Abstract
Harmine is a naturally occurring monoamine oxidase inhibitor that has recently been shown to selectively inhibit the dual-specificity tyrosine-(Y)-phosphorylation-regulated kinase 1A (DYRK1A). We investigated the cognitive effects of 1 mg (low) Harmine and 5 mg (high) Harmine using the delayed-match-to-sample (DMS) asymmetrical 3-choice water maze task to evaluate spatial working and recent memory, and the Morris water maze task (MM) to test spatial reference memory. Animals were also tested on the visible platform task, a water-escape task with the same motor, motivational, and reinforcement components as the other tasks used to evaluate cognition, but differing in its greater simplicity and that the platform was visible above the surface of the water. A subset of the Harmine-high treated animals showed clear motor impairments on all behavioral tasks, and the visible platform task confirmed a lack of competence to perform the procedural components of water maze testing. After excluding animals from the high dose group that could not perform the procedural components of a swim task, it was revealed that both high- and low-dose treatment with Harmine enhanced performance on the latter portion of DMS testing, but had no effect on MM performance. Thus, this study demonstrates the importance of confirming motor and visual competence when studying animal cognition, and verifies the one-day visible platform task as a reliable measure of ability to perform the procedural components necessary for completion of a swim task.
Keywords: Harmine, Hippocampal, Cognition, Working memory, Reference memory
1. Introduction
Harmine is a beta-carboline alkaloid found in dozens of plant species that is most well known as a naturally occurring inhibitor of monoamine oxidase. It has a long history of use among South American Indian tribes as a major constituent of Ayahuasca, a hallucinogenic brew used in religious rituals for its psychotropic effects [1]. Recently, Harmine has also been shown to be a potent and selective inhibitor of the DYRK1A protein kinase [2].
The DYRK1A protein kinase plays a key role in neurodevelopment, and has been hypothesized to contribute to abnormal brain development and early onset of dementia and neurodegeneration in Down syndrome [3]. In addition, increased DYRK1A immunoreactivity has been associated with neurofibrillary tangle pathology in patients with Down syndrome and in sporadic Alzheimer's disease [4]. DYRK1A has also been shown to directly phosphorylate tau protein at multiple sites [5–7]. Interestingly, DYRK1A haploinsufficiency has also been shown to produce cognitive impairments on both spatial and non-spatial tasks [8], suggesting that both over- and under- expression of DYRK1A may not be optimal for cognitive functioning. It is crucial to note that, in both Down Syndrome individuals and DYRK1A haploinsufficient mice, the over- or under- expression of DYRK1A is present for the entire lifespan, including during development. How manipulations of DYRK1A activity during adulthood might influence cognitive function remains unknown.
We have recently found that Harmine reduces expression of tau phosphorylated at AD-relevant sites in vitro [9]. Whether these in vitro effects translate to learning and memory alterations has not yet been determined. However, it has been shown that DYRK1A overexpression results in impairment of hippocampal dependent memory tasks [10], suggesting that inhibition of DYRK1A activity could provide cognitive benefit. Thus, we hypothesize that Harmine, an inhibitor of DYRK1A activity, will enhance cognition in the rodent model. There is some evidence of this, as Harmine administration enhanced short-term memory on the non-spatial object recognition task at doses of 1 mg/kg, 2.5 mg/kg and 5 mg/kg in rodents [11]. It is unclear whether these cognitive benefits will extend to spatial working or reference memory, or tasks with higher cognitive complexity. The present study evaluated the effects of Harmine administration on cognition in aged rodents using spatial working, recent, and reference memory tasks. We tested animals on the win-stay delayed match-to-sample (DMS) 3-choice task to evaluate spatial working and recent memory, and the Morris water maze (MM) to measure spatial reference memory [12] following Harmine administration.
Harmine is structurally similar to the compound 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which creates a Parkinsonian-like phenotype in rodents via metabolism to the toxic N-methyl-4-phenylpyridinium ion (MPP+) [13]. MPTP dose-dependently induces tremors in mice, rats, and rabbits [14,15] and is known for its psychoactive effects in humans. Because of Harmine's potential to produce impairments in motor function, we used both a low and high dose, and we took precautions by including a task to identify animals that would need to be excluded from cognitive interpretations because they lack the ability to perform the procedural components of the water escape cognitive tasks. Indeed, our goal was to determine whether cognitive benefits could be achieved with a dose of Harmine that was below the threshold for motor impairments. To accomplish this goal, we also evaluated the animals' performance using an adaptation of the cue-navigation visible platform version of the spatial MM task previously used to dissociate visual and motor acuity from place memory [12]. This task is “matched for motor requirements, motivation and reinforcement” to the tasks we used to test cognitive aptitude [12 (pp 682), 16–18], and has previously been utilized as a control measure to ensure that a treatment does not impact motor function or visual acuity required to navigate through an environment.
