the DNA binding fluorophores, the 5′ nuclease, adjacent linear and hairpin oligonucleotide probes, and self-fluorescing amplicons) are used to detect the PCR product during real-time PCR (63). Among these, the most widely used format is the 5′→3′ nuclease oligonucleotide probe (TaqMan assay). The TaqMan real-time PCR is highly specific due to the sequence-specific hybridization of the probe. Along with the development of fluorophores, nucleotide labeling chemistries, and instrumentation, it has the potential to develop multiplex PCR protocol with up to four fluorophores in a single tube. Ideally, a four-color multiplex TaqMan real-time RT-PCR protocol could be developed to detect and differentiate the four dengue virus serotypes. Table 1 summarizes the various procedures for group- and serotype-specific real-time RT-PCR targeted at different regions of the dengue virus genome. It is important to emphasize that the primers and probes that have been reported previously may not be able to detect all dengue virus strains (23, 73). Indeed, the sensitivity of each of the primers and probes available depends on the sequence homology between the primers and probes and the targeted gene sequence of the particular virus analyzed. Therefore, it is always a good practice to use multiple primers and probes targeted at different gene regions in order to avoid the false-negative results caused by sequence variations among different strains and potential mutants. In contrast to the TaqMan assay, the SYBR Green real-time RT-PCR assay has the advantage of simplicity in primer design and uses universal RT-PCR protocols suitable for the detection of multiple target sequences, although it is theoretically less specific (53, 84). In an attempt to develop a universal diagnostic real-time RT-PCR protocol for arbovirus, we have successfully developed a one-step RT-PCR system that can be used to detect and differentiate several flaviviruses, including dengue virus, Japanese encephalitis (JE) virus, yellow fever virus, and West Nile virus (84; unpublished data). Finally, great care should be taken to avoid the false-positive results that may occur due to sample and/or reagent contamination during the performance of the RT-PCR.
TABLE 1.
Summary of the various procedures for dengue virus identification by real-time RT-PCR
| Authors (year) | Primer specificity | Chemistry | Target region (positions) | Reference |
|---|---|---|---|---|
| Shu et al. (2003) | Dengue group | SYBR Green | C (135-305) | 84 |
| Dengue serotype 1 | C (135-325) | |||
| Dengue serotype 2 | C (135-338) | |||
| Dengue serotype 3 | C (135-336) | |||
| Dengue serotype 4 | C (135-268) | |||
| Warrilow et al. (2002) | Dengue group | TaqMan | 3′ UTRa (10578-10685) | 96 |
| Wang et al. (2002) | Dengue serotype 2 | TaqMan | C (141-234) | 95 |
| Drosten et al. (2002) | Dengue group | TaqMan | 3′ UTR (10615-10694) | 19 |
| Callahan et al. (2001) | Dengue group | TaqMan | 3′ UTR (10589-10699) | 9 |
| Dengue serotype 1 | NS5 (8586-8692) | |||
| Dengue serotype 2 | C (237-305) | |||
| Dengue serotype 3 | C (118-241) | |||
| Dengue serotype 4 | C (187-293) | |||
| Chen et al. (2001) | Dengue serotype 2 | TaqMan | E | 13 |
| Houng et al. (2001) | Dengue serotypes 1 to 4 | TaqMan | 3′ UTR | 42 |
| Laue et al. (1999) | Dengue serotypes 1 to 4 | TaqMan | NS5 (9959-10119) | 58 |
UTR, untranslated region.
Serological diagnosis.
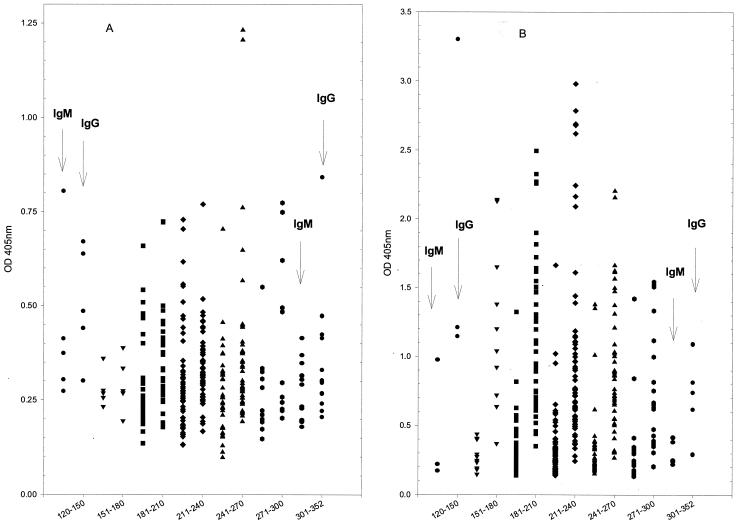
The serological diagnosis of dengue virus infection is rather complicated for the following reasons: (i) patients may have multiple and sequential infections with the four dengue virus serotypes due to a lack of cross-protective neutralization antibodies; (ii) multiple and sequential flavivirus infections make differential diagnosis difficult due to the presence of preexisting antibodies and original antigenic sin (many B-cell clones responding to the first flavivirus infection are restimulated to synthesize early antibody with a greater affinity for the first infecting virus than for the present infecting virus in every subsequent flavivirus infection) in regions where two or more flaviviruses are cocirculating; (iii) IgG antibodies have high degrees of cross-reactivity to homologous and heterologous flavivirus antigens; and (iv) the serodiagnosis of past, recent, and present dengue virus infections is difficult due to the long persistence of IgG antibodies (≥10 months, as measured by E/M-specific capture IgG ELISA, or life long, as measured by E/M antigen-coated indirect IgG ELISA) in many dengue patients with secondary infections (31, 46) (see Fig. 2B). Thus, among the viral infections that can be diagnosed by serology, dengue virus infection is among the most challenging. However, great advances in analyzing the complicated viral antigens and antibody responses have recently been made by the development of various methods that target different structural and NS proteins for serodiagnosis and seroepidemiological studies of dengue virus infection.
FIG. 2.
Persistence of IgM and IgG antibody levels in postinfection sera from dengue patients with primary (A) or secondary (B) dengue virus infection. Serum samples were collected from 120 to 352 days postinfection (as indicated on the x axis) and were measured by E/M-specific capture IgM and IgG ELISAs at a 1:100 dilution. See reference 85 for details about the method.
(i) Antigen detection.
