INTRODUCTION
Exhaled nitric oxide (eNO) concentration is a non-invasive measure of airway inflammation that can be determined by analyzing gas collected from a single breath.1–4 eNO levels are elevated in chronic asthma5–7 and correlate with other markers of inflammation and impaired pulmonary function.8–11 High levels can be associated with relapse and exacerbations,12–15 and greater airway responsiveness.7,11,16,17 eNO levels may also change abruptly after rapid alteration of lower airway caliber. In studies of subjects with stable asthma, eNO levels decreased after methacholine, histamine, and exercise18–21 and increased after short-acting beta-agonists (SABA);11,21 but changes after SABA are less consistent during acute exacerbations.21,22,23,24
If eNO levels during acute treatment are associated with important outcomes such as need for hospitalization, this information may aid clinicians in management. Few studies have investigated eNO in children in emergency department (ED) settings, 22–26 and only two examined association between eNO and disposition.24,25 Although these authors reported eNO levels were not associated with hospitalization, both studies had limitations. One was stopped early due to poor eNO measurement reproducibility,24 and the other did not assess reproducibility.25 Additionally both studies allowed administration of SABA in the ED before initial eNO measurement, which may have influenced results.24,25
In this study, we measured eNO levels in children with acute asthma exacerbations, examined reproducibility and examined how eNO levels measured before treatment and when disposition was decided relate to ED disposition. We hypothesized that levels would be higher for those hospitalized, reflecting greater airway inflammation. Additionally, we examined how eNO levels changed after treatment and whether change differed according to ED disposition. Considering eNO levels may vary depending on treatment, we also examined if eNO levels and change differed according to treatment before arrival and in the ED.
MATERIALS AND METHODS
Study Design and Setting
We conducted this prospective exploratory study in an urban tertiary-care children's hospital ED, enrolling subjects from January 2006 to October 2007. The annual census was approximately 58,000, and most patients with asthma exacerbations were African American, had Medicaid insurance, and resided in urban zip codes. The institutional review board approved study procedures, and we completed the informed consent process for all subjects.
Participant Recruitment and Enrollment
We enrolled subjects when research assistants were available to perform study procedures. Eligibility criteria were age 6 through 17 years, parental report of asthma diagnosis, not born before 34 weeks gestational age, no other chronic pulmonary conditions, and plan to receive ≥ 1 inhaled albuterol treatment according to the clinical team. We chose this age group assuming that younger children would have limited ability to correctly perform eNO measurements, as is often the case with peak expiratory flow rate (PEFR) measurement.
Asthma Management
ED clinicians independently directed treatment and determined subjects' dispositions. There is an ED asthma treatment algorithm routinely used that is based on the National Asthma Education and Prevention Program (NAEPP) guidelines27 and outlines standard recommendations including weight-based doses of inhaled albuterol and ipratropium bromide and systemic corticosteroids. No medications were mandated or withheld due to study participation.
Outcomes Measured
eNO Measurement
We collected eNO samples using a single-breath off-line technique. The sampling device (General Electric Analytical/Ionics, Boulder, Colorado, United States) consisted of a rigid tube with filtered mouthpiece, inspiratory filter (nitric oxide scrubber), pressure gauge, and 1.5 liter Mylar bag. While seated, subjects inhaled through the device to total lung capacity and immediately exhaled for 8–10 seconds at pressure of 4–8 cmH2O.3 The exhalate was partitioned to avoid collecting dead space gas by closing a valve after the first five seconds of exhalation and diverting the remainder of exhalate into a Mylar bag. We collected 2 replicate samples per subject per measurement time (initial and final), and eNO concentrations (parts per billion, ppb) in the mylar bags were analyzed within 6 hours of collection using the NiOX® system (Aerocrine AB, Stockholm, Sweden). eNO values were not made available to the ED clinicians.
Timing of eNO Measurements
We measured initial eNO levels after subjects were evaluated by ED clinicians but before they received medications, and measured final levels as soon as possible after disposition decisions were made without interfering with care. We chose these time points as they corresponded to when clinicians were making decisions about severity, treatment plans, and response to treatment.
For patient safety and to avoid interfering with care, we obtained from the ED attending permission to enroll each subject before he received treatment. While we sought to enroll subjects representing a range of acute severity levels, there were potential subjects whom attendings decided were too severe to allow delay of treatment to complete enrollment procedures and initial measurement. As a result, we acknowledged a severity spectrum bias toward mild and moderate exacerbations. Shortly after study inception, we began collecting clinical data and final eNO levels for subjects deemed unable to await treatment (severe group).