2. Material and methods
2.1. Subjects
Thirty 17 month-old Fischer-344 male rats raised at the National Institute on Aging colony at Harlan Laboratories (Indianapolis, IN) were used in the study. After arrival, rats were pair-housed, had food and water ad-lib, and were maintained on a 12-h light/dark cycle. Procedures were approved by the Institutional Animal Care and Use Committee, and adhered to National Institutes of Health standards.
2.2. Experimental design and drug treatments
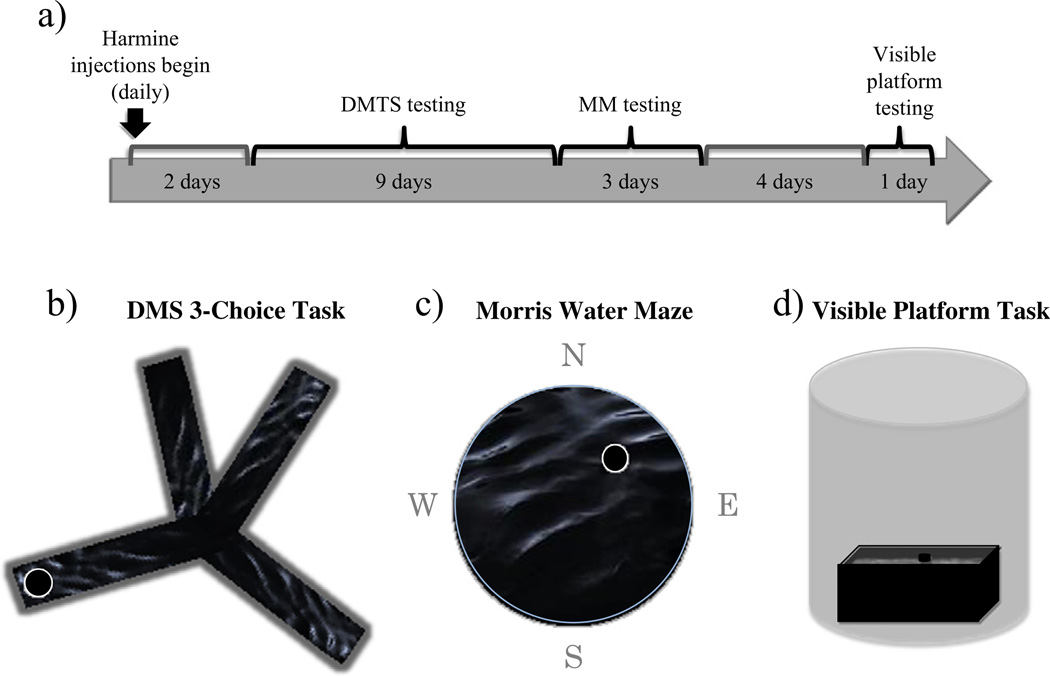
Rats were randomly divided into three treatment groups (n at start of study, n included in final behavioral analyses): vehicle (10, 10), 1 mg/day (low) Harmine (10, 10), or 5 mg/day (high) Harmine (10, 6). There were no differences in body weights between treatment groups (Table 1). Nine days after arrival, animals started receiving daily subcutaneous injections at a volume of 0.5 ml. Harmine (Acros Organics, Geel, Belgium, Harmine hydrochloride hydrate 98%) was prepared daily, and dissolved in saline (NaCl 0.9%). Behavioral testing began after the second injection day (Fig. 1a), and testing commenced approximately 30–45 min after the end of injections and lasted for 6–8 h. Animals were divided into three testing squads. Squad 1 was tested approximately 30–45 min after the end of injections, Squad 2 began testing approximately 3 to 4 h after injections ended, Squad 3 began testing approximately 6 to 7 h after the end of injections. Of critical importance, treatment groups were counterbalanced across testing squad; two to three animals from each treatment group were included in each testing squad, resulting in some of each treatment group being tested across the entire testing day.
Table 1.
Mean ± SEM body weights for each treatment group. There were no body weight differences between groups.
| Treatment group | Control | Harmine-low | Harmine-high |
|---|---|---|---|
| Body weights (g) | 476.10 ± 18.52 | 485.00 ± 10.52 | 480.89 ± 6.67 |
Figure 1.
Timeline detailing study procedures and schematics of the behavioral tasks utilized.