Progress toward the detection of antigen in acute-phase serum samples by serology has been slow due to the low sensitivity of the assay for patients with secondary infections, as such patients have preexisting virus-IgG antibody immunocomplexes. However, recent studies that used ELISA and dot blot assays directed to the E/M antigen (the denKEY kit; Globio Co., Beverly, Mass.) and the NS1 antigen (1, 49, 102) demonstrated that high concentrations of the E/M and NS1 antigens in the forms of an immune complex could be detected in the acute-phase sera of both patients with primary dengue virus infections and patients with secondary dengue virus infections up to 9 days after the onset of illness. Koraka et al. (49) recently reported on the detection by a dot blot immunoassay of immune complex-dissociated NS1 antigen in patients with acute dengue virus infections. It was concluded that NS1 antigen detection by dot blot immunoassay in both nondissociated and dissociated serum and plasma samples from patients with primary and secondary dengue virus infections results in the highest number of dengue antigen-positive patients compared with the numbers obtained by RT-PCR and with the denKEY kit. Although the results demonstrated the potential of NS1 antigen detection for the serodiagnosis of acute dengue virus infection, the relatively low rate of positive results by the RT-PCR assay reported in the study suggested that it was underestimated (49, 84). In addition, many RT-PCR-positive samples showed negative results when they were analyzed by this NS1 antigen detection method. Further studies are needed to evaluate and compare the sensitivities and specificities of RT-PCR and NS1 antigen detection assays for the early diagnosis of dengue virus infection with acute-phase serum samples. Despite this concern, the results suggest that assays for NS1 antigen detection could be a potential means for the early diagnosis of dengue virus infection.
(ii) Antibody detection.
Several methods have been described for the serological detection of dengue virus-specific antibodies, including the hemagglutination inhibition (HI) test (16), the neutralization test (77), the indirect immunofluorescent-antibody test (90), ELISA (6), complement fixation (30), dot blotting (10), Western blotting (51), and the rapid immunochromatography test (for which many commercial kits are available). Among these, capture IgM and/or IgG ELISA, antigen-coated indirect IgM and/or IgG ELISA, and the HI test are the most commonly used serological techniques for the routine diagnosis of dengue virus infections. Traditionally, the HI test was used to detect and differentiate primary and secondary dengue virus infections due to its simplicity, sensitivity, and reproducibility. Patients are classified as having secondary dengue virus infections when the HI test titer in their sera is greater than or equal to 1:2,560 and are classified as having primary dengue virus infection if the HI test titer is less than 1:2,560 (99). The HI test has recently become less popular and has gradually been replaced by the E/M-specific capture IgM and IgG ELISA due to the inherent disadvantages of the HI test (46, 85).
The E/M-specific capture IgM and IgG ELISA has become the most powerful assay for the serodiagnosis of dengue virus infection due to its high sensitivity, specificity, simplicity, and feasibility for automation (5, 6, 27, 46, 52, 85). Many commercial kits with good sensitivities and specificities are now available (27, 65). Anti-dengue virus IgM antibody is produced transiently during primary and secondary infections. In patients with primary dengue virus infections, IgM antibodies develop rapidly and are detectable on days 3 to 5 of illness in half of the hospitalized patients. Studies of the dynamic antibody response showed that anti-dengue virus IgM levels peak at about 2 weeks postinfection and then decline to undetectable levels over 2 to 3 months (46, 99). Anti-dengue virus IgG appears shortly afterwards.
In patients with secondary dengue virus infections, while the kinetics of IgM production are similar to those observed in patients with primary infections, IgM levels are significantly lower (46, 99). Anti-dengue virus IgM antibodies also peak at about 2 weeks postinfection, begin to wane thereafter, and are detectable in about 30% of patients 2 months after the onset of symptoms. In contrast to primary infection, secondary infection with dengue virus results in the earlier appearance of high titers of cross-reactive IgG antibodies before or simultaneously with the IgM responses (99).
Innis et al. (46) first proposed classification of primary and secondary infections by determining the ratio of the units of dengue virus IgM antibodies to the units of dengue virus IgG antibodies. They showed that the acute-phase sera of patients with primary dengue virus infections had higher IgM/IgG ratios, whereas patients with secondary infections had lower IgM/IgG ratios. This method has made a great contribution to the analysis of the immune status of patients with dengue. We have recently modified and simplified this method so that differentiation of primary and secondary dengue virus infection can be made by using the ratio of IgM/IgG readings directly (≥1.2 or <1.2, respectively) without calculating the antibody units through the use of a standard control (85).
To compare the dynamic distributions of serum IgM and IgG antibodies induced by primary and secondary dengue virus infections, we have recently analyzed dengue virus-specific IgM and IgG antibodies using serum samples collected from patients in the acute, convalescent, and postinfection stages. Figure 1 shows the dynamics of the serum IgM and IgG antibody levels measured by an E/M-specific capture IgM and IgG ELISA in patients with primary and secondary dengue virus infections whose sera were collected during the acute and convalescent phases, whereas Fig. 2 shows the dynamics of the serum IgM and IgG antibody levels in sera collected between 120 and 352 days postinfection. Figure 2A and B presents the results for patients with primary and secondary dengue virus infections, respectively. The results show that most patients with primary dengue virus infection would not have detectable IgM or IgG antibodies at 4 months postinfection. This is in contrast to the high IgG titers detected in many of the patients with secondary dengue even 10 months after infection.
FIG. 1.
Dynamics of IgM and IgG antibody levels in acute- and convalescent-phase serum samples from dengue patients with primary or secondary infection. Serum samples collected at the acute phase (3 to 7 days after the onset of illness), the early convalescent phase (8 to 13 days), and the late convalescent phase (14 to 30 days) were analyzed by E/M-specific capture IgM and IgG ELISA at a 1:100 dilution. See reference 85 for details about the method.
Although detection of IgM antibody to dengue virus by an E/M-specific capture IgM ELISA usually indicates an active or recent infection, the most reliable way to demonstrate active infection would be a significant (fourfold or greater) rise in IgM and/or IgG antibody titers between the acute- and the convalescent-phase sera. This could best be analyzed by an E/M-specific capture IgM ELISA (for IgM antibodies) and an E/M antigen-coated indirect IgG ELISA (for IgG antibodies) with serially diluted serum samples. For routine analysis, significant increases in IgM and/or IgG antibody levels, from negative or low optical density (OD) values in acute-phase serum to positive and high OD values in convalescent-phase serum, can be conveniently determined. We strongly recommend the analysis of paired serum samples from both the acute and the convalescent phases by an E/M-specific capture IgM and IgG ELISA to avoid false-positive results in areas where dengue is highly endemic because of the long persistence of dengue virus-specific IgG antibodies in many patients with secondary infections.
