Abstract
Purpose/Objectives
To examine the role of apolipoprotein E (APOE) genotype in the cognitive function of post-menopausal women with early-stage breast cancer prior to initiation of adjuvant therapy and over time with treatment.
Design
Longitudinal, genetic association study.
Setting
Urban university cancer center.
Sample
Three cohorts of postmenopausal women: 37 women with breast cancer receiving chemotherapy and anastrozole, 41 women with breast cancer receiving anastrozole alone, and 50 healthy women.
Methods
Cognitive function was evaluated three times during a 12-month period using a comprehensive neuropsychological test battery. Participants were genotyped and classified based on the presence or absence of at least one APOE ε4 allele. Multiple linear regression was used to determine if APOE genotype accounted for observed variability in cognitive function data.
Main Research Variables
APOE genotype, breast cancer treatment, and cognitive function.
Findings
Performance or changes in performance on tasks of executive function, attention, verbal learning and memory, and visual learning and memory were found to be influenced by APOE genotype and/or interactions between APOE genotype and study cohort.
Conclusions
The results indicate that cognitive function in postmenopausal women with breast cancer is modified by APOE genotype and the combination of APOE genotype and treatment.
Implications for Nursing
APOE genotype, along with other biomarkers, may be used in the future to assist nurses in identifying women with breast cancer most at risk for cognitive decline.
Keywords: breast neoplasms, cognition, genes, biologic markers
Breast cancer is the most prevalent form of cancer, excluding skin cancer, among women in the United States, with an estimated 232,340 new cases of invasive breast cancer and 64,640 new cases of carcinoma in situ diagnosed in 2013 (American Cancer Society, 2013). Fortunately, in the United States, the overall five-year relative survival rate for women with breast cancer, inclusive of all stages, is 89% (Howlader et al., 2011), making women with breast cancer the largest group of cancer survivors in the United States at 2.9 million women (American Cancer Society, 2013). However, survivorship comes with long-term and late effects related to cancer and/or cancer treatment for a large number of breast cancer survivors.
One of the most common and problematic phenomenon experienced by breast cancer survivors is adjuvant therapy-related cognitive decline (Bender et al., 2006; Downie, Mar Fan, Houédé-Tchen, Yi, & Tannock, 2006; Hurria et al., 2006; Jenkins et al., 2006; Mehnert et al., 2007; Schagen et al., 1999; Schilder et al., 2009; Shilling & Jenkins, 2007). A large body of evidence exists to objectively support these reported deficits (Falleti, Sanfilippo, Maruff, Weih, & Phillips, 2005). In addition, growing evidence suggests that women with breast cancer have poorer cognitive function compared to healthy women prior to the initiation of adjuvant therapy (Hermelink et al., 2007; Schilder et al., 2010; Wefel et al., 2004; Wefel, Saleeba, Buzdar, & Meyers, 2010). Even small changes in cognitive function can have a major impact on a survivor’s quality of life, affecting relationships with family and friends, educational and career decisions, job performance, emotional state, the ability to make informed treatment decisions, and adherence to cancer therapy (Boykoff, Moieni, & Subramanian, 2009; Munir, Burrows, Yarker, Kalawsky, & Bains, 2010; Myers, 2012; Stilley, Bender, Dunbar-Jacob, Sereika, & Ryan, 2011; Tchen et al., 2003; Von Ah, Habermann, Carpenter, & Schneider, 2013).
However, discrepancies remain in the percentage of women with breast cancer exhibiting cognitive changes, the severity of the change, and the specific cognitive domains affected (Falleti et al., 2005; Janelsins et al., 2012). It also remains unclear if all women with breast cancer or only a subset of these women are at risk for poorer cognitive function at pretreatment or for cognitive decline with therapy. Therefore, understanding the variability in cognitive changes in women with breast cancer is key to better predict which women are most at risk for poorer pretreatment cognitive function, as well as cognitive decline with adjuvant therapy, and to tailor and personalize interventions to mitigate the effects of cognitive changes for these women.
Potential Mechanisms Related to Cognitive Decline
Oxidative Stress
A potential mechanism to account for the poorer pre-therapy cognitive function and the cognitive changes observed in women with breast cancer is oxidative stress. Oxidative stress has been implicated in other, more severe cognitive conditions including mild cognitive impairment, Parkinson disease, and Alzheimer disease (Bonda et al., 2010; Mariani, Polidori, Cherubini, & Mecocci, 2005). Oxidation refers to the removal of an electron from an atom or molecule and occurs normally in humans as part of mechanisms such as mitochondrial and peroxisomal metabolism, but also can be the result of exogenous exposures to various agents including ultraviolet light, chemotherapeutics, and environmental toxins (Finkel & Holbrook, 2000).
One of the byproducts of oxidation is free radicals. Free radicals that contain oxygen, or reactive oxygen species (ROS), are of particular interest within biologic systems. ROS are positively charged, unstable atoms or molecules that try to achieve stability by taking electrons from other atoms or molecules. This process of stealing electrons can result in cellular and DNA damage along with the creation of additional free radicals, generating a chain reaction of even more damage that can ultimately result in neuronal dysfunction (Finkel & Holbrook, 2000). To combat excessive ROS burden, humans have antioxidant defenses, including specific enzymes, peptides, and vitamins. Therefore, oxidative stress is the sum of ROS production and antioxidant capability for ROS elimination (Azzi, 2007; Finkel & Holbrook, 2000).