2.3. Delayed-match-to-sample asymmetrical 3-choice task
Two forms of short-term memory, spatial working and recent memory, were evaluated using a win-stay water-escape DMS asymmetrical place-learning task. The maze was an eight-arm apparatus (each arm 38.1 × 12.7 cm) that was adapted as described below, filled with opaque, room temperature water. Four of the eight arms were blocked using plastic inserts that were identical to the interior of the maze (solid black) and the maze containing a submerged platform (10 cm diameter) in one of the four open arms (Fig. 1b). This task was identical to the win-stay DMS plus maze used previously [19,20], except that the four open arms were configured asymmetrically, rather than in a plus shape (see Fig. 1b). Animals were released into a different start arm at the beginning of each trial, varying semi-randomly such that the animals were released from each of the three non-platformed arms twice within a day of testing. The platform remained in the same location within a day, but changed location across days. Animals received six trials/day for nine days with 90 s to locate the platform, 15 s on the platform and a 30 second intertrial-interval in a heated cage. Trial 1 was the information trial, trial 2 was the working memory trial and trials 3–6 were considered recent memory trials. Entry into any non-platformed arm was counted as an error. An arm entry was counted when the tip of a rat's snout reached a mark on the outside of the arm(not visible from the inside of the maze; 11 cm into the arm).
2.4. Morris water maze
Spatial reference memory was evaluated using the MM. The apparatus was a round tub (188 cm diameter) filled with opaque room temperature water containing a submerged platform (10 cm diameter) (Fig. 1c). The platform remained in a fixed location across all days and trials, testing spatial reference memory [12]. Testing consisted of six trials/day for three days. Animals were dropped off at different starting points (north, south, east or west) for each trial, varying semi-randomly. Animals had 60 s to locate the platform where they remained for 15 s before being placed back into a heated cage awaiting the next trial. The inter-trial-interval was approximately 5–8 min. To evaluate whether animals spatially localized the platform, a seventh probe trial was given on the third day of testing, during which the platform was removed and animals were given 60 s to swim freely in the maze. A video camera and tracking system tracked and measured each rat's swim pathway (Ethovision; Noldus Instruments, Wageningen, The Netherlands).
2.5. Visible platform task
Four days after MM testing, we evaluated motor and visual competence using the visible platform task. This was an adaptation of the cue-navigation version of the spatial MM task previously used to dissociate visual and motor acuity from place memory [12]. This task is ideal due to its similarity to other spatial water-maze tasks with respect to motor and visual requirements, differing only in that animals are not required to associate the location of the platform with distal cues [12]. Animals were given four days after completion of the DMS and MM tasks to rest before the visible platform task was administered; this was done to ensure that any differences observed on the visible platform task were due to the administration of the treatment, and not to exhaustion from repeated water maze testing. The apparatus was a rectangular tub (39 × 23 in) filled with clear room temperature water. A black platform (10 cm wide) was positioned 1.5″ above the surface of the water, following previously published methods [16]. Opaque curtains surrounded the maze to block distal cues (Fig. 1d). Animals were given six trials in one day. The drop off location remained the same across trials, and the platform location for each trial varied semi-randomly across three locations. Each rat had 90 s to locate the platform, where it remained for 15 s before being placed back into a heated cage awaiting the next trial. The inter-trial-interval was approximately 5–8 min.
2.6. Procedure for determining visual and motoric competence for water maze testing
Because we counterbalanced treatment groups across squads, we were able to evaluate Harmine treatment at different timepoints relative to when injection occurred. Two Harmine-high animals had to be repeatedly removed from the first maze tested (DMS) because they were incapable of keeping their heads above the surface of the water. These animals were removed from the study before completion of the first maze task, and were therefore not tested on the MM or visible platform task. Two additional Harmine-high treated subjects demonstrated motor difficulties impacting swim ability. These animals were impacted to a lesser degree, such that it was not necessary to remove them from the maze because they could maintain their heads above the water while swimming; however, they were unable to swim to the platform on their own. These two animals had to be led to the platform on the majority of their trials, often without having entered any arms, and therefore receiving an error score of zero. Because these motor challenges would impact interpretation of performance, we removed these two animals from all statistical analyses. All four of these animals received Harmine-high treatment; all remaining animals were able to swim well and locate the platform on their own by day 5 of testing. All four of the Harmine-high treated animals described above that were ultimately excluded from analyses were tested at the beginning of the day, in close temporal proximity to injections. The two animals that were physically unable to complete testing were the first two animals to be tested each day and both showed persistent and severe motor problems during maze testing, such that they were unlikely to survive behavioral testing, had it continued. It is important to note that these four animals exclusively comprised the subset of Harmine-high treated animals in the first testing squad; all of these animals were tested daily within 90 min of injection. Most Harmine-high treated animals demonstrated similar impairments lasting roughly 1–2 h after injections, followed by qualitatively normal behavior until the next round of injections. Because maze testing continued beyond this 2-hour post-injection window of time, we were able to evaluate the remaining Harmine-high animals later in the day, when their swimming ability was no longer impacted to a degree that hindered the procedural aspects of solving a water escape task. We took extra caution to include only animals that either located the platform on their own, or made at least multiple incorrect arm entries during their allotted trial time in our analyses, to avoid any possibility of interpreting motor deficits as superior maze performance.