Emergency Department Clinical Data
We collected data on initial vital signs, medications administered, PEFR and pulmonary score (PS) when conducted, disposition (hospitalized or discharged), and return visits within 72 hours. The amount of albuterol was recorded as total milligrams (mg) and mg per kilogram (kg). PEFR was recorded as percent predicted based on height. The PS is used routinely and has 3 domains (respiratory rate, wheeze, and degree of retractions) with 3 possible points per domain (score range 0 to 9).28
Chronic Severity
We collected data regarding chronic severity using 4 questions designed for the study that assessed symptoms during the 4 weeks preceding the exacerbation. The first asked “how often did you have cough/wheeze, shortness of breath, or chest tightness (asthma symptoms)?” with response options: “2 or fewer times per week”; “3 to 6 times per week”; “daily”; and “continuously”. The other questions asked “how often were you awakened from sleep because of asthma symptoms?”; “how often did you have asthma symptoms while exercising or playing?”; and “how often did asthma keep you from doing what you wanted?”. For these three questions, response options were: “2 or fewer times per month”; “3 to 4 times per month”; “5 to 9 times per month”; or “10 or more times per month”. Responses were ranked from 1 to 4 in their order presented, and 1 of 4 severity categories based on NAEPP guidelines27 (intermittent, mild persistent, moderate persistent, and severe persistent) was assigned based on the highest response for any question (e.g. if 4th response was chosen for any question, severity assignment was severe persistent).
Medications Used Before Arrival
We collected data on medications used before arrival including use of inhaled corticosteroids (ICS) and/or montelukast during the preceding 4 weeks, ICS during the preceding 24 hours, oral corticosteroids (OCS) during the preceding 4 days, and the number of SABA treatments during the preceding 24 hours, including timing of last SABA treatment.
Analysis
We performed statistical analysis using SPSS 12.0 software (SPSS Inc, Chicago, Illinois, United States). Nominal data are reported as number with percentage and compared using chi-square or Fisher’s exact test. Continuous data are reported as mean with standard deviation (SD) or 95% confidence interval (CI) and compared using t-tests or analysis of variance. To assess reproducibility, we determined rank correlations and coefficients of variability (CV) of the 2 replicate eNO samples at each measurement time (initial and final) and compared CV by measurement time. Individual initial and final eNO levels were the mean values of the 2 samples collected at that measurement time. Initial levels were compared according to treatment before arrival, chronic severity, and demographics, and rank correlations were determined between initial level and heart rate and respiratory rate and between initial level and PEFR. For the relationship between eNO levels and disposition, initial and final levels were analyzed on a log scale to better fit the requirements of parametric tests. Change in eNO was analyzed as arithmetic ppb difference between initial and final levels and as percent difference, to take into account variability in initial levels, and compared according to disposition. Change in eNO was also compared according to demographics, chronic severity category, and treatment before arrival. Rank correlations were determined between eNO change and amount of albuterol administered in the ED and between eNO change and PEFR change. Receiver operating characteristic (ROC) curves and area under the curve (AUC) statistics for discriminating disposition were determined for initial and final eNO levels and change between. When we designed the study there were no consistent data for baseline eNO levels for children with acute exacerbations in ED settings or for clinically meaningful eNO levels or change, and we did not perform a sample size estimation. Our goal was to include 100 subjects, and we stopped enrollment after accruing 107 total subjects which included a small over-enrollment number allowing for possible missing data.
RESULTS
Subjects with Initial and Final eNO Measurements
Demographic and Clinical Characteristics
We enrolled 83 subjects for collection of both initial and final eNO levels. Two did not complete final measurements. Both were discharged; one did not await final measurement and the other declined. Characteristics of enrolled subjects by disposition are shown in Table 1. None had return visits within 72 hours.
Table 1.
Characteristics of Subjects Enrolled for Initial and Final eNO Measurements According to Emergency Department Disposition
| Hospitalized (n=9) |
Discharged (n=74) |
|
|---|---|---|
| Age, years | 12.1 (2.4) | 11.1 (2.8) |
| Gender, male | 5 (56%) | 39 (53%) |
| Race | ||
| African American | 8 (89%) | 69 (93%) |
| White/Caucasian | 1 (11%) | 5 (7%) |
| Height, centimeters | 150.1 (11.8) | 152.3 (14.6) |
| Weight, kilograms | 56.6 (26.4) | 53.1 (22.9) |
| Home medicines | ||
| Albuterol | 9 (100%) | 71 (96%) |
| Any inhaled corticosteroid | 6 (67%) | 33 (45%) |
| Montelukast | 4 (44%) | 22 (30%) |
| Medicines used before emergency department visit | ||
| SABA during preceding 24 hours | 9 (100%) | 64 (87%) |
| Number of SABA doses | 2.9 (1.8) | 2.5 (1.7) |
| SABA during preceding 4 hours | 6 (67%) | 30 (47%) |
| Inhaled corticosteroid during preceding 24 hours | 4 (44%) | 17 (23%) |
| Oral corticosteroid during preceding 4 days | 5 (56%) | 11 (15%) |
| Asthma severity | ||
| Intermittent | 1 (11%) | 23 (31%) |
| Mild persistent | 2 (22%) | 25 (34%) |
| Moderate persistent | 3 (33%) | 13 (18%) |
| Severe persistent | 3 (33%) | 13 (18%) |
| Initial vital signs | ||
| Heart rate, beats per minute | 108.8 (19.7) | 101.2 (16.3) |
| Respiratory rate, breaths per minute | 30.0 (10.2) | 24.4 (5.7) |
| Pulse oximetry % (room air) | 96.7 (2.3) | 97.6 (2.0) |
| n=9 | n=58 | |
| PEFR (% predicted) before treatment | 49.2 (26.4) | 59.7 (22.6) |
| n=8 | n=58 | |
| Pulmonary Score before treatment | 3.1 (1.9) | 2.4 (1.3) |
| Medications administered in emergency department | ||
| Albuterol nebulizer dose total, milligrams | 29.7 (11.8) | 12.1 (9.1) |
| Albuterol nebulizer dose total, milligram/kilogram | 0.61 (0.33) | 0.24 (0.17) |
| Ipratropium bromide | 9 (100%) | 67 (91%) |
| Oral corticosteroid | 9 (100%) | 52 (70%) |
| n=8 | n=36 | |
| PEFR % predicted change, final-initial | 12.6 (14.4) | 21.8 (15.9) |
Categorical data reported as number (%). Other data reported as mean (SD).