The cellular environment of a woman with breast cancer is one of increased oxidative stress. Research has shown that individuals with cancer have higher levels of oxidative stress markers prior to treatment than healthy controls (Amin, Mohamed, El-Wakil, & Ibrahem, 2012; Blasiak et al., 2004; Hamed, Zakhary, & Maximous, 2012). In addition, chemotherapy serves as an exogenous source of ROS (Conroy et al., 2012; Joshi et al., 2005; Kasapovic et al., 2010), and anti-estrogen therapies such as aromatase inhibitors essentially block the production of estrogen, which performs an antioxidant role in the brain (Strehlow et al., 2003; Unfer, Conterato, Da Silva, Duarte, & Emanuelli, 2006). Because of high metabolic demands and low antioxidant capacity, brain cells are particularly vulnerable to oxidative damage. For additional detail on the role of chemotherapy and estrogen in cognitive decline, the authors recommend a review article by Walker, Drew, Antoon, Kalueff, and Beckman (2012).
Considering the role oxidative stress plays in poorer cognitive function, the potential increased oxidative stress influence on the brain cells of women with breast cancer, and the variability seen between women with respect to cognitive changes, exploring genetic underpinnings of this observed variability is logical, starting with candidate genes known to influence and/or modify the response to oxidative stress.
Apolipoprotein E
Evidence suggests that apolipoprotein E (APOE) performs antioxidant activities throughout the body (Hayek, Oiknine, Brook, & Aviram, 1994), in addition to its better known function as a regulatory protein involved in cholesterol and phospholipid metabolism (Mahley, Innerarity, Rall, & Weisgraber, 1984). Three functionally distinct APOE isoforms exist in humans, E2, E3, and E4, which correspond to the three normal variant alleles, ε2, ε3, and ε4, respectively. These allele variants differ from each other at two amino acid sites (Mahley et al., 1984). The antioxidant ability of APOE appears to be isoform-dependent with the E2 isoform having the greatest antioxidant capacity and the E4 isoform having the least antioxidant capacity (i.e., E2 > E3 > E4) (Jolivalt et al., 2000; Miyata & Smith, 1996; Pedersen, Chan, & Mattson, 2000). Additional information about APOE genotype and oxidative stress can be found in Jofre-Monseny, Minihane, and Rimbach (2008).
In addition, a well-established relationship exists between the presence of one or more ε4 alleles and increased risk of Alzheimer disease (Farrer et al., 1997; Richard & Amouyel, 2001; Sadigh-Eteghad, Talebi, & Farhoudi, 2012). Numerous studies also have found a relationship between the ε4 allele and poorer cognitive functioning in healthy middle-aged and older adult populations (Flory, Manuck, Ferrell, Ryan, & Muldoon, 2000; Hofer et al., 2002; Izaks et al., 2011; Wehling, Lundervold, Standnes, Gjerstad, & Reinvang, 2007). However, only one previous study has investigated the association between APOE genotype and cognitive change in women with breast cancer. In this cross-sectional study of 80 long-term breast cancer and lymphoma survivors, who had previously received standard dose chemotherapy and were now an average of 8.8 years post-treatment, Ahles et al. (2003) found that the presence of at least one ε4 allele was associated with poorer performance in visual memory, spatial ability, and psychomotor functioning compared to survivors who did not possess an ε4 allele. However, the interpretations of these findings are limited by the lack of pretreatment data, longitudinal assessment, and healthy control group for comparison. In addition, the substantial length of time post-treatment does not inform the immediate effects of APOE genotype and treatment on cognitive function.
Therefore, because of the presumed increase in oxidative stress from cancer, chemotherapy, and anti-estrogen therapy, combined with the known impact of oxidative stress on cognitive function and the variability in antioxidant capacity by APOE isoform, the purpose of the current study was to explore the role of APOE genotype in the cognitive function of postmenopausal women with early-stage breast cancer prior to the initiation of adjuvant chemotherapy and/or anti-estrogen therapy and over time through the first year of adjuvant treatment.
Methods
Participants and Setting
Participants were recruited for this exploratory, genetic ancillary study from the Anastrozole Use in Menopausal Women (AIM) study (R01 CA107408), a longitudinal prospective cohort study investigating the impact of the anti-estrogen therapy, anastrozole, on changes in cognitive function in postmenopausal women with breast cancer. The final sample for this ancillary study (N = 128) was comprised of three cohorts of postmenopausal women: (a) women with breast cancer who received chemotherapy plus anastrozole (n = 37), (b) women with breast cancer who received anastrozole alone (n = 41), and (c) healthy, control women matched on age and years of education to the participants with breast cancer (n = 50).
Women with breast cancer were recruited from the Comprehensive Breast Cancer Program of the University of Pittsburgh Cancer Institute. Healthy women were recruited using a variety of approaches including referral from women in the breast cancer cohorts, advertisements, and random digit dialing through the University Center for Social and Urban Research.
Participants currently undergoing data collection for the AIM study were simultaneously recruited to obtain a genetic sample for the ancillary study. Participants who previously completed data collection for the AIM study, and gave permission to be recontacted, were contacted for the purpose of procuring a genetic sample. Both the AIM study and ancillary study were approved by the University of Pittsburgh Institutional Review Board. Informed consent was obtained from all study participants for the parent study and the ancillary genetic study.
Inclusion criteria for all participants include being postmenopausal, having a maximum age of 75 years, having the ability to speak and read English, and completion of a minimum of eight years of education. An additional inclusion criterion for women with breast cancer was newly diagnosed early-stage breast cancer (i.e., stages I, II, or IIIa) based on the Tumor, Nodes, Metastasis (TNM) Classification of Malignant Tumors (Edge et al., 2010). Exclusion criteria for all participants include self-reported hospitalization for a psychiatric illness within the past two years and a history of neurologic illness or cancer. In addition, women with breast cancer with clinical evidence of distant metastases were deemed ineligible.