2.7. Statistical analyses
In an initial analysis, Harmine-low and Harmine-high groups were compared. There were no statistical differences between the two Harmine-treated groups for any measure on the DMS or MM tasks. Therefore, for final analyses, both Harmine treatment groups were combined so that the final treatment groups were as follows (final n in parentheses): Vehicle (10), Harmine (16).
DMS testing was divided into four testing blocks consisting of two consecutive days each (Block 1 = Days 2 and 3, Block 2 = Days 4 and 5, Block 3 = Days 6 and 7, Block 4 = Days 8 and 9). DMS data were analyzed with an omnibus ANOVA with treatment as the between groups variable and total number of errors for each trial as repeated measures within each testing block.
MM data were analyzed with an omnibus ANOVA with treatment as the between groups variable, and swim distance (cm) to the platform as repeated measures. For MM probe trial data, percent distance in the previously platformed (target) quadrant was compared with the diagonally opposite quadrant, using repeated measures (quadrant) ANOVA. Rats that spatially localized the platform should spend a greater percent distance in the target vs. opposite quadrant.
3. Results
3.1. DMS asymmetrical 3-choice task
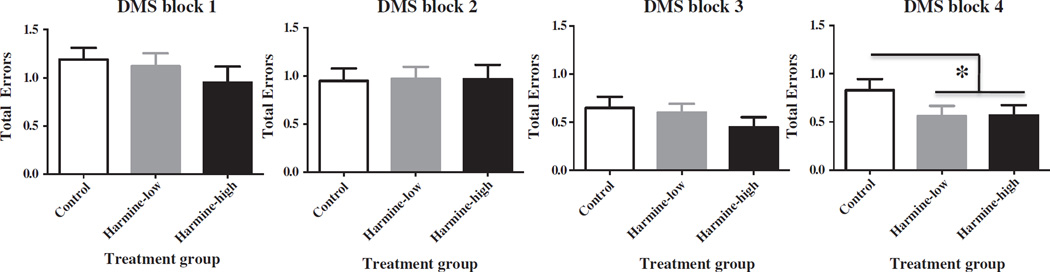
For testing block 4, the lattermost portion of testing, there was a main effect of Harmine treatment for trials 2–6 (F(1,24) = 5.04, p < 0.05), with Harmine-treated animals making fewer total errors relative to animals treated with saline, indicating that Harmine treatment enhanced working and recent memory (Fig. 2). There were no effects of Harmine treatment for testing blocks 1, 2 or 3.
Figure 2.
Mean ± SEM total errors made on each block of the DMTS task. There were no effects of Harmine on DMTS performance for testing blocks 1–3. For testing block 4, the lattermost portion of testing, there was a main effect of Harmine treatment for trials 2–6 (F(1,24)= 5.04, p < 0.05), with Harmine-treated animals making fewer total errors than animals treated with saline.
3.2. Morris water maze
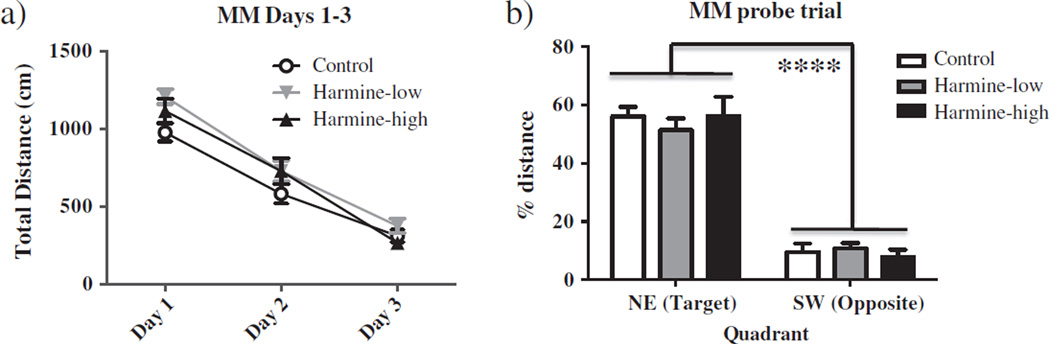
There were no Harmine treatment effects for distance on days 1–3 (Fig. 3a). For the probe trial, a higher percent distance was spent in the previously platformed vs. the opposite quadrant (Quadrant main effect: F(1,24) = 154.84; p <.0001). This was in the absence of a Treatment × Quadrant interaction (F(1,24) = 0.03, p = 0.87; NS), indicating that all groups localized to the previously platformed quadrant (Fig. 3b).