SABA = short-acting-beta-agonist, PEFR = peak expiratory flow rate.
eNO Measurement Reproducibility
Values of replicate initial eNO samples were highly correlated (r = 0.98, p<0.001; mean difference = −0.24 ppb, 95% CI −1.02 to 0.54, p=0.94). Replicate final samples were also correlated (r = 0.99, p<0.001; mean difference = 0.31 ppb, 95% CI −0.35 to 0.98, p=0.95). CV were similar by measurement time (initial = 0.059 ± 0.057, final = 0.061 ± 0.070; p=0.81).
Initial eNO Levels
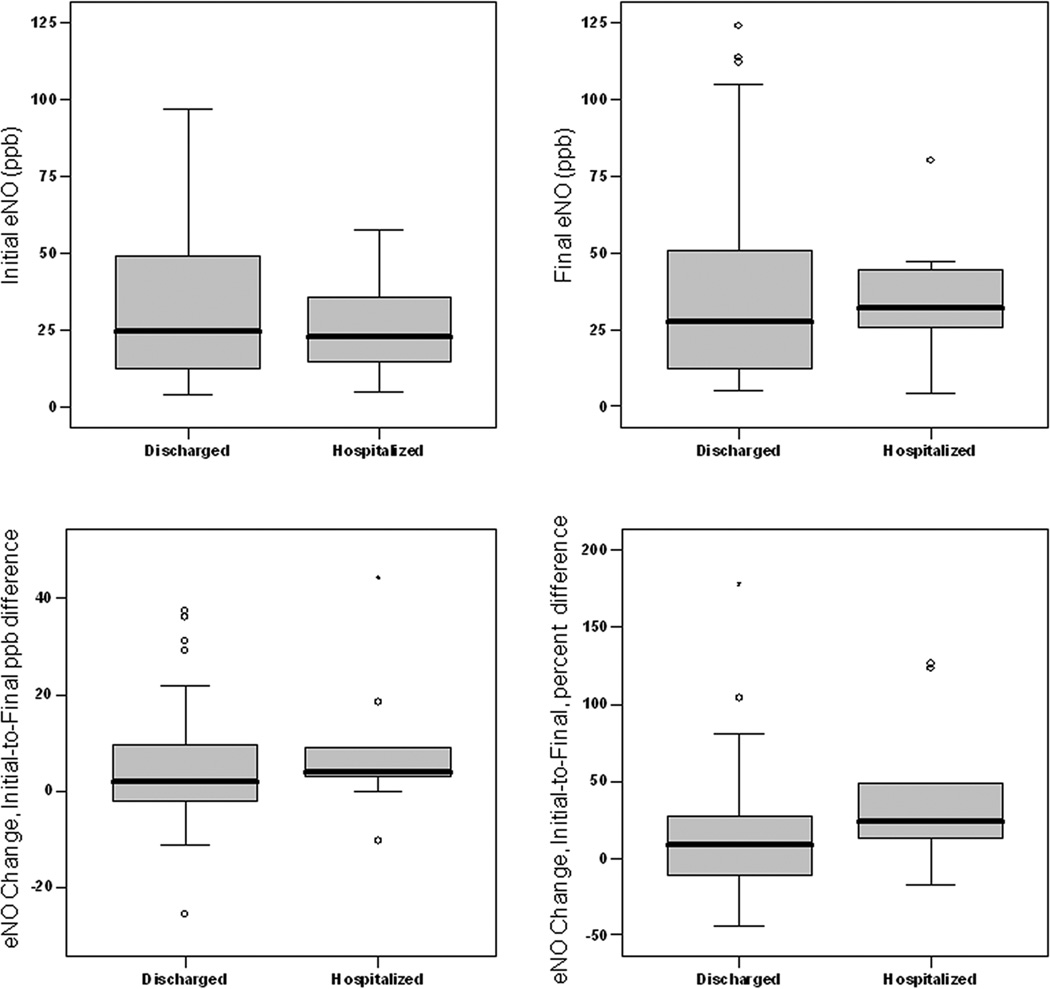
Overall, the mean initial eNO level was 32.1 ppb (95% CI 26.8 to 37.3; range 4.1 to 96.7), and 61% of subjects had levels > 20 ppb. Initial levels were similar according to disposition (hospitalized = 25.0 ppb ± 16.4, discharged = 32.9 ± 24.6; mean difference = −8.0 ppb,95% CI −24.8 to 8.9, p=0.44) (Figure 1). The ROC curve AUC statistic (p = 0.53) indicated initial eNO level did not consistently discriminate hospitalization from discharge.