Many laboratories have used E/M antigen-coated indirect IgM and IgG ELISAs (especially the indirect IgG ELISA) to detect dengue virus-specific IgM and IgG antibodies. Due to the higher sensitivity of this assay for the detection of IgG compared to that of the E/M-specific capture IgG ELISA, it can be reliably used to determine whether a fourfold or greater increase in the levels of specific IgG antibodies is present (14, 31, 67). Some laboratories have replaced the HI test with the antigen-coated indirect IgG ELISA for the detection and differentiation of primary and secondary dengue virus infections (14).
It is worth emphasizing that although E/M-specific IgG antibodies are highly cross-reactive among various flaviviruses during a secondary response, the E/M- and NS1-specific anti-dengue virus IgM antibodies have limited cross-reactivities (45, 46, 64, 81). Therefore, the cross-reactivity of dengue-specific IgM antibodies found in a few dengue patients may actually indicate a recent infection with another flavivirus. Indeed, studies have shown the successful development of tests, based on IgM antibodies, with a panel of viral antigens for the differential diagnosis of acute or recent flavivirus infection (66, 78). We have found that the E/M-specific capture IgM and IgG ELISA can reliably be used for the differential diagnosis of JE, dengue, yellow fever, and West Nile encephalitis by using virus-infected culture supernatants as the source of viral antigens and flavivirus-specific mouse monoclonal antibodies as the secondary antibody (86; unpublished data).
Some investigators have studied dengue virus-specific IgA and IgE antibody responses. Talarmin et al. (88) reported on the use of an IgA- and IgM-specific capture ELISA for the diagnosis of dengue virus infection. The results showed that IgM appears more rapidly and lasts longer (over 2 to 3 months) than IgA (about 40 days). They concluded that the capture IgA ELISA is a simple method that can be performed together with the capture IgM ELISA and that can help in interpreting the serology of DF. More recently, Balmaseda et al. (2) reported on the detection of specific IgM and IgA antibodies in serum and saliva. They concluded that dengue virus-specific IgA in serum is a potential diagnostic target. Koraka et al. (48) reported on the development of a capture IgE ELISA for the detection of total and dengue virus-specific IgE antibody responses. The results showed that dengue virus-specific IgE titers were significantly higher in patients with DHF and/or DSS than in patients with DF and non-dengue virus infections. They concluded that analysis of dengue virus-specific IgE ELISA might contribute to the understanding of the pathogenesis of dengue virus.
Previous studies directed toward the analysis of NS proteins have shown that the NS1, NS3, and NS5 antigens are the most immunogenic in inducing dengue virus-specific antibody responses (15, 21, 22, 51, 72, 80, 89). Among these, NS1-specific antibody responses were studied by using synthetic peptides, recombinant protein, or native antigens from either mouse brain-derived or virus-infected culture supernatants (15, 21, 22, 43, 44, 51, 81, 83, 85). We have developed an NS1 isotype- and serotype-specific ELISA that can be easily and reliably used to differentiate (i) JE virus and dengue virus infections, (ii) JE vaccination and JE infection, and (iii) primary and secondary dengue virus infections and (iv) for serotyping of dengue virus in patients with primary dengue virus infections (81, 82, 83, 85). More recently, a retrospective seroepidemiological study with serum samples collected from Liuchiu Hsiang, Pingtung County, in southern Taiwan demonstrated that the NS1 serotype-specific IgG ELISA could replace the neutralization test for seroepidemiological studies for the differentiation JE virus and dengue virus infections and for the serotyping of dengue virus in patients with primary infections (83). Furthermore, this assay can be used to differentiate secondary and tertiary or quaternary dengue virus infections if very early acute-phase sera (from days 1 to 3 after the onset of illness) are available for analysis. Serum samples from patients with secondary infections collected from days 1 to 3 after the onset of illness would show a primary NS1 serotype-specific IgG response, while serum samples from patients with tertiary or quaternary infections would show strong and complex NS1 serotype-specific IgG responses.
Wong et al. (98) recently reported on an immunoassay that targets the NS5 antigen for the differentiation of West Nile virus infection from dengue virus and St. Louis encephalitis virus infections and from vaccination against a flavivirus. The results showed that the NS5-based assay could reliably be used to discriminate between West Nile virus infections and dengue virus or St. Louis encephalitis virus infections.
Many rapid test kits that use the principle of immunochromatography are commercially available. Most of these kits can simultaneously detect IgM and IgG antibodies to dengue virus in human whole blood, serum, or plasma within 5 to 30 min. Some of these kits claim that it is possible to differentiate primary and secondary dengue virus infections, although our experience suggests that this is not always reliable. Several evaluations that offer conclusions in favor or against these commercial kits are available (3, 12, 17, 56, 91, 92). We have done preliminary evaluations of five rapid test kits available to us (unpublished data). The results showed that these kits generally have higher sensitivities for IgG detection but lower sensitivities for IgM detection and various specificities compared to the results of the E/M-specific capture IgM and IgG ELISA. Although the rapid test has the advantages of easy performance and the rapid provision of results, it should best serve as a screening test for clinicians in hospitals. Furthermore, these kits should not be used for surveillance for dengue disease in public health settings or in seroepidemiological studies due to the high sensitivity of this assay for the detection of IgG and the long persistence of cross-reactive flavivirus IgG antibodies in the general population in many areas where dengue is endemic.
DENGUE VIRUS SEROTYPING
Dengue virus serotype analysis is important in epidemiological and pathological studies. Among the available methods, virus isolation followed by type-specific monoclonal antibody immunofluorescence staining, the neutralization test, and RT-PCR are widely used by many laboratories studying dengue virus (57, 77, 99). Several studies have addressed whether the E/M-specific capture IgM ELISA can be used accurately to identify the dengue virus serotype (7, 33, 47, 69). This problem remains unsettled and points to the difficulty of dengue virus serotyping by the capture IgM ELISA. Burke (7) investigated the serotype specificity of IgM to dengue virus using convalescent-phase serum and antigens of the four dengue virus serotypes. He found serotype-specific IgM responses in all 16 patients with primary dengue virus infection and 9 of 16 patients with secondary dengue virus infection. Nawa et al. (69) analyzed serum samples from 14 patients with confirmed dengue without knowledge of their immune status. They found that IgM responses were generally cross-reactive among the serotypes but that in most cases IgM levels were highest against the infecting dengue virus serotype.