Evaluation of Cognitive Function
Cognitive function was evaluated at three time points in all study participants. For women with breast cancer receiving chemotherapy plus anastrozole, cognitive function was assessed after primary surgery but prior to the initiation of chemotherapy (T0), prior to the initiation of anastrozole (T1), and six months after the initiation of anastrozole (T2). Cognitive function was evaluated in women who received anastrozole alone prior to the initiation of anastrozole (T0), six months after the initiation of anastrozole (T1), and 12 months after the initiation of anastrozole (T2). Healthy controls were assessed at comparable time points: baseline (T0), six months after T0 (T1), and 12 months after T0 (T2).
Knowledge Translation.
Possession of one or more apolipoprotein E (APOE) ε4 alleles has been associated with decreased antioxidant capacity and increased risk of Alzheimer disease.
Variability in APOE genotype may partially explain observed variation in cognitive changes in women with and receiving treatment for breast cancer.
Potential modifications of cancer- and treatment-related cognitive changes in women with breast cancer by genetic variation should be further investigated.
Cognitive function was measured using a comprehensive battery of neuropsychological tests encompassing six cognitive domains: attention, learning and memory, psychomotor speed, mental flexibility, executive function, and visuospatial ability. Neuropsychological tests were selected based on test validity, reliability, and sensitivity, as well as on the availability of alternative, equivalent forms to minimize the influence of practice effects. The battery was administered to study participants by research nurses trained by a clinical neuropsychologist. The average time for completion was 90 minutes. The neuropsychological tests comprising the battery and the reduction of the dimensionality of the cognitive function data has been described in detail previously (Bender et al., 2013). The six resulting composite cognitive function factors and the neuropsychological tests comprising each factor are detailed in Table 1. All cognitive measures have been demonstrated to be sensitive to changes in cognitive function in women with breast cancer (Bender et al., 2010).
Table 1.
Neuropsychological Tests According to Cognitive Function Factors
| Factor | Neuropsychological Test |
|---|---|
| Attention | CANTAB Spatial Working Memory Test (Owen et al., 1995) CANTAB Stockings of Cambridge Test (Owen et al., 1995) Digit Vigilance Test (Matthews, 1964) |
| Executive function | Delis Kaplan Color Word Interference Test (Delis et al., 2001) Verbal Fluency Test (Delis et al., 2001) Trail Making Test B (Reitan & Wolfson, 1985) |
| Psychomotor efficiency | Grooved Pegboard Test (Matthews, 1964) Digit Symbol Substitution Test (Wechsler, 1981) |
| Verbal learning and memory | Rivermead Behavioral Memory Test (Wilson et al., 1989) Rey Auditory Verbal Learning Test (Rey, 1964) |
| Visual learning and memory | CANTAB Paired Associates Learning Test (Owen et al., 1995) Rey Complex Figure Test (Osterrieth, 1944) |
| Visuospatial ability | CANTAB Rapid Visual Information Processing Test (Owen et al., 1995) |
CANTAB—Cambridge Neuropsychological Test Automated Battery
Covariates and Confounders
Age and estimated verbal intelligence (National Adult Reading Test-Revised) (Nelson, 1981) were measured at T0. Time-dependent covariates including depression (Beck Depression Inventory–II) (Beck, Steer, & Brown, 1996), anxiety (Profile of Mood States [POMS] tension-anxiety subscale) (McNair, Lorr, & Droppleman, 1992), fatigue (POMS fatigue-inertia subscale) (McNair et al., 1992), and pain (Brief Pain Inventory) (Cleeland, 1989) were assessed at each time point.
Genotyping for Apolipoprotein E
A sample of 3 cc of whole blood or 2 cc of saliva was collected from each participant. DNA was extracted from peripheral leukocytes using a simple salting out procedure (Miller, Dykes, & Polesky, 1988) or from saliva using the protocol and reagents supplied with the Oragene DNA collection kits (DNA Genotek, 2012). Genotypes were determined for the two functional single nucleotide polymorphisms (SNPs) for the APOE gene, rs429358 and rs7412, that comprise the ε2, ε3, and ε4 alleles. Genotype for rs429358 was determined via TaqMan® allelic discrimination, and genotype for rs7412 was determined by inclusion in an i-PLEX® MassARRAY® multiplex assay. Positive and negative controls were included. Genotype data were double blind culled by two individuals, and discrepancies were rectified by review of raw data. SNP genotypes for rs429358 and rs7412 were combined for each participant, as detailed in Table 2, to determine APOE genotype. Participant genotypes were then classified based on the presence (i.e., ε4/ε4, ε2/ε4, and ε3/ε4) or absence (i.e., ε2/ε2, ε2/ε3, and ε3/ε3) of one or more APOE ε4 alleles.
Table 2.
APOE Genotype Determination
| APOE Genotype | rs429358 Allele | rs7412 Allele |
|---|---|---|
| ε2/ε2 | T | T |
| ε3/ε3 | T | C |
| ε2/ε3 | T | CT |
| ε2/ε4 | CT | CT |
| ε3/ε4 | CT | C |
| ε4/ε4 | C | C |
APOE—apolipoprotein E
Statistical Analysis
The statistical analysis was carried out using StataSE®, version 12. A detailed descriptive analysis of all data, including demographic data, was initially performed. Data were screened for all assumptions required for the planned linear regression analysis (e.g., linearity, normality), and sources of missing data were investigated. The comparability of baseline covariate and confounder data and baseline cognitive ability between participants included in the ancillary analysis and remaining participants from the parent study was assessed using independent t tests to evaluate equality of means. In addition, the comparability of demographic and baseline covariate and confounder data among APOE ε4 status and study cohorts was assessed using analysis of variance and Pearson’s chi-square tests of independence.