Figure 1. Initial and Final Exhaled Nitric Oxide (eNO) Levels and Change in eNO Levels by Emergency Department Disposition.
Initial and Final exhaled nitric oxide (eNO) levels and difference between Initial and Final levels by disposition. Data reported as parts per billion (ppb) or percent difference for eNO change. Box-plots depict medians and interquartile ranges.
Initial levels were lower but not clearly different for subjects reporting OCS use within 4 days (20.0 ppb ± 10.6 versus 34.9 ± 25.4, p =0.055), and initial levels were similar according to disposition when adjusted for OCS use (p=0.77, logistic regression). Initial levels were also similar according to use of SABA, montelukast, and ICS before arrival and chronic severity; and initial levels did not correlate with initial heart rate, respiratory rate, pulse-oximetry, PEFR or PS (all p-values > 0.05).
Final eNO Levels
Overall, the mean final eNO level was 36.3 ppb (95% CI 29.8 to 42.9; range 4.6 to 124.0). Final levels were similar according to disposition (hospitalized = 33.8 ppb ± 22.2, discharged = 36.6 ± 30.6; mean difference = −2.8 ppb,95% CI −23.8 to 18.2, p=0.96). (Figure 1). The ROC curve AUC statistic (p = 0.87) indicated final eNO level did not consistently discriminate hospitalization from discharge.
Change in eNO between Initial and Final Measurement
Overall mean final level was higher than initial level (36.3 ppb ± 29.7 versus 31.5 ± 23.9, p<0.001) (Figure 2). Mean ppb change was 4.8 (95% CI 2.3 to 7.3; range = −25.5 to 44.3), and mean percent change was 16% (95% CI 7% to 24%; range = −44% to 178%). eNO ppb change was similar according to disposition (hospitalized = 8.9 ppb ± 15.3, discharged = 4.3 ± 10.9; mean difference = 4.6 ppb,95% CI −3.4 to 12.6, p=0.27), as was percent change (hospitalized = 41% ± 52%, discharged = 12% ± 36%; mean difference = −0.28%,95% CI −0.55% to −0.02%; p=0.12) (Figure 1). The ROC curve AUC statistics for ppb change (p = 0.27) and percent change (p=0.12) indicated eNO change did not consistently discriminate hospitalization from discharge.
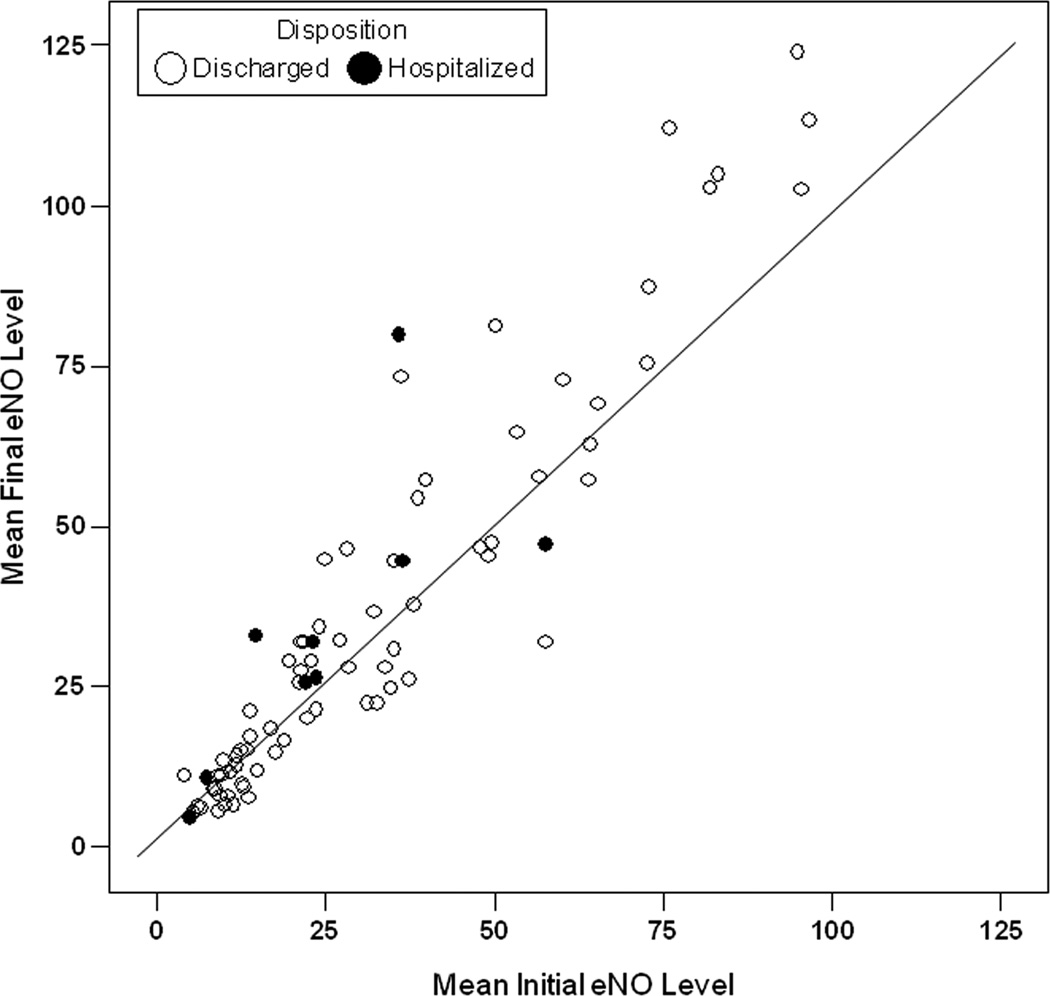
Figure 2. Initial and Final Exhaled Nitric Oxide (eNO) Levels for Individual Subjects.
Figure shows data points for Initial and Final exhaled nitric oxide (eNO) levels for each individual subjects measured in parts per billion (ppb) with line of identify.
Fifty-one (63%) subjects had increased final levels to some degree, and the hospitalization rate was similar according to eNO change direction (7/51 versus 2/30, p=0.33). Thirty-seven (46%) subjects had final levels more than 10% and 5 ppb different from their initial levels; 28 had such increases and 9 had such decreases, and proportions hospitalized in these groups were similar (p=0.79).