We have recently developed E/M and NS1 serotype-specific capture IgM ELISAs by using culture supernatants of serotype 1, 2, 3, and 4 dengue virus-infected Vero cells as the antigen source to detect and differentiate the dengue virus serotypes in convalescent-phase serum samples. Dual analyses by both the E/M and the NS1 serotype-specific capture IgM ELISAs showed that positive serotype specificity could be correctly identified in 98.6 and 61.9% of all serum samples from patients with primary and secondary dengue virus infections, respectively (86). It is emphasized that equal amounts of each of the four dengue virus antigens should be added to the microtiter wells in order to obtain reliable results.
As described above, the NS1 serotype-specific IgG ELISA could also be reliably used for dengue virus serotype analysis with convalescent-phase sera from patients with primary infections and acute-phase sera from patients with secondary infections (which would detect the serotype that caused the first infection) but not with convalescent-phase sera from patients with secondary infections (85).
Ludolfs et al. (62) reported on the serological differentiation of infections with dengue virus serotypes 1 to 4 by using recombinant antigens. Immunoblot strips dotted with the B domains of dengue virus serotypes 1 to 4 expressed in Escherichia coli were used to detect serotype-specific antibodies in paired serum samples from 41 patients with primary and secondary dengue virus infections. The results showed that the correct serotype could be identified in 31 of 33 patients with primary dengue virus infection. However, this immunoblot strip method is not reliable for serotyping of the virus causing secondary dengue virus infections.
CONCLUSIONS
The laboratory diagnosis of dengue virus infection has been greatly improved during the last decade. The rapid detection of the dengue virus genomic sequence by real-time one-step RT-PCR has become a trend. This assay has the advantages of simplicity, rapidity, and a low contamination rate compared to the characteristics of the nested RT-PCR method, which, however, has a sensitivity similar to that of the real-time RT-PCR. For acute-phase serum samples, the real-time one-step RT-PCR by either the TaqMan assay or SYBR Green method has been developed and successfully applied to the clinical diagnosis of dengue virus infections. Future developments based on a four-color multiplex protocol may revolutionize this field and eventually replace the conventional RT-PCR as the new gold standard for the rapid diagnosis of dengue virus infection. It should be pointed out that multiple primers and probes targeted to different regions of the dengue virus gene are needed to increase the sensitivity and avoid false-negative results. In addition, good quality control should be followed to avoid false-positive results caused by sample and/or reagent contamination.
For serological diagnosis, detection of the NS1 antigen in acute-phase serum samples has shown promising results. Further studies are needed to evaluate its usefulness and to compare it with real-time one-step RT-PCR with respect to its sensitivity and specificity. For antibody detection, the E/M-specific capture IgM and IgG ELISA has become the new standard in serodiagnosis due to its high degrees of sensitivity and specificity and its simplicity. Careful analysis showed that the differential diagnosis of flavivirus infection could be made by tests with a panel of viral antigens. Finally, an easy, sensitive, and specific NS1 serotype-specific IgG ELISA has been developed and has reliably been used for the serodiagnosis and seroepidemiological study of dengue virus infection. The advantage of the NS1 serotype-specific IgG ELISA is that dengue virus serotyping is possible for patients with primary dengue virus infection if convalescent-phase or postinfection sera are available.
In conclusion, present advances in molecular and serological diagnostic methods have greatly improved the sensitivity and specificity of diagnosis of dengue virus infection. It is expected that the successful application of these assays will contribute significantly to the clinical treatment, etiologic investigation, and control of dengue virus infections.
REFERENCES
- 1.Alcon, S., A. Talarmin, M. Debruyne, A. Falconar, V. Deubel, and M. Flamand. 2002. Enzyme-linked immunosorbent assay to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40:376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balmaseda, A., M. G. Guzman, S. Hammond, G. Robleto, C. Flores, Y. Tellez, E. Videa, S. Saborio, L. Perez, E. Sandoval, Y. Rodriguez, and E. Harris. 2003. Diagnosis of dengue virus infection by detection of specific immunoglobulin M (IgM) and IgA antibodies in serum and saliva. Clin. Diagn. Lab. Immunol. 10:317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branch, S. L., and P. N. Levett. 1999. Evaluation of four methods for detection of immunoglobulin M antibodies to dengue virus. Clin. Diagn. Lab. Immunol. 6:555-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bravo, J. R., M. G. Guzman, and G. P. Kouri. 1987. Why dengue haemorrhagic fever in Cuba? I. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS). Trans. R. Soc. Trop. Med. Hyg. 81:816-820. [DOI] [PubMed] [Google Scholar]
- 5.Bundo, K., and A. Igarashi. 1985. Antibody-capture ELISA for detection of immunoglobulin M antibodies in sera from Japanese encephalitis and dengue hemorrhagic fever patients. J. Virol. Methods 11:15-22. [DOI] [PubMed] [Google Scholar]
- 6.Burke, D. S., A. Nisalak, and M. A. Ussery. 1982. Antibody capture immunoassay detection of Japanese encephalitis virus immunoglobulin M and G antibodies in cerebrospinal fluid. J. Clin. Microbiol. 15:1034-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke, D. S. 1983. Rapid methods in the laboratory diagnosis of dengue virus infections, p. 72-84. In T. Pang and R. Pathmananathan (ed.), Proceedings of the International Conference Dengue/Dengue Hemorrhagic Fever. University of Malaysia, Kuala Lumpur, Malaysia.