Figure 3.
a) Mean ± SEM total distance swam for all days of the MM. There were no Harmine treatment effects for distance on days 1–3. b) Mean ± SEM percent of total distance swam in the NE versus the SW quadrant of the MM during the probe trial. A higher percent distance was spent in the previously platformed vs. the opposite quadrant (Quadrant main effect: F(1,24) = 154.84; p < .0001) with no Treatment × Quadrant interaction (F(1,24) = 0.03, p = 0.87; NS).
3.3. Visible platform task
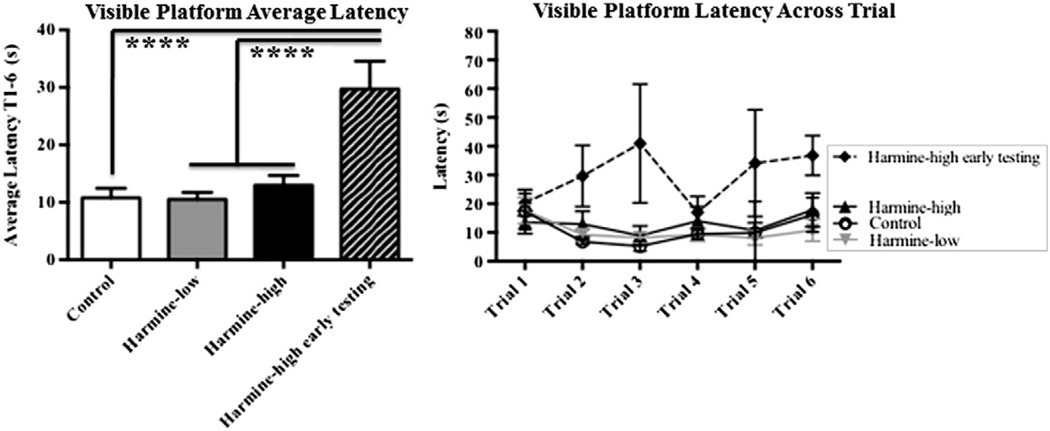
The visible platform task was used to confirm that animals have the ability to perform the procedural components of water-escape maze testing, including the visual and motor capacities necessary to swim toward and climb onto a platform. To verify that the animals excluded from analyses were experiencing treatment-induced impairments impacting procedural ability to perform the task, we included these animals in a separate group (Harmine-high early testing) for our initial visible platform analysis. There was a main effect of Treatment (F(3,25) = 7.49, p < 0.001), with post-hoc analyses revealing that the Harmine-high early testing animals had greater escape latencies than the Control (Fisher, p < 0.0001), and Harmine (Fisher, p < 0.001) groups. Harmine-high early testing animals had latencies that were, on average, three times larger than those of the other animals. The average escape time for the Harmine-high early testing animals was outside of 2 standard deviations of the average escape time of the rest of the animals in the Harmine-high treatment group, indicating that, at the time of their behavioral testing, these animals were experiencing motor and/or visual impairments that precluded them from effectively performing the procedural components of a swim water escape task.
Harmine-low and Harmine-high treatment groups did not differ from the Control group on latency to reach the platform (Fisher, p > 0.05), indicating that our final groups did not differ in the motor or visual capacity necessary to complete a water maze task (Fig. 4). This finding was especially crucial for interpretation of the other water maze tasks, as we assume that differences in our water maze represent memory differences, and not differences on other factors such as motor or visual ability.
Figure 4.
Mean ±SEM latency to reach the platform on the visible platform task. There was a main effect of Treatment (F(3,25)= 7.49, p < 0.001), with post-hoc analyses revealing that the Harmine-high early testing animals had greater escape latencies than the Control (Fisher, p < 0.0001), and Harmine (Fisher, p < 0.001) treatment groups. Harmine-low and Harmine-high treatment groups did not differ from the Control group on latency to reach the platform (Fisher, p > 0.05).