eNO change (as both ppb and percent difference) was similar according to demographics, chronic severity, or SABA or OCS use before arrival (p-values > 0.05). eNO change correlated positively with change in PEFR % predicted (ppb change, r = 0.509, p=0.002; percent change, r = 0.486, p=0.003). Mean time between initial and final eNO measurement was 100 minutes ± 57 (range 20 to 292), and eNO change correlated positively with greater elapsed time (ppb change, r = 0.338, p = 0.002 ; percent change, r = 0.363, p = 0.001). eNO change did not correlate with amount of albuterol administered in the ED.
Subjects with Final eNO Measurement Only (Severe Group)
Twenty-four subjects comprised the severe group deemed unable to await treatment for study procedures. Compared to the main sample (with initial and final eNO levels), this severe group had relatively higher initial respiratory rate (29 ± 8 breaths per minute versus 25 ± 6; difference of means = 1.17; 95% CI 1.06 to 1.20), heart rate (115 ± 120 beats per minute versus 102 ± 17; difference of means = 1.12; 95% CI 1.04 to 1.21) and PS (3.8 ± 2.0 versus 2.5 ± 1.4); received more albuterol (total mg = 27 ± 14 versus 14 ± 11; difference of means = 2.22; 95% CI 1.53 to 3.20; mg/kg = 0.56 ± 0.32 versus 0.28 ± 0.22; difference of means = 2.23; 95% CI 1.59 to 3.15); more frequently received intravenous magnesium sulfate (8 versus 0); and had a higher hospitalization rate (42% versus 11%). Other characteristics (as listed in Table 1) were similar between these groups. Final replicate eNO samples remained highly correlated (r = 0.99, p<0.001) with good CV (0.59 ± 0.66) when data for this severe group were included in analysis.
Although the severe group had slightly higher mean final eNO level compared to the main sample of subjects (40.1 ppb ± 24.5 versus 36.3 ± 29.7, p=0.22), final levels remained similar according to disposition with data for severe group included in analysis (mean difference between hospitalized and discharged = 1.42 ppb, 95% CI −13.0 to 15.8, p=0.47).
DISCUSSION
We measured eNO levels in children treated for acute asthma exacerbations to investigate how levels related to ED disposition. If eNO levels could distinguish those needing hospitalization, this may help improve care for many children with asthma. However, we found initial and final levels and change between did not differ significantly according to whether the child was hospitalized or discharged.
While our findings are consistent with the two other pediatric studies reporting eNO levels are not associated with hospitalization during treatment of acute exacerbations,24,25 our results add precision to this conclusion. Reproducibility of eNO measurement is an important consideration when examining association with outcomes. Gill and colleagues stopped their study after interim analysis revealed unacceptably poor reproducibility (median CV=12%),24 and Kwok and colleagues collected only one sample per subject at each measurement time.25 In our study we collected two samples per subject per measurement time, using a single-breath off-line collection method, and had good reproducibility (CV = 6% for both initial and final measurements).