Multiple linear regression was used to investigate the effect of APOE genotype on all six cognitive factors, both cross-sectionally for each time point (i.e., T0, T1, and T2) and longitudinally using change scores (i.e., T0–T1, T0–T2, and T1–T2). To obtain minimally confounded estimates of effect, all evaluated predictors were included in each model. Age, estimated intelligence, and study cohort were incorporated as fixed covariates and confounders. Time-dependent covariates and confounders (i.e., depression, anxiety, fatigue, and pain scores) for a particular assessment time point, or the change in a time-dependent covariate and confounder from assessment to assessment, were incorporated into each model as appropriate. Because the authors were interested in how the effect of APOE genotype on cognitive function may be modified by the prescribed treatment regimen, interactions between APOE ε4 absence or presence and study cohort were initially examined. If no significant interactions were observed, a main effects model, considering only APOE ε4 absence/ presence and study cohort, was fit for each cognitive function factor. Women with no ε4 alleles and the healthy control cohort served as the reference groups in the regression analysis. Unstandardized regression coefficients and significance tests at a two-tailed significance level of 0.05 were used to determine if APOE ε4 genotype status or APOE ε4 genotype by study cohort interactions improved model fit and, therefore, account for observed variability in the cognitive function data.
For each regression model, the authors examined the residuals to identify any sources of model misspecification or outliers and influential observations that may have impacted the validity of the regression findings. The screening of residuals identified several models that did not meet normality or homogeneous variance assumptions and/or contained ill-fitted observations. In cases of nonnormality or heterogeneous variance, a series of data transformations were conducted in an attempt to induce normality and homoscedasticity. To evaluate the robustness of findings, a regression model excluding points determined to be influential, as well as a robust regression model using Huber and biweight iterations, was generated. Models eliminating potentially influential multivariate-outlier cases or diminishing the weight of potentially influential univariate-outlier cases were created, as needed, to conclude the sensitivity analysis. Unstandardized regression coefficients, p values, and the correlations of fitted values were compared between the models.
Findings
Genetic samples were collected from 137 (37%) of the 366 participants from the AIM parent study. Of the 137, 5 participants (4%) had indeterminable genotypes and 4 participants (3%) had incomplete cognitive function or covariate and confounder information at T0. The women included in the APOE analysis (n = 128) were marginally younger (p = 0.048) and better educated (p = 0.032) than AIM study participants not included in the APOE analysis (n = 238) (see Table 3). Women in the APOE analysis also had higher unadjusted mean baseline visual learning and memory (p = 0.015) and psychomotor efficiency (p = 0.016) factor z scores than the remaining AIM study participants. No relationship was observed between study cohort and ε4 genotype status (χ2 = 1.192, p = 0.551). Study cohort by ε4 absence/presence groups differed slightly on estimated intelligence (p = 0.002) (see Table 4). The study cohorts did not differ on age, years of education, or baseline levels of depression, anxiety, fatigue, and pain. In general, study participants were Caucasian (97%), married (67%), and had one or more child (85%).
Table 3.
Comparison of T0 Characteristics of AIM Study Participants Included and Not Included in the APOE Analysis (N = 366)
| Characteristic | Included (n = 128)
|
Not Included (n = 238)
|
pa | ||
|---|---|---|---|---|---|
| X̄ | SD | X̄ | SD | ||
| Age (years) | 59.31 | 5.699 | 60.66 | 6.432 | 0.048* |
|
| |||||
| Years of education | 15.22 | 3.157 | 14.55 | 2.66 | 0.032* |
|
| |||||
| Estimated intelligenceb | 110.25 | 9.184 | 108.33 | 9.149 | 0.057 |
|
| |||||
| Depressionc | 4.8 | 5.161 | 6.1 | 6.608 | 0.055 |
|
| |||||
| Anxietyd | 7.64 | 5.698 | 7.59 | 5.784 | 0.931 |
|
| |||||
| Fatiguee | 5.2 | 5.77 | 5.67 | 5.575 | 0.456 |
|
| |||||
| Painf | 1.25 | 1.98 | 1.51 | 2.292 | 0.262 |
|
| |||||
| Visual learning and memoryg | 0.107 | 0.785 | −0.1139 | 0.839 | 0.015* |
|
| |||||
| Executive functiong | 0.1316 | 0.598 | 0.0827 | 0.707 | 0.506 |
|
| |||||
| Verbal learning and memoryg | −0.0591 | 0.843 | −0.2237 | 0.809 | 0.068 |
|
| |||||
| Attentiong | −0.1119 | 0.695 | −0.2652 | 0.739 | 0.054 |
|
| |||||
| Psychomotor efficiencyg | 0.0558 | 0.738 | −0.1555 | 0.829 | 0.016* |
|
| |||||
| Visuospatial abilityg | −0.0902 | 1.018 | −0.0847 | 0.899 | 0.958 |
p < 0.05
Independent t tests were used to compare means between AIM study participants included and not included in the APOE analysis.
National Adult Reading Test–Revised verbal IQ score
Beck Depression Inventory–II
Profile of Mood States tension/anxiety subscale
Profile of Mood States fatigue/inertia subscale
Brief Pain Inventory Pain Right Now score
Z score
AIM—Anastrozole Use in Menopausal Women; APOE—apolipoprotein E
Table 4.