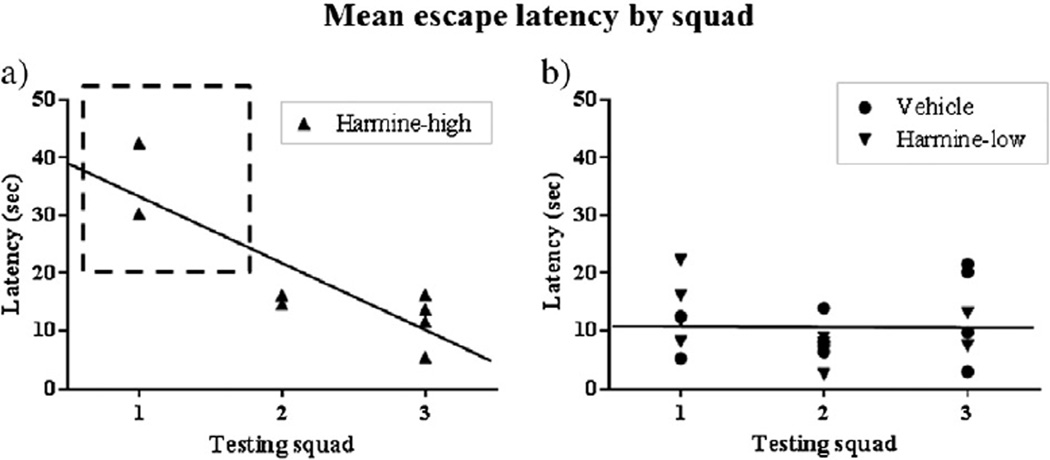
Visible platform data were further analyzed using a regression analysis with testing squad (1–3) as the predictor and mean escape latency across trials 1–6 as the criterion. Fig. 5 shows average swim time across all 6 trials on the visible platform task for each subject in order of testing squad. For Harmine-high treated animals, testing squad (1–3) was a significant predictor of mean escape latency (β = −11.567, SE = 2.751, p < 0.01, R2= 0.75) with mean escape latency decreasing by an average of roughly 11.6 s with each squad tested, and testing squad accounting for 75% of the total variance in mean escape latency (Fig. 5a). Testing squad was not a significant predictor of mean escape latency in the Harmine-low (β = −2.968, SE = 2.255, p > 0.05, NS, R2 = 0.18) or Control (β = 2.696, SE = 2.619, p > 0.05 NS, R2 = 0.12) groups. To simplify presentation, Harmine-low and Control groups are presented together (β = −0.08, SE = 1.702, p > 0.05, NS, R2 < 0.01) (Fig. 5b). Once the two remaining Harmine-high animals in testing squad 1 were excluded, testing squad was no longer a significant predictor of mean escape latency within the Harmine-high treated animals, further supporting the assertion that these animals were impaired in performing the procedural components of a swim task (β = −3.659, SE = 3.485, p > 0.05, NS, R2 = 0.21, data not shown).
Figure 5.
Swim time across all 6 trials on the visible platform task for each subject in order of testing squad.
4. Discussion
The current study found that: (1) Harmine is a significant enhancer of short-term working and recent memory, (2) Harmine does not affect spatial reference memory at doses of 1 mg or 5 mg per day, and (3) high doses of Harmine produce clear but transient motor side-effects, impacting ability to meet procedural requirements of testing.
Harmine treatment enhanced working and recent memory on the DMS task on the lattermost portion of testing. It is noteworthy that this enhancement was only apparent for the final portion of DMS testing (Block 4), and did not emerge in earlier testing sessions; it is possible that this difference emerged on this lattermost portion of testing due to a slight (non-significant) increase in the error rate of the control group. However, Harmine also elicited obvious motoric effects within 2 h of injection. Only Harmine-high animals that were tested more than 2 h after injection were included in these analyses. Many of these animals also showed motor deficits earlier in the day, closer in time to injections, which were resolved by the time they were tested in the DMS and MM tasks. The plasma half-life of Harmine has been reported as varying from 1 to 3 h [21], which corresponds to the length of time that animals showed the most severe motor deficits following injections each day. Because we found corresponding performance effects on our water maze control task, the visible platform task, which was administered after the animals had several days with no water maze testing or otherwise physically challenging activities, we conclude that these motoric deficits were induced solely by the Harmine treatment. This study demonstrates the importance of verifying motoric and visual competence in swim tasks such as water maze testing and indicates the visible platform task as a task that is sensitive to non-mnemonic differences.
Harmine is known to inhibit a large number of protein targets, including the 5-hydroxytryptamine receptor subtypes 5-HT2 and 5-HT1A [22], the NMDA receptor [23], monoamine oxidase-A (MAO-A) [24], and dopaminergic signaling pathways [25–27]. While the specific functionally-relevant target(s) mediating cognitive effects of Harmine have yet to be fully defined, one possibility is that Harmine may be exerting cognitive benefits through its inhibition of DYRK1A activity.