Another difference between these two studies and ours is timing of eNO measurements. SABA may rapidly change eNO levels,11,21 and both other studies allowed albuterol in the ED before eNO measurement. This occurred for 33% of subjects in the study by Gill and colleagues, 24 and this proportion was not reported by Kwok and colleagues25; and neither paper reported results adjusted for this potential confounder. We measured initial levels before any ED treatment and analyzed results according to treatment before arrival. In this sample, we found initial levels were similar according to prior treatment. Timing of final eNO measurements also differed between studies. Gill and colleagues measured subsequent levels after one hour of treatment, and Kwok and colleagues measured eNO again before subjects left the ED, but at least 1 hour after systemic steroids.24,25 We measured final levels when disposition was determined to see how levels related to this important clinical decision. Considering results of these studies collectively, eNO levels are not associated with hospitalization regardless of when measured.
Hospitalization is an important patient-oriented outcome potentially influenced by many factors including acute severity and treatment response, as well as past history and social and environmental concerns. In chronic asthma, high eNO levels are associated with risk of exacerbation,12–15 and many of our subjects had high initial levels (although we lacked pre-enrollment data to which to compare). However, initial levels in our sample did not differ according to chronic severity, and high levels were not associated with needing hospitalization. Although initial levels were relatively high in our sample compared to results reported by Kwok and colleagues (median eNO approximately 9 ppb, range up to 70),25 our data are consistent with results of three other pediatric studies in which mean baseline levels were 39 ± 20, 38 ± 19, and 48 ± 8 ppb.22,23,26 We found a broad range of initial levels that may reflect variability due to prior medication use as well as chronic severity. However, initial levels were similar according to medication use before arrival. Although eNO levels are reported to decline after OCS use,26 and initial levels in our subjects using OCS within 4 days were relatively lower, levels were similar according to disposition when adjusted for this variable. Considering other factors related to acute severity, we found no association between initial levels and vital signs, pulse-oximetry, and PEFR or PS (although data were limited), results consistent with other studies.24,25
Predicting hospitalization for children with asthma exacerbations is challenging, and many authors conclude the most accurate predictors are oxygen saturation and examination after treatments.29–35 We found final eNO levels obtained after treatment (including data from the severe group) and initial-to-final change were similar according to disposition, and our analysis with percent change took into consideration variability of initial levels. While most subjects had increased final levels to some degree, 39% had decreased levels, and the hospitalization rate was similar according to direction of change. This inconsistency and variability of eNO change challenge clinical application in predicting disposition.
Studies of subjects with stable asthma report increased eNO levels after SABA,11,21 but investigations during acute exacerbations have less consistent results, possibly due to variability in study protocols, acute severity and degree of bronchodilation. Small but statistically significant eNO increases after bronchodilators were reported by two studies, although one involved inhaled and intravenous medications21, and the other did not describe treatment in detail.24 Two other studies reported no significant change after inhaled terbutaline.22,23 Our finding that overall final eNO level was higher than initial and most subjects had increased levels may be a result of collecting more native eNO previously trapped in lower airways which is available for sampling after bronchodilation.21,36 Although we had limited PEFR data, we found eNO change correlated positively with PEFR change. Other studies have reported no correlation24 or negative correlation between changes in eNO and pulmonary function measures.21,22,23
While studies have analyzed and reported eNO change in terms of statistical significance, there is no literature defining clinically meaningful levels or changes during acute exacerbations. However, there are guidelines for reliability and acceptable differences between samples during routine measurement. For online eNO measurement for which values are immediately available, samples are typically repeated until 2 or 3 levels are within 10% or not different by > 5 ppb (to account for lower levels).3 If these criteria were considered clinically significant, fewer than half of subjects had such change, whether increased or decreased, and hospitalization rates were similar according to such change.
Our study has some limitations not yet discussed. It was conducted at a single site, and subjects may not be representative of all children with acute exacerbations limiting application of results to other settings and populations. We had a relatively small proportion of subjects with initial eNO levels who were eventually hospitalized. ED clinicians independently directed treatment, and not all subjects received identical therapy. Although differences in treatment may influence eNO change, the treatment algorithm is based on national guidelines,27 and treatments administered were based on clinical judgment allowing examination of eNO levels with respect to medication administered in standard practice. While we attempted to enroll a clinically relevant sample of subjects, we did not exclude children with upper respiratory tract infections, gastroesophageal reflux, tobacco smoke exposure, or recent intake of food or drink, and we did not collect information about these characteristics that may affect eNO levels.