Sample Characteristics (N = 128)
| Characteristic | Chemotherapy Plus Anastrozole (n = 37)
|
Anastrozole Alone (n = 41)
|
Healthy Controls (n = 50)
|
pa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ε4 (n = 11)
|
No ε4 (n = 26)
|
ε4 (n = 9)
|
No ε4 (n = 32)
|
ε4 (n = 16)
|
No ε4 (n = 34)
|
||||||||
| X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | X̄ | SD | ||
| Age (years) | 58.64 | 4.61 | 58.5 | 5.666 | 61.56 | 4.613 | 61.03 | 5.608 | 60.25 | 6.557 | 57.5 | 5.594 | 0.112 |
| Years of education | 16.27 | 3.349 | 15.42 | 2.595 | 15.67 | 2.958 | 14.97 | 3.605 | 15 | 2.633 | 14.94 | 3.428 | 0.847 |
| Estimated intelligence (NART) | 110.58 | 7.299 | 108.2 | 9.754 | 109.22 | 6.378 | 105.92 | 9.516 | 115.368 | 9.373 | 113.52 | 7.375 | 0.002* |
| T0 depression (BDI–II) | 3.09 | 2.625 | 5.85 | 5.655 | 3 | 2.291 | 5.34 | 6.378 | 4.06 | 4.669 | 4.88 | 4.848 | 0.548 |
| T0 anxiety (POMS tension/anxiety) | 10.64 | 7.514 | 9.45 | 5.896 | 6.44 | 3.245 | 7.16 | 4.712 | 6.13 | 5.084 | 6.79 | 6.188 | 0.145 |
| T0 fatigue (POMS fatigue/inertia) | 3.91 | 4.061 | 6.23 | 6.855 | 2.78 | 3.346 | 5.69 | 6.188 | 5 | 6.501 | 5.12 | 5.139 | 0.674 |
| T0 pain (BPI Right Now) | 0.91 | 1.375 | 1.69 | 2.112 | 1.56 | 1.74 | 1.28 | 1.955 | 0.63 | 1.544 | 1.21 | 2.307 | 0.638 |
|
| |||||||||||||
| Characteristic | n | n | n | n | n | n | pa | ||||||
|
| |||||||||||||
| Married | 7 | 20 | 5 | 24 | 8 | 22 | 0.433 | ||||||
| Children | 8 | 23 | 7 | 27 | 13 | 31 | 0.678 | ||||||
| Caucasian | 10 | 25 | 9 | 32 | 15 | 33 | 0.672 | ||||||
| Cancer stage | |||||||||||||
| • I | 8 | 11 | 8 | 25 | – | – | – | ||||||
| • IIa | 3 | 10 | – | 7 | – | – | |||||||
| • IIb | – | 3 | 1 | – | – | – | |||||||
| • IIIa | – | 2 | – | – | – | – | |||||||
p < 0.05
One-way analysis of variance was used to compare study cohort means of continuous variables. Pearson’s chi-square tests of independence was used to examine the general associations between categorical variables.
BDI–II—Beck Depression Inventory–II; BPI—Brief Pain Inventory; NART—National Adult Reading Test–Revised verbal IQ score; POMS—Profile of Mood States
Note. At baseline, participants were not experiencing depression, anxiety, fatigue, or pain. Although not significant, women in the chemotherapy plus anastrozole group had somewhat higher anxiety scores at baseline.
Cross-Sectional Time Point Analysis
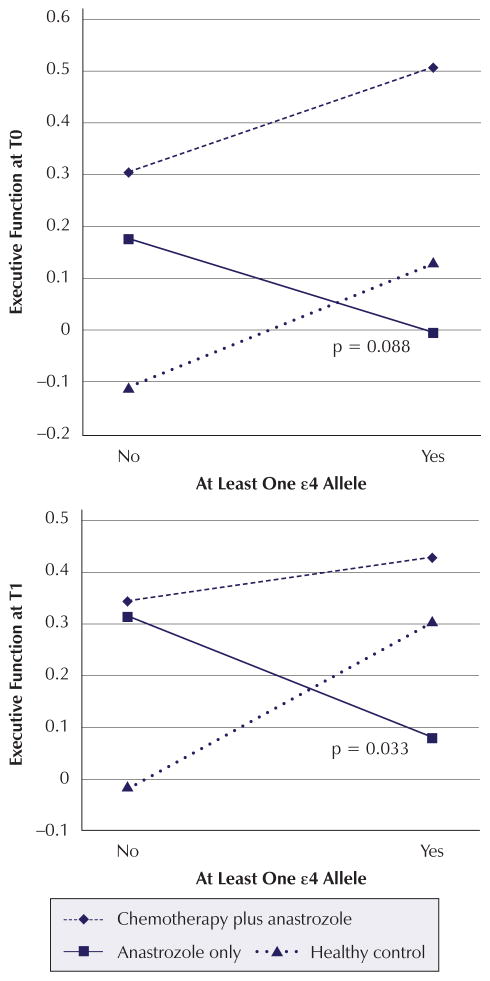
Significant time point analysis findings are summarized in Table 5. The time point analysis indicated that possession of one or more ε4 alleles contributes to poorer verbal learning and memory performance at T0 (β = −0.334, p = 0.031) and T1 (β = −0.3222, p = 0.038) regardless of cancer or treatment status. Although not statistically significant, this trend extends to T2 (β = −0.2891, p = 0.064). The combination of anastrozole-alone group membership and possession of one or more ε4 alleles contributes negatively to executive function performance both at T0 (β = −0.4448, p = 0.088) and T1 (β = −0.5771, p = 0.033) (see Figure 1).
Table 5.