The overexpression of DYRK1A has been shown to impair hippocampal-dependent memory performance [10]. In addition to cognitive phenotypes, the overexpression of DYRK1A in Down syndrome patients is associated with increased phosphorylation of Tau protein, increased DYRK1A-positive neurofibrillary tangles (NFTs), neurofibrillary degeneration, increased amyloid precursor protein (APP) cleavage and increased β-amyloidosis [4], suggesting that DYRK1A could be an important target for cognitive enhancing compounds relevant to Alzheimer's disease [28]. Given that Harmine is currently the most potent and selective known inhibitor of DYRK1A [2], further studies examining the cognitive impact of Harmine administration in DYRK1A and transgenic Alzheimer's disease mouse models, with careful consideration of potential side effects, are warranted.
HIGHLIGHTS.
Old rats given 1 or 5 mg Harmine were tested on a maze battery.
Motor impairment was seen 1–2 h post-treatment with 5, but not 1, mg.
Visible platform task identified rats unable to perform maze procedural components.
Harmine enhanced working and recent memory in motor unimpaired rats.
Illustrates necessity of control tasks for accurate interpretation of maze cognition
Acknowledgments
This research was funded by grants awarded to HAB-N from the National Institute on Aging (AG028084), the state of Arizona, ADHS, a Diversity Supplement to a National Institute on Aging grant (AG028084), the APA Diversity Program in Neuroscience, the NIH Initiative for Maximizing Student Development (IMSD) program (R25GM099650), the More Graduate Education at Mountain States Alliance (NSF), and the Western Alliance to Expand Student Opportunities Louis Stokes Alliance for Minority Participation Bridge to the Doctorate (WAESO-LSAMP-BD) National Science Foundation Cooperative Agreement HRD-1025879.
References
- 1.Freedland CS, Mansbach RS. Behavioral profile of constituents in ayahuasca, an Amazonian psychoactive plant mixture. Drug Alcohol Depend. 1998;54:183–194. doi: 10.1016/s0376-8716(98)00154-9. [DOI] [PubMed] [Google Scholar]
- 2.Becker W, Sippl W. Activation, regulation and inhibition of DYRK1A. Fed Eur Biochem Soc J. 2010;278:246–256. doi: 10.1111/j.1742-4658.2010.07956.x. http://dx.doi.org/10.1111/j.1742-4658.2010.07956.x. [DOI] [PubMed] [Google Scholar]
- 3.Park J, Song W, Chung KC. Function and regulation ofDYRK1A: towards understanding Down syndrome. Cell Mol Life Sci. 2009;66:3235–3240. doi: 10.1007/s00018-009-0123-2. http://dx.doi.org/10.1007/s00018-009-0123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wegiel J, Dowjat K, Kaczmarski W, Kuchna I, Nowicki K, Frackowiak J, et al. The role of overexpressed DYRK1A protein in the early onset of neurofibrillary degeneration in Down syndrome. Acta Neuropathol. 2008;116(4):391–407. doi: 10.1007/s00401-008-0419-6. http://dx.doi.org/10-1007/s00401-008-0419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azorsa DO, Robeson RH, Frost D, et al. High-content siRNA screening of the kinome identifies kinases involved in Alzheimer's disease-related tau hyperphosphorylation. BMC Genomics. 2010;11:25. doi: 10.1186/1471-2164-11-25. http://dx.doi.org/10.1186/1471-2164-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J, Yang EJ, Yoon JH, Chung KC. Dyrk1A overexpression in immortalized hippocampal cells produces the neuropathological features of Down syndrome. Mol Cell Neurosci. 2007;36:270–279. doi: 10.1016/j.mcn.2007.07.007. http://dx.doi.org/10.1016/j.mcn.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Ryoo SR, Jeong HK, Radnaabazar C, et al. DYRK1A-mediated hyperphosphorylation of Tau. A functional link between Down syndrome and Alzheimer disease. J Biol Chem. 2007;282:34850–34857. doi: 10.1074/jbc.M707358200. http://dx.doi.org/10.1074/jbc.M707358200. [DOI] [PubMed] [Google Scholar]