In conclusion, we found eNO measurement was feasible for children age 6 to 17 years with acute asthma exacerbations. However, eNO levels measured before treatment and when disposition was decided did not differ significantly between hospitalized and discharged children, and eNO levels appear to be poor predictors of the need for hospitalization. eNO increased after treatment for most subjects, and eNO change correlated positively with PEFR change, but there was inconsistency and variability of change and no association with other severity indicators. Considering these results and those of other studies, eNO measurement is unlikely to be useful during ED management of children with acute asthma exacerbations.
Acknowledgement
Funding Source: National Heart Lung Blood Institute (R01 HL 072919)
The authors would like to acknowledge and thank Phillip Blanks for his diligence in enrollment and data collection for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jobsis Q, Raatgeep HC, Hop WC, et al. Controlled low flow offline sampling of exhaled nitric oxide in children. Thorax. 2001;56:285–289. doi: 10.1136/thorax.56.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barreto M, Pia Villa M, Martella S, et al. Off-Line Exhaled Nitric Oxide Measurements in Children. Pediatr Pulmonol. 2001;32:159–167. doi: 10.1002/ppul.1102. [DOI] [PubMed] [Google Scholar]
- 3.ATS/ERS Recommendations for Standardized Procedures for the Online and Offline Measurement of Exhaled Lower Respiratory Nitric Oxide and Nasal Nitric Oxide – 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 4.Jobsis Q, Schellekens SL, Kroesbergen A, et al. Off-line sampling of exhaled air for nitric oxide measurement in children: methodological aspects. Eur Respir J. 2001;17:898–903. doi: 10.1183/09031936.01.17508980. [DOI] [PubMed] [Google Scholar]
- 5.Kharitonov SA, Yates D, Robbins RA, et al. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133–135. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 6.Artlich A, Hagenah JU, Jonas S, et al. Exhaled nitric oxide in childhood asthma. Eur J Pediatr. 1996;155:698–701. doi: 10.1007/BF01957156. [DOI] [PubMed] [Google Scholar]
- 7.Zietkowski Z, Bodzenta-Lukaszyk A, Tomasiak MM, et al. Comparison of exhaled nitric oxide measurement with conventional tests in steroid-naive asthma patients. J Investig Allergol Clin Immunol. 2006;16:239–246. [PubMed] [Google Scholar]
- 8.Rosias PP, Dompeling E, Dentener MA, et al. Childhood Asthma: Exhaled Markers of Airway Inflammation, Asthma Control Score, and Lung Function Tests. Pediatr Pulmonol. 2004;38:107–114. doi: 10.1002/ppul.20056. [DOI] [PubMed] [Google Scholar]
- 9.Warke TJ, Fitch PS, Brown V, et al. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax. 2002;57:383–387. doi: 10.1136/thorax.57.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuburai T, Tsurikisawa N, Taniguichi M, et al. The relationship between exhaled nitric oxide measured with an off-line method and airway reversible obstruction in Japanese adults with asthma. Allergol Int. 2007;56:37–43. doi: 10.2332/allergolint.O-06-439. [DOI] [PubMed] [Google Scholar]
- 11.Colon-Semidey AJ, Marshik P, Crowley M, et al. Correlation between reversibility of airway obstruction and exhaled nitric oxide levels in children with stable bronchial asthma. Pediatr Pulmonol. 2000;30:385–392. doi: 10.1002/1099-0496(200011)30:5<385::aid-ppul4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Harkins MS, Flato KL, Iwamoto GK. Exhaled nitric oxide predicts asthma exacerbations. J Asthma. 2004;41:471–476. doi: 10.1081/jas-120033990. [DOI] [PubMed] [Google Scholar]
- 13.Pijnenburg MW, Hofhuis W, Hop WC, et al. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax. 2005;60:215–218. doi: 10.1136/thx.2004.023374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritsch M, Uxa S, Horak F, Jr, et al. Exhaled nitric oxide in the management of childhood asthma: a prospective 6-month study. Pediatr Pulmonol. 2006;41:855–862. doi: 10.1002/ppul.20455. [DOI] [PubMed] [Google Scholar]
- 15.Gelb AF, Flynn Taylor C, Shinar CM, et al. Role of spirometry and exhaled nitric oxide to predict exacerbations in treated asthmatics. Chest. 2006;129:1492–1499. doi: 10.1378/chest.129.6.1492. [DOI] [PubMed] [Google Scholar]
- 16.Langley SJ, Goldthorpe S, Custovic A, et al. Relationship among pulmonary function, bronchial reactivity, and exhaled nitric oxide in a large group of asthmatic patients. Ann Allergy Asthma Immunol. 2003;91:398–404. doi: 10.1016/S1081-1206(10)61688-2. [DOI] [PubMed] [Google Scholar]