- 8.Burke, D. S., A. Nisalak, D. E. Johnson, and R. M. Scott. 1988. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 38:172-180. [DOI] [PubMed] [Google Scholar]
- 9.Callahan, J. D., S. J. Wu, A. Dion-Schultz, B. E. Mangold, L. F. Peruski, D. M. Watts, K. R. Porter, G. R. Murpgy, W. Suharyono, C. C. King, C. G. Hayes, and J. J. Temenak. 2001. Development and evaluation of serotype- and group-specific fluorogenic reverse transcriptase PCR (TaqMan) assays for dengue virus. J. Clin. Microbiol. 39:4119-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardosa, M. J., T. Phaik, and N. Sham. 1988. Development of a dot enzyme immunoassay for dengue 3: a sensitive method for the detection of anti-dengue antibodies. J. Virol. Methods 22:81-88. [DOI] [PubMed] [Google Scholar]
- 11.Chambers, T. J., C. S. Hahn, R. Galler, and C. M. Rice. 1990. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44:649-688. [DOI] [PubMed] [Google Scholar]
- 12.Charrel, R. N., and X. de Lamballerie. 2002. Low specificity of an immunochromatographic serological assay for diagnosis of dengue fever in travelers returning with malaria. Clin. Diagn. Lab. Immunol. 9:1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, R. F., W. T. Yeh, M. Y. Yang, and K. D. Yang. 2001. A model of the real-time correlation of viral titers with immune reactions in antibody-dependent enhancement of dengue-2 infections. FEMS Immunol. Med. Microbiol. 30:1-7. [DOI] [PubMed] [Google Scholar]
- 14.Chungue, E., G. Marche, R. Plichart, J. P. Boutin, and J. Roux. 1989. Comparison of immunoglobulin G enzyme-linked immunosorbent assay (IgG-ELISA) and haemagglutination inhibition (HI) test for the detection of dengue antibodies. Prevalence of dengue IgG-ELISA antibodies in Tahiti. Trans. R. Soc. Trop. Med. Hyg. 83:708-711. [DOI] [PubMed] [Google Scholar]
- 15.Churdboonchart, V., N. Bhamarapravati, S. Peampramprecha, and S. Sirinavin. 1991. Antibodies against dengue viral proteins in primary and secondary dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 44:481-493. [DOI] [PubMed] [Google Scholar]
- 16.Clarke, D. H., and J. Casals. 1958. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne virus. Am. J. Trop. Med. Hyg. 7:561-573. [DOI] [PubMed] [Google Scholar]
- 17.Cuzzubbo, A. J., T. P. Endy, A. Nisalak, S. Kalayanarooj, D. W. Vaughn, S. A. Ogata, D. E. Clements, and P. L. Devine. 2001. Use of recombinant envelope proteins for serological diagnosis of dengue virus infection in an immunochromatographic assay. Clin. Diagn. Lab. Immunol. 8:1150-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deubel, V., R. M. Kinney, and D. W. Trent. 1988. Nucleotide sequence and deduced amino acid sequence of the nonstructural proteins of dengue type 2 virus, Jamaica genotype: comparative analysis of the full-length genome. Virology 165:234-244. [DOI] [PubMed] [Google Scholar]
- 19.Drosten, C., S. Gottig, S. Schilling, M. Asper, M. Panning, H. Schmitz, and S. Gunther. 2002. Rapid detection and quantitation of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J. Clin. Microbiol. 40:2323-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endy, T. P., S. Chunsuttiwat, A. Nisalak, D. H. Libraty, S. Green, A. L. Rothman, D. W. Vaughn, and F. A. Ennis. 2002. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am. J. Epidemiol. 156:40-51. [DOI] [PubMed] [Google Scholar]
- 21.Falkler, W. A., A. R. Diwan, and S. B. Halstead. 1973. Human antibody to dengue soluble complement-fixing (SCF) antigens. J. Immunol. 111:1804-1809. [PubMed] [Google Scholar]
- 22.Garcia, G., D. W. Vaughn, and R. M. Del Angel. 1997. Recognition of synthetic oligopeptides from nonstructural proteins NS1 and NS3 of dengue-4 virus by sera from dengue virus-infected children. Am. J. Trop. Med. Hyg. 56:466-470. [DOI] [PubMed] [Google Scholar]
- 23.Gardner, S. N., T. A. Kuczmarski, E. A. Vitalis, and T. R. Slezak. 2003. Limitations of TaqMan PCR for detecting divergent viral pathogens illustrated by hepatitis A, B, C, and E viruses and human immunodeficiency virus. J. Clin. Microbiol. 41:2417-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gentry, M. K., E. A. Henchal, J. M. McCown, W. E. Brandt, and J. M. Dalrymple. 1982. Identification of distinct determinants on dengue-2 virus using monoclonal antibodies. Am. J. Trop. Med. Hyg. 31:548-555. [DOI] [PubMed] [Google Scholar]
- 25.Gibbons, R. V., and D. W. Vaughn. 2002. Dengue: an escalating problem. BMJ 324:1563-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green, S., D. W. Vaughn, S. Kalayanarooj, S. Nimmannitya, S. Suntayakorn, A. Nisalak, R. Lew, B. L. Innis, I. Kurane, A. L. Rothman, and F. A. Ennis. 1999. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J. Infect. Dis. 179:755-762. [DOI] [PubMed] [Google Scholar]
- 27.Groen, J., P. Koraka, J. Velzing, C. Copra, and A. D. M. E. Osterhaus. 2000. Evaluation of six immunoassays for detection of dengue virus-specific immunoglobulin M and G antibodies. Clin. Diagn. Lab. Immunol. 7:867-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gubler, D. J., and L. Rosen. 1976. A simple technique for demonstrating transmission of dengue virus by mosquitoes without the use of vertebrate hosts. Am. J. Trop. Med. Hyg. 25:146-150. [DOI] [PubMed] [Google Scholar]
- 29.Gubler, D. J., D. Reed, L. Rosen, and J. C. J. Hitchcock. 1978. Epidemiologic, clinical, and virologic observations on dengue in the kingdom of Tonga. Am. J. Trop. Med. Hyg. 27:581-589. [DOI] [PubMed] [Google Scholar]
- 30.Gubler, D. J., and G. E. Sather. 1988. Laboratory diagnosis of dengue and dengue hemorrhagic fever, p. 291-322. In A. Homma and J. F. Cunha (ed.), Proceedings of the International Symposium on Yellow Fever and Dengue. Bio-Manguinhos, Rio de Janeiro, Brazil.
- 31.Gubler, D. J. 1996. Serologic diagnosis of dengue/dengue haemorrhagic fever. Dengue Bull. 20:20-23. [Google Scholar]
- 32.Gubler, D. J. 1997. Dengue and dengue hemorrhagic fever: its history and resurgence as a global public health problem, p. 1-22. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, N.Y.