Cognitive Factors With Significant Cross-Sectional Assessment Results
| Time and Model | APOE ε4 Presence
|
APOE ε4 Presence by Chemotherapy Plus Anastrozole Interaction
|
APOE ε4 Presence by Anastrozole Alone Interaction
|
|||
|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |
| Executive Function | ||||||
|
| ||||||
| T0 (n = 128) | ||||||
| Interaction | 0.2675 | 0.102 | −0.0654 | 0.795 | −0.4448 | 0.088* |
| Main effects | 0.1257 | 0.244 | ||||
|
| ||||||
| T1 (n = 125) | ||||||
| Interaction | 0.3292 | 0.047* | −0.2429 | 0.353 | −0.5771 | 0.033* |
| Main effects | 0.1047 | 0.341 | ||||
|
| ||||||
| T2 (n = 112) | ||||||
| Interaction | 0.1237 | 0.537 | 0.086 | 0.793 | −0.3331 | 0.323 |
| Main effects | 0.0679 | 0.62 | ||||
|
| ||||||
| Verbal Learning and Memory | ||||||
|
| ||||||
| T0 (n = 128) | ||||||
| Interaction | −0.0522 | 0.882 | −0.633 | 0.079 | −0.3464 | 0.349 |
| Main effects | −0.334 | 0.031* | ||||
|
| ||||||
| T1 (n = 125) | ||||||
| Interaction | −0.0899 | 0.417 | −0.0993 | 0.789 | −0.3895 | 0.309 |
| Main effects | −0.3222 | 0.038* | ||||
|
| ||||||
| T2 (n = 112) | ||||||
| Interaction | −0.1778 | 0.436 | −0.3244 | 0.384 | −0.0774 | 0.84 |
| Main effects | −0.2891 | 0.064* | ||||
p < 0.1; estimates controlled for age, estimated intelligence, depression, anxiety, fatigue, and pain scores.
APOE–apolipoprotein E
Note. The healthy control cohort and women with no ε4 alleles served as the reference groups in the analysis.
Figure 1. Mean Z Scores for Interaction Effects: Executive Function.
Note. Mean Z scores were calculated for each apolipoprotein E ε4 status and treatment combination based on mean covariate and confounder values. P values for the significant or marginally significant interactions are displayed in each graph.
Longitudinal Change Score Analysis
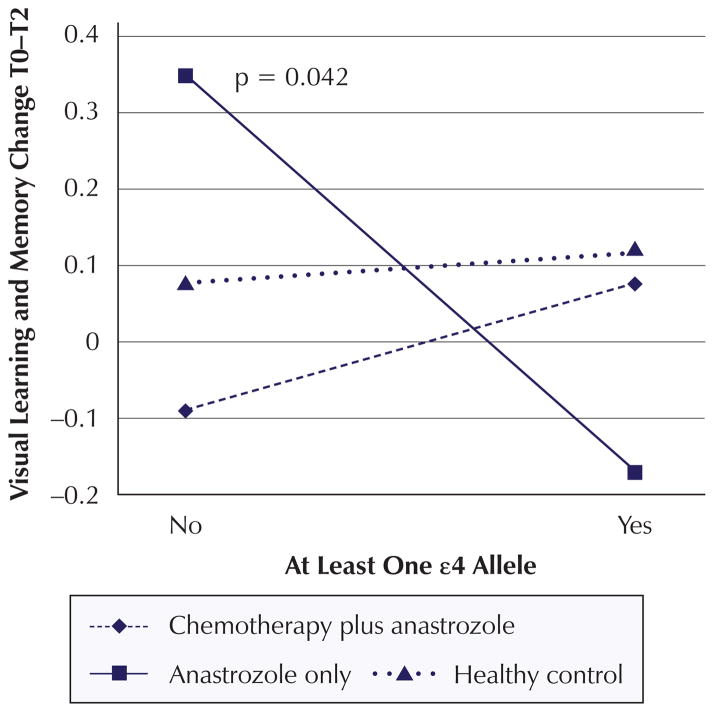
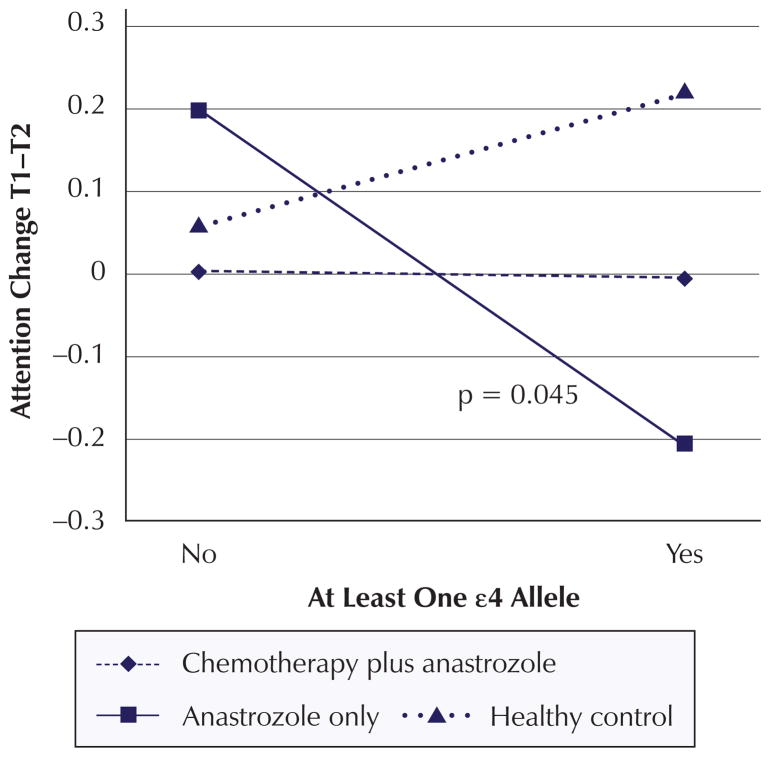
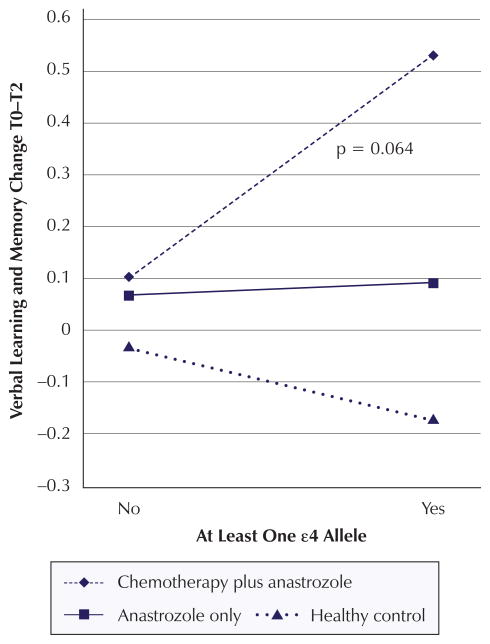
Significant change score analysis findings are summarized in Table 6. The change score analysis revealed a significant decline in visual learning and memory from T1 to T2 (β = −0.269, p = 0.027) for women with one or more ε4 alleles compared to women with no ε4 alleles regardless of cancer or treatment status. In addition, the combination of anastrozole-alone group membership and possession of one or more ε4 alleles negatively impacted change in visual learning and memory from T0 to T2 (β = −0.567, p = 0.042) (see Figure 2). The combination of anastrozole-alone group member and possession of one or more ε4 alleles contributes negatively to the change in attention from T1 to T2 (β = −0.5715, p = 0.045) (see Figure 3). In addition, the combination of chemotherapy plus anastrozole group membership and possession of one or more ε4 alleles had a positive impact on verbal learning and memory scores from T0 to T2 (β = 0.5468, p = 0.064) (see Figure 4).