- 8.Arqué G, Fotaki V, Fernández D, Martínez de Lagrán M, Arbonés ML, Dierssen M. Impaired spatial learning strategies and novel object recognition in mice haploinsufficient for the dual specificity tyrosine-regulated kinase-1A (Dyrk1A) PLoS One. 2008;3:e2575. doi: 10.1371/journal.pone.0002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frost D, Meechoovet B, Wang T, Gately S, Giorgetti M, Shcherbakova I, et al. β-carboline compounds, including harmine, inhibit DYRK1A and tau phosphorylation at multiple Alzheimer's disease-related sites. PLoS One. 2011 doi: 10.1371/journal.pone.0019264. http://dx.doi.org/10.1371/journal.pone.0019264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn KJ, Jeong HK, Choi HS, et al. DYRK1A BAC transgenic mice show altered synaptic plasticity with learning and memory defects. Neurobiol Dis. 2006;22:463–472. doi: 10.1016/j.nbd.2005.12.006. http://dx.doi.org/10.1016/j.nbd.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Moura DJ, Rorig C, Vieira DL, Henriques JAP, Roesler R, Saffi J, et al. Effects of β-carboline alkaloids on the object recognition task in mice. Life Sci. 2006;79:2099–2104. doi: 10.1016/j.lfs.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 13.Albores R, Neafsey EJ, Drucker G, Fields JZ, Collins MA. Mitochondrial respiratory inhibition by N-methylated β-carboline derivatives structurally resembling N-methyl-4-phenylpyridine. Proc Natl Acad Sci. 1990;87:9368–9372. doi: 10.1073/pnas.87.23.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawanishi K, Eguchi N, Hayashi T, Hashimoto Y. Relationship between occurrence of tremor/convulsions and level of β-carbolines in the brain after administration of β-carbolines into mice. Pharmacol Biochem Behav. 1993;47(3):689–699. doi: 10.1016/0091-3057(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 15.Fuentes JA, Longo VG. An investigation on the central effects of Harmine, Harmaline and related β-carbolines. Neuropharmacology. 1971;10:15–23. doi: 10.1016/0028-3908(71)90004-9. [DOI] [PubMed] [Google Scholar]
- 16.Hunter CL, Bimonte HA, Granholm AC. Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory. Behav Brain Res. 2003;138:121–131. doi: 10.1016/s0166-4328(02)00275-9. http://dx.doi.org/10.1016/S0166-4328(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 17.Rummel J, Epp JR, Galea LA. Estradiol does not influence strategy choice but place strategy choice is associated with increased cell proliferation in the hippocampus of female rats. Horm Behav. 2010;58:582–590. doi: 10.1016/j.yhbeh.2010.07.009. http://dx.doi.org/10.1016/j.yhbeh.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, et al. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem. 2010;93:444–453. doi: 10.1016/j.nlm.2010.01.002. http://dx.doi.org/10.1016/j.nlm.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engler-Chiurazzi LB, Tsang CW, Nonnenmacher S, Liang WS, Corneveaux JJ, Prokai L, et al. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol Aging. 2011;32:680–697. doi: 10.1016/j.neurobiolaging.2009.09.005. http://dx.doi.org/10.1016/j.neurobiolaging.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1994;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- 21.Baselt R. Disposition of toxic drugs and chemicals in man. 8th ed. Foster City, CA: Biomedical Publications; 2008. pp. 727–728. [Google Scholar]
- 22.Glennon RA, Dukat M, Grella B, et al. Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors. Drug Alcohol Depend. 2000;60:121–132. doi: 10.1016/s0376-8716(99)00148-9. [DOI] [PubMed] [Google Scholar]
- 23.Du W, Aloyo VJ, Harvey JA. Harmaline competitively inhibits [3H] MK-801 binding to the NMDA receptor in rabbit brain. Brain Res. 1997;770:26–29. doi: 10.1016/s0006-8993(97)00606-9. [DOI] [PubMed] [Google Scholar]
- 24.Herraiz T, Gonzalez D, Ancin-Azpilicueta C, Aran VJ, Guillen H. beta-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO) Food Chem Toxicol. 2010;48:839–845. doi: 10.1016/j.fct.2009.12.019. http://dx.doi.org/10.1016/j.fct.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Arib O, Rat P, Molimard R, et al. Electrophysiological characterization of harmane-induced activation of mesolimbic dopamine neurons. Eur J Pharmacol. 2010;629:47–52. doi: 10.1016/j.ejphar.2009.12.012. http://dx.doi.org/10.1016/j.ejphar.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Yang YJ, Lee JJ, Jin CM, Lim SC, Lee MK. Effects of harman and norharman on dopamine biosynthesis and L-DOPA-induced cytotoxicity in PC12 cells. Eur J Pharmacol. 2008;587:57–64. doi: 10.1016/j.ejphar.2008.03.050. http://dx.doi.org/10.1016/j.ejphar.2008.03.050 [10]. [DOI] [PubMed] [Google Scholar]
- 27.Pimpinella G, Palmery M. Interaction of beta-carbolines with central dopaminergic transmission in mice: structure–activity relationships. Neurosci Lett. 1995;189:121–124. doi: 10.1016/0304-3940(95)11469-d. [DOI] [PubMed] [Google Scholar]
- 28.Wegiel J, Gong CX, Hwang YW. The role of DYRK1A in neurodegenerative diseases. Fed Eur Biochem Soc J. 2011;278:236–245. doi: 10.1111/j.1742-4658.2010.07955.x. http://dx.doi.org/10.1111/j.1742-4658.2010.07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]