- 33.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guzman, M. G., G. Kouri, J. Bravo, M. Soler, S. Vazquez, and L. Morier. 1990. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am. J. Trop. Med. Hyg. 42:179-184. [DOI] [PubMed] [Google Scholar]
- 35.Guzman, M. G., and G. Kouri. 1996. Advances in dengue diagnosis. Clin. Diagn. Lab. Immunol. 3:621-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guzman, M. G., and G. Kouri. 2003. Dengue: an update. Lancet Infect. Dis. 2:33-42. [DOI] [PubMed] [Google Scholar]
- 37.Halstead, S. B., H. Shotwell, and J. Casals. 1973. Studies on the pathogenesis of dengue infection in monkeys. II. Clinical laboratory responses to heterologous infection. J. Infect. Dis. 128:15-22. [DOI] [PubMed] [Google Scholar]
- 38.Halstead, S. B. 1988. Pathogenesis of dengue: challenge to molecular biology. Science 239:476-481. [DOI] [PubMed] [Google Scholar]
- 39.Harris, E., T. G. Roberts, L. Smith, J. Selle, L. D. Kramer, S. Valle, E. Sandoval, and A. Balmaseda. 1998. Typing of dengue viruses in clinical specimens and mosquitoes by single-tube multiplex reverse transcriptase PCR. J. Clin. Microbiol. 36:2634-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henchal, E. A., J. M. McCown, M. C. Seguin, M. K. Gentry, and W. E. Brandt. 1983. Rapid identification of dengue virus isolates by using monoclonal antibodies in an indirect immunofluorescence assay. Am. J. Trop. Med. Hyg. 32:164-169. [DOI] [PubMed] [Google Scholar]
- 41.Henchal, E. A., S. L. Polo, V. Vorndam, C. Yaemsiri, B. L. Innis, and C. H. Hoke. 1991. Sensitivity and specificity of a universal primer set for the rapid diagnosis of dengue virus infections by polymerase chain reaction and nucleic acid hybridization. Am. J. Trop. Med. Hyg. 45:418-428. [DOI] [PubMed] [Google Scholar]
- 42.Houng, H. H., R. C. M. Chen, D. W. Vaughn, and N. Kanesa-thasan. 2001. Development of a fluorogenic RT-PCR system for quantitative identification of dengue virus serotypes 1-4 using conserved and serotype-specific 3′noncoding sequences. J. Virol. Methods 95:19-32. [DOI] [PubMed] [Google Scholar]
- 43.Huang, J. H., J. J. Wey, C. Chin, and Y. C Wu. 1999. Antibody responses to an immunodominant nonstructural 1 synthetic peptide in patients with dengue fever and dengue hemorrhagic fever. J. Med. Virol. 57:1-8. [PubMed] [Google Scholar]
- 44.Huang, J. L., J. H. Huang, R. H. Shyn, C. W. Teng, Y. L. Lin, M. D. Kuo, C. W. Yao, and M. F. Shaio. 2001. High-level expression of immunodominant recombinant dengue viral NS1 protein and its potential use as a diagnostic antigen. J. Med. Virol. 65:553-560. [PubMed] [Google Scholar]
- 45.Hung, N. T., H. Y. Lei, N. T. Lan, Y. S. Lin, K. J. Huang, L. B. Lien, C. F. Lin, T. M. Yeh, D. Q. Ha, V. T. Q. Huong, L. C. Chen, J. H. Huang, L. T. My, C. C. Liu, and S. B. Halstead. 2004. Dengue hemorrhagic fever in infants: a study on clinical and cytokine profiles. J. Infect. Dis. 221-232. 189: [DOI] [PubMed]
- 46.Innis, B. L., A. Nisalak, S. Nimmannitya, S. Kusalerdchariya, V. Chongswasdi, S. Suntayakorn, P. Puttisri, and C. H. Hoke. 1989. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am. J. Trop. Med. Hyg. 40:418-427. [DOI] [PubMed] [Google Scholar]
- 47.Innis, B. L. 1997. Antibody responses to dengue virus infection, p. 221-243. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, N.Y.
- 48.Koraka, P., B. Murgue, X. Deparis, T. E. Setiati, C. Suharti, E. C. van Gorp, C. E. Hack, A. D. Osterhaus, and J. Groen. 2003. Elevated levels of total and dengue virus-specific immunoglobulin E in patients with varying disease severity. J. Med. Virol. 70:91-98. [DOI] [PubMed] [Google Scholar]
- 49.Koraka, P., C. P. Burghoorn-Maas, A. Falconar, T. E. Setiati, K. Djamiatun, J. Groen, and A. D. M. E. Osterhaus. 2003. Detection of immune-complex-dissociated nonstructural-1 antigen in patients with acute dengue virus infections. J. Clin. Microbiol. 41:4154-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuberski, T., and L. Rosen. 1977. A simple technique for the detection of dengue antigen in mosquitoes by immunofluorescence. Am. J. Trop. Med. Hyg. 26:533-537. [DOI] [PubMed] [Google Scholar]
- 51.Kuno, G., A. V. Vorndam, D. J. Gubler, and I. Gomez. 1990. Study of anti-dengue NS1 antibody by Western blot. J. Med. Virol. 32:102-108. [DOI] [PubMed] [Google Scholar]
- 52.Kuno, G., I. Gomez, and D. J. Gubler. 1991. An ELISA procedure for the diagnosis of dengue infections. J. Virol. Methods 33:101-113. [DOI] [PubMed] [Google Scholar]
- 53.Kuno, G. 1998. Universal diagnostic RT-PCR protocol for arboviruses. J. Virol. Methods 72:27-41. [DOI] [PubMed] [Google Scholar]
- 54.Kurane, I., A. L. Rothman, P. G. Livingston, S. Green, S. J. Gagnon, J. Janus, B. L. Innis, S. Nimmannitya, A. Nisalak, and F. A. Ennis. 1994. Immunopathologic mechanisms of dengue hemorrhagic fever and dengue shock syndrome. Arch. Virol. Suppl. 9:59-64. [DOI] [PubMed] [Google Scholar]
- 55.Lam, S. K. 1986. Isolation of dengue viruses by intracerebral inoculation of mosquito larvae. J. Virol. Methods 14:133-140. [DOI] [PubMed] [Google Scholar]
- 56.Lam, S. K., and P. L. Devine. 1998. Evaluation of capture ELISA and rapid immunochromatographic test for the determination of IgM and IgG antibodies produced during dengue infection. Clin. Diagn. Virol. 10:75-81. [DOI] [PubMed] [Google Scholar]
- 57.Lanciotti, R. S., C. H. Calisher, D. J. Gubler, G. J. Chang, and A. V. Vorndam. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laue, T., P. Emmerich, and H. Schmitz. 1999. Detection of dengue virus RNA in patients after primary or secondary dengue infection by using the TaqMan automated amplification system. J. Clin. Microbiol. 37:2543-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lei, H. Y., T. M. Yeh, H. S. Liu, Y. S. Lin., S. H. Chen, and C. C. Liu. 2001. Immunopathogenesis of dengue virus infection. J. Biomed. Sci. 8:377-388. [DOI] [PubMed] [Google Scholar]