Table 6.
Cognitive Factors With Significant Longitudinal Change Score Results
| Time and Model | APOE ε4 Presence
|
APOE ε4 Presence by Chemotherapy Plus Anastrozole Interaction
|
APOE ε4 Presence by Anastrozole Alone Interaction
|
|||
|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |
| Visual Learning and Memory | ||||||
|
| ||||||
| T0–T1 (n = 124) | ||||||
| Interaction | 0.1375 | 0.371 | 0.209 | 0.402 | −0.1525 | 0.548 |
| Main effects | 0.154 | 0.133 | ||||
|
| ||||||
| T0–T2 (n = 112) | ||||||
| Interaction | 0.0498 | 0.76 | 0.1082 | 0.681 | −0.567 | 0.042* |
| Main effects | −0.0604 | 0.592 | ||||
|
| ||||||
| T1–T2 (n = 111) | ||||||
| Interaction | −0.087 | 0.622 | −0.1782 | 0.542 | −0.5112 | 0.088* |
| Main effects | −0.269 | 0.027* | ||||
|
| ||||||
| Verbal Learning and Memory | ||||||
|
| ||||||
| T0 –T1 (n = 124) | ||||||
| Interaction | −0.0651 | 0.722 | 0.4485 | 0.133 | −0.0911 | 0.763 |
| Main effects | 0.0347 | 0.777 | ||||
|
| ||||||
| T0–T2 (n = 112) | ||||||
| Interaction | −0.1261 | 0.486 | 0.5468 | 0.064* | 0.1539 | 0.616 |
| Main effects | 0.0717 | 0.562 | ||||
|
| ||||||
| T1–T2 (n = 111) | ||||||
| Interaction | −0.0428 | 0.811 | 0.053 | 0.857 | 0.1105 | 0.713 |
| Main effects | 0.0005 | 0.997 | ||||
|
| ||||||
| Attention | ||||||
|
| ||||||
| T0–T1 (n = 124) | ||||||
| Interaction | 0.0409 | 0.785 | −0.258 | 0.29 | 0.1385 | 0.576 |
| Main effects | 0.0069 | 0.945 | ||||
|
| ||||||
| T0–T2 (n = 112) | ||||||
| Interaction | 0.1523 | 0.375 | −0.2949 | 0.289 | −0.3997 | 0.171 |
| Main effects | −0.0336 | 0.773 | ||||
|
| ||||||
| T1–T2 (n = 111) | ||||||
| Interaction | 0.1539 | 0.359 | −0.1669 | 0.547 | −0.5715 | 0.045* |
| Main effects | −0.0408 | 0.722 | ||||
p < 0.1; estimates controlled for age, estimated intelligence, depression, anxiety, fatigue, and pain change scores.
APOE–apolipoprotein E
Note. The healthy control cohort and women with no ε4 alleles served as the reference groups in the analysis.
Figure 2. Mean Z Scores for Interaction Effects: Visual Learning and Memory Change.
Note. Mean Z scores were calculated for each apolipoprotein E ε4 status and treatment combination based on mean covariate and confounder values. P values for the significant or marginally significant interactions are displayed in each graph.
Figure 3. Mean Z Scores for Interaction Effects: Attention Change.
Note. Mean Z scores were calculated for each apolipoprotein E ε4 status and treatment combination based on mean covariate and confounder values. P values for the significant or marginally significant interactions are displayed in each graph.
Figure 4. Mean Z Scores for Interaction Effects: Verbal Learning and Memory Change.
Note. Mean Z scores were calculated for each apolipoprotein E ε4 status and treatment combination based on mean covariate and confounder values. P values for the significant or marginally significant interactions are displayed in each graph.