- 17.Strunk RC, Szefler SJ, Phillips BR, et al. Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol. 2003;112:883–992. doi: 10.1016/j.jaci.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 18.de Gouw HW, Hendriks J, Woltman AM, et al. Exhaled nitric oxide (NO) is reduced shortly after bronchoconstriction to direct and indirect stimuli in asthma. Am J Respir Crit Care Med. 1998;158:315–319. doi: 10.1164/ajrccm.158.1.9703005. [DOI] [PubMed] [Google Scholar]
- 19.Ho LP, Wood FT, Robson A, et al. The current single exhalation method of measuring exhaled nitric oxide is affected by airway calibre. Eur Respir J. 2000;15:1009–1013. doi: 10.1034/j.1399-3003.2000.01506.x. [DOI] [PubMed] [Google Scholar]
- 20.Piacentini GL, Bodine A, Peroni DG, et al. Reduction in exhaled nitric oxide immediately after methacholine challenge in asthmatic children. Thorax. 2002;57:771–773. doi: 10.1136/thorax.57.9.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsufuji H, Kobayashi H, Imasaki T, et al . Acute changes in bronchoconstriction influences exhaled nitric oxide level. Jpn J Physiol. 2001;51:151–157. doi: 10.2170/jjphysiol.51.151. [DOI] [PubMed] [Google Scholar]
- 22.Lee MY, Tsai YG, Yang KD, et al. Comparison of the effects of nebulized terbutaline with or without intravenous betamethasone on exhaled nitric oxide in children with acute asthma attack. J Microbiol Immunol Infect. 2006;39:33–38. [PubMed] [Google Scholar]
- 23.Tsai YG, Lee MY, Yang KD, et al. A single dose of nebulized budesonide decreases exhaled nitric oxide in children with acute asthma. J Pediatr. 2001;139:433–437. doi: 10.1067/mpd.2001.116295. [DOI] [PubMed] [Google Scholar]
- 24.Gill M, Walker S, Khan A, et al. Exhaled nitric oxide levels during acute asthma exacerbation. Acad Emerg Med. 2005;12:579–586. doi: 10.1197/j.aem.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Kwok MY, Walsh-Kelly CM, Gorelick MH. The Role of Exhaled Nitric Oxide in Evaluation of Acute Asthma in a Pediatric Emergency Department. Acad Emerg Med. 2009;16:21–28. doi: 10.1111/j.1553-2712.2008.00304.x. [DOI] [PubMed] [Google Scholar]
- 26.Lanz MJ, Leung DY, White CW. Comparison of exhaled nitric oxide to spirometry during emergency treatment of asthma exacerbations with glucocorticoids in children. Ann Allergy Asthma Immunol. 1999;82:161–164. doi: 10.1016/S1081-1206(10)62591-4. [DOI] [PubMed] [Google Scholar]
- 27.National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health, National Heart Lung Blood Institute; 2007. Oct, Publication No. 08-5846. [Google Scholar]
- 28.Smith SR, Baty JD, Hodge D., III Validation of the Pulmonary Score. An Asthma Severity Score for Children. Acad Emerg Med. 2002;9:99–104. doi: 10.1111/j.1553-2712.2002.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 29.Ownby DR, Abarzua J, Anderson JA. Attempting to predict hospital admission in acute asthma. Am J Dis Child. 1984;138:1062–1066. doi: 10.1001/archpedi.1984.02140490062015. [DOI] [PubMed] [Google Scholar]
- 30.Kerem E, Tibshirani R, Canny G, et al. Predicting the need for hospitalization in children with acute asthma. Chest. 1990;98:1355–1361. doi: 10.1378/chest.98.6.1355. [DOI] [PubMed] [Google Scholar]
- 31.Geelhoed GC, Landau LI, Le Souef PN. Evaluation of SaO2 as a predictor of outcome in 280 children presenting with acute asthma. Ann Emerg Med. 1994;23:1236–1241. doi: 10.1016/s0196-0644(94)70347-7. [DOI] [PubMed] [Google Scholar]
- 32.Kunitoh H, Nagatomo A, Okamoto H, et al. Predicting the need for hospital admission in patients with acute bronchial asthma. J Asthma. 1996;33:105–112. doi: 10.3109/02770909609054538. [DOI] [PubMed] [Google Scholar]
- 33.Chey T, Jalaludin B, Hanson R, et al. Validation of a predictive model for asthma admission in children: how accurate is it for predicting admissions? J Clin Epidemiol. 1999;52:1157–1163. doi: 10.1016/s0895-4356(99)00111-0. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigo G, Rodrigo C. A new index for early prediction of hospitalization in patients with acute asthma. Am J Emerg Med. 1997;15:8–13. doi: 10.1016/s0735-6757(97)90039-5. [DOI] [PubMed] [Google Scholar]
- 35.Kelly AM, Kerr D, Powell C. Is severity assessment after one hour of treatment better for predicting the need for admission in acute asthma? Respir Med. 2004;98:777–781. doi: 10.1016/j.rmed.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Kharitonov SA, Chung KF, Evans D, et al. Increased exhaled nitric oxide in asthma is mainly derived from the lower respiratory tract. Am J Respir Crit Care Med. 1996;153:1773–1780. doi: 10.1164/ajrccm.153.6.8665033. [DOI] [PubMed] [Google Scholar]