- 60.Leyssen, P., E. D. Clercq, and J. Neyts. 2000. Perspectives for the treatment of infections with Flaviviridae. Clin. Microbiol. Rev. 13:67-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin, C. F., H. Y. Lei, A. L. Shiau, C. C. Liu, H. S. Liu, T. M. Yeh, S. T. Wang, T. I. Zang, F. C. Sheu, C. F. Kuo, and Y. S. Lin. 2001. Generation of IgM anti-platelet autoantibody in dengue patients. J. Med. Virol. 63:143-149. [PubMed] [Google Scholar]
- 62.Ludolfs, D., S. Schilling, J. Altenschmidt, and H. Schmitz. 2002. Serological differentiation of infections with dengue virus serotypes 1 to 4 by using recombinant antigens. J. Clin. Microbiol. 40:4317-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackay, I. M., K. E. Arden, and A. Nitsche. 2002. Real-time PCR in virology. Nucleic Acids Res. 30:1292-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Makino, Y., M. Tadano, M. Saito, N. Maneekarn, N. Sittisombut, V. Sirisanthana, B. Poneprasert, and T. Fukunaga. 1994. Studies on serological cross-reaction in sequential flavivirus infections. Microbiol. Immunol. 38:951-955. [DOI] [PubMed] [Google Scholar]
- 65.Mantke, O. D., K. Lemmer, S. S. Biel, J. Groen, H. Schmitz, J. P. Durand, H. Zeller, and M. Niedrig. 2004. Quality control assessment for the serological diagnosis of dengue virus infections. J. Clin. Virol. 29:105-112. [DOI] [PubMed] [Google Scholar]
- 66.Martin, D. A., B. J. Biggerstaff, B. Allen, A. J. Johnson, R. S. Lanciotti, and J. T. Roehrig. 2002. Use of immunoglobulin M cross-reactions in differential diagnosis of human flaviviral encephalitis infections in the United States. Clin. Diagn. Lab. Immunol. 9:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miagostovich, M. P., R. M. Nogueira, F. B. dos Santos, H. G. Schatzmayr, E. S. Araujo, and V. Vorndam. 1999. Evaluation of an IgG enzyme-linked immunosorbent assay for dengue diagnosis. J. Clin. Virol. 14:183-189. [DOI] [PubMed] [Google Scholar]
- 68.Morita, K., M. Tanaka, and A. Igarashi. 1991. Rapid identification of dengue virus serotypes by using polymerase chain reaction. J. Clin. Microbiol. 29:2107-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nawa, M., K. I. Yamada, T. Takasaki, T. Akatsuka, and I. Kurane. 2000. Serotype-cross-reactive immunoglobulin M responses in dengue virus infections determined by enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 7:774-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nimmannitya, S. 1987. Clinical spectrum and management of dengue haemorrhagic fever. Southeast Asian J. Trop. Med. Public Health 18:392-397. [PubMed] [Google Scholar]
- 71.Oliveira, D. P. S., L. D. Malta, M. Clotteau, N. R. R. J. Pires, and F. B. A. Lopes. 2003. Improved detection of dengue-1 virus from IgM-positive serum samples using C6/36 cell cultures in association with RT-PCR. Intervirology 46:227-231. [DOI] [PubMed] [Google Scholar]
- 72.Patarapotikul, J., S. Pothipunya, R. Wanotayan, A. Hongyantarachai, and S. Tharavanij. 1993. Western blot analysis of antigens specifically recognized by natural immune responses of patients with Japanese encephalitis infections. Southeast Asian J. Trop. Med. Public Health 24:269-276. [PubMed] [Google Scholar]
- 73.Reynes, J. M., S. Ong, C. Mey, C. Ngan, S. Hoyer, and A. A. Sall. 2003. Improved molecular detection of dengue virus serotype 1 variants. J. Clin. Microbiol. 41:3864-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rice, C. M., E. M. Lenches, S. R. Eddy, S. J. Shin, R. L. Sheets, and J. H. Strauss. 1985. Nucleotide sequence of yellow fever virus. Implications for flavivirus expression and evolution. Science 229:726-733. [DOI] [PubMed] [Google Scholar]
- 75.Rosen, L., and D. Gubler. 1974. The use of mosquitoes to detect and propagate dengue viruses. Am. J. Trop. Med. Hyg. 23:1153-1160. [DOI] [PubMed] [Google Scholar]
- 76.Rosen, L. 1977. The Emperor's New Clothes revisited, or reflections on the pathogenesis of dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 26:337-343. [DOI] [PubMed] [Google Scholar]
- 77.Russell, P. K., A. Nisalak, P. Sukhavachana, and S. Vivona. 1967. A plaque reduction test for dengue virus neutralizing antibodies. J. Immunol. 99:285-290. [PubMed] [Google Scholar]
- 78.Schmitz, H., and P. Emmerich. 1984. Detection of specific immunoglobulin M antibody to different flaviviruses by use of enzyme-labeled antigens. J. Clin. Microbiol. 19:664-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seah, C. L. L., V. T. K. Chow, H. C. Tan, and Y. C. Chan. 1995. Rapid single-step RT-PCR typing of dengue viruses using NS3 gene primers. J. Virol. Methods 51:193-200. [DOI] [PubMed] [Google Scholar]
- 80.Se-Thoe, S. Y., M. M. Ng, and A. E. Ling. 1999. Retrospective study of Western blot profiles in immune sera of natural dengue virus infections. J. Med. Virol. 57:322-330. [DOI] [PubMed] [Google Scholar]
- 81.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, T. H. Lin, and J. H. Huang. 2000. Dengue NS1-specific antibody responses: isotype distribution and serotyping in patients with dengue fever and dengue hemorrhagic fever. J. Med. Virol. 62:224-232. [DOI] [PubMed] [Google Scholar]
- 82.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, T. H. Lin, and J. H. Huang. 2001. Antibody to the nonstructural protein NS1 of Japanese encephalitis virus: potential application of mAb-based indirect ELISA to differentiate infection from vaccination. Vaccine 19:1753-1763. [DOI] [PubMed] [Google Scholar]
- 83.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, H. H. Yang, T. H. Lin, and J. H. Huang. 2002. Potential application of nonstructural protein NS1 serotype-specific immunoglobulin G enzyme-linked immunosorbent assay in the seroepidemiologic study of dengue virus infection: correlation of results with those of the plaque reduction neutralization test. J. Clin. Microbiol. 40:1840-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shu, P. Y., S. F. Chang, Y. C. Kuo, Y. Y. Yueh, L. J. Chien, C. L. Sue, T. H. Lin, and J. H. Huang. 2003. Development of group- and serotype-specific one-step SYBR Green I-based real-time reverse transcription-PCR assay for dengue virus. J. Clin. Microbiol. 41:2408-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, T. H. Lin, and J. H. Huang. 2003. Comparison of capture immunoglobulin M (IgM) and IgG enzyme-linked immunosorbent assay (ELISA) and nonstructural protein NS1 serotype-specific IgG ELISA for differentiation of primary and secondary dengue virus infections. Clin. Diagn. Lab. Immunol. 10:622-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shu, P. Y., L. K. Chen, S. F. Chang, C. L. Sue, L. J. Chien, C. Chin, T. H. Lin, and J. H. Huang. 2004. Dengue virus serotyping based on envelope/membrane (E/M) and nonstructural protein NS1 serotype-specific capture immunoglobulin M (IgM) enzyme-linked immunosorbent assays (ELISA). J. Clin. Microbiol. 42:2489-2494. [DOI] [PMC free article] [PubMed]
- 87.Sudiro, T. M., H. Ishiko, S. Green, D. W. Vaughn, A. Nisalak, S. Kalayanarooj, A. L. Rothman, B. Raengsakulrach, J. Janus, I. Kurane, and F. A. Ennis. 1997. Rapid diagnosis of dengue viremia by reverse transcriptase-polymerase chain reaction using 3′-noncoding region universal primers. Am. J. Trop. Med. Hyg. 56:424-429. [DOI] [PubMed] [Google Scholar]
- 88.Talarmin, A., B. Labeau, J. Lelarge, and J. L. Sarthou. 1998. Immunoglobulin A-specific capture enzyme-linked immunosorbent assay for diagnosis of dengue fever. J. Clin. Microbiol. 36:1189-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Valdes, K., M. Alvarez, M. Pupo, S. Vazquez, R. Rodriguez, and M. G. Guzman. 2000. Human dengue antibodies against structural and nonstructural proteins. Clin. Diagn. Lab. Immunol. 7:856-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vathanophas, K., W. M. Hammon, R. W. Atchison, and G. E. Sather. 1973. Attempted type specific diagnosis of dengue virus infection by the indirect fluorescent antibody method directed at differentiating IgM and IgG responses. Proc. Soc. Exp. Biol. Med. 142:697-702. [DOI] [PubMed] [Google Scholar]
- 91.Vaughn, D. W. 1998. Serological investigation of a febrile outbreak in Delhi, India, using a rapid immunochromatographic test. J. Clin. Microbiol. 36:2795-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vaughn, D. W., A. Nisalak, S. Kalayanarooj, T. Solomon, N. M. Dung, A. Cuzzubbo, and P. L. Devine. 1998. Evaluation of a rapid immunochromatographic test for diagnosis of dengue virus infection. J. Clin. Microbiol. 36:234-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vaughn, D. W., S. Green, S. Kalayanarooj, B. L. Innis, S. Nimmannitya, S. Suntayakorn, T. P. Endy, B. Raengsakulrach, A. L. Rothman, F. A. Ennis, and A. Nisalak. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181:2-9. [DOI] [PubMed] [Google Scholar]
- 94.Vorndam, V., and G. Kuno. 1997. Laboratory diagnosis of dengue virus infections, p. 313-334. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, N.Y.
- 95.Wang, W. K., T. L. Sung, Y. C. Tsai, C. L. Kao, S. M. Chang, and C. C. King. 2002. Detection of dengue virus replication in peripheral blood mononuclear cells from dengue virus type 2-infected patients by a reverse transcription-real-time PCR assay. J. Clin. Microbiol. 40:4472-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Warrilow, D., J. A. Northill, A. Pyke, and G. A. Smith. 2002. Single rapid TaqMan fluorogenic probe based PCR assay that detects all four dengue serotypes. J. Med. Virol. 66:524-528. [DOI] [PubMed] [Google Scholar]
- 97.Win, T. 1982. Detection of dengue viruses by immunofluorescence of the intracerebral inoculation of mosquitoes. Lancet i:57-64. [DOI] [PubMed] [Google Scholar]
- 98.Wong, S. J., R. H. Boyle, V. L. Demarest, A. N. Woodmansee, L. D. Kramer, H. Li, M. Drebot, R. A. Koski, E. Fikrig, D. A. Martin, and P. Y. Shi. 2003. Immunoassay targeting nonstructural protein 5 to differentiate West Nile virus infection from dengue and St. Louis encephalitis virus infections and from flavivirus vaccination. J. Clin. Microbiol. 41:4217-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.World Health Organization. 1997. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control, 2nd ed. World Health Organization, Geneva, Switzerland.
- 100.World Health Organization. 1999. Strengthening implementation of the global strategy for dengue fever and dengue haemorrhagic fever, prevention and control. Report of the informal consultation, 18-20 October. World Health Organization, Geneva, Switzerland.
- 101.Wu, S. J., E. M. Lee, R. Putvatana, R. N. Shurtliff, K. R. Porter, W. Suharyono, D. M. Watts, C. C. King, G. S. Murphy, C. G. Hayes, and J. W. Romano. 2001. Detection of dengue viral RNA using a nucleic acid sequence-based amplification assay. J. Clin. Microbiol. 39:2794-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Young, P., R., P. A. Hilditch, C. Bletchly, and W. Halloran. 2000. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 38:1053-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]