Discussion
This exploratory study investigated the role of APOE genotype in cognitive function of postmenopausal women with early-stage breast cancer and represents the first study to examine the effect of APOE genotype, breast cancer, and breast cancer treatment simultaneously on cognitive function over time. In the individual time point analysis, the authors found significant or moderately significant associations between the possession of one or more ε4 alleles and poorer verbal learning and memory performance, regardless of cancer or treatment status, at all three assessment time points. Study cohort by ε4 status interactions also were observed at baseline and at the first post-treatment assessment time point for the executive function factor, with the combination of anastrozole-alone group membership and possession of one or more ε4 alleles contributing to poorer performance on executive function tasks. When the authors assessed the effect of possession of one or more ε4 alleles on changes in cognitive function over time, a significant main effect was found that was indicative of a decrease in visual learning and memory performance from T1–T2, regardless of cancer or treatment status, as well as two significant interaction effects. Specifically, anastrozole-alone group membership in combination with ε4 carrier status contributed to a decrease in attention scores from the first post-treatment (six months post-anastrozole initiation) to the second post-treatment assessment (12 months post-anastrozole initiation), and chemotherapy plus anastrozole group membership in combination with ε4 carrier status contributed to an improvement in verbal learning and memory from baseline to the second post-treatment assessment.
Consistent with findings previously reported in the literature on the relationship between APOE genotype and memory in the general adult population, the authors found that possession of one or more ε4 alleles was associated with poorer verbal learning and memory performance across all study participants, regardless of study cohort or treatment status, at every assessment time point (Caselli et al., 2011; Flory et al., 2000; Hofer et al., 2002; Nilsson, Nyberg, & Bäckman, 2002; Wehling et al., 2007). The authors propose that the marginally significant findings observed at T2 could be a reflection of practice effects (Lezak, Howieson, & Loring, 2004).
Executive function was the other cognitive factor found to have significant cross-sectional APOE genotype effects. Of note, while the main effect β coefficient contributes positively to the model for all participants, the interaction β coefficient contributes negatively to the model, nullifying the main effect and contributing an overall negative input to the baseline executive function performance for women prescribed anastrozole possessing one or more ε4 alleles. This latter finding, in particular, not only adds to the literature supporting the notion that women with breast cancer have poorer cognitive function prior to the initiation of adjuvant therapy compared to healthy controls, but also extends the knowledge, suggesting that cognitive changes are potentially augmented by genetic variation and the biologic characteristics of a woman’s breast cancer that determine treatment regimens (Ahles & Saykin, 2007; Vardy, Wefel, Ahles, Tannock, & Schagen, 2008). A similar finding was observed at the first post-treatment assessment, lending support to the proposed increased oxidative stress hypothesis; however, this trend did not significantly extend to the second post-treatment assessment.
Of note, the authors found that a chemotherapy plus anastrozole treatment regimen in combination with possession of one or more ε4 alleles actually positively contributed to verbal learning and memory performance from baseline to the second post-treatment assessment; this same trend is observed for anastrozole treatment regimen in combination with ε4 carrier status. Although unexpected based on the proposed oxidative stress hypothesis, which postulates that women with breast cancer receiving chemotherapy (i.e., highest amount of oxidative stress) who also possessed one or more ε4 alleles (i.e., least antioxidant capacity) would experience the greatest cognitive decline, this result is not entirely unfounded. In fact, evidence suggests that possession of one or more ε4 alleles may be cognitively advantageous early in life (Hubacek et al., 2001; Yu, Lin, Chen, Hong, & Tsai, 2000). Mondadori et al. (2007) found the ε4 allele to be associated with better episodic memory performance when compared to ε2 and ε3 alleles in healthy, young (X̄ age = 22.8 years, SD = 4) adults. In addition, results from the functional magnetic resonance imaging component of the study suggest that the ε4 allele is associated with more economic use of neural learning resources (Mondadori et al., 2007). Several studies considering the effect of the ε4 allele in healthy, middle-aged adults report minimal if any difference in cognitive function performance between heterozygous ε4 carriers and noncarriers (Han & Bondi, 2008; Izaks et al., 2011; Jorm et al., 2007); however, although comparable in neuropsychological task performance, cognitively intact middle- and older-aged ε4 carriers demonstrate greater brain activity during learning and memory tests than their matched ε3 counterparts (Bondi, Houston, Eyler, & Brown, 2005; Wishart et al., 2006). Therefore, this unanticipated longitudinal improvement may be partially accounted for by an undefined protective function of the ε4 allele, more efficient learning (i.e., practice effects), and an increased magnitude and extent of neural resource use by the chemotherapy plus anastrozole cohort on verbal learning and memory tasks. As the current study did not incorporate brain imaging, the two latter hypotheses could not be explored. Alternatively, treatment of the underlying cancer (of which cancers prescribed chemotherapy and anastrozole are more aggressive) may result in improvement of symptoms, including cognitive function, over time.
To the authors’ knowledge, only one study has previously examined the effect of APOE genotype on cognitive function in individuals with breast cancer. Ahles et al. (2003) reported significantly poorer performance on tasks of visual memory, spatial ability, and psychomotor functioning in long-term breast cancer and lymphoma survivors treated with chemotherapy with one or more ε4 alleles compared to those with no ε4 alleles. The results from Ahles et al. (2003) are difficult to compare to the current study because of the use of a cross-sectional design, the focus on long-term (X̄ = 8.8 years post-treatment) cognitive functioning, inclusion of lymphoma survivors, and inability to examine treatment effects. One other study has explored genetic modification of cancer- and therapy-related cognitive changes in women with breast cancer. Small et al. (2011) investigated the influence of catechol-O-methyltransferase (COMT) genotype on cognitive performance six months after completion of treatment in women with breast cancer who received (a) chemotherapy with or without radiotherapy or (b) radiotherapy only and (c) healthy controls with no history of cancer. The results of the study indicated that COMT valine carriers treated with chemotherapy performed more poorly on tasks of attention than healthy controls who were also valine carriers. The results from these studies and the current study all provide evidence for the modification of cancer- and treatment-related cognitive changes in women with breast cancer by genetic variation.
Limitations
Although the results of this exploratory study are informative, a number of limitations should be acknowledged. First, the study sample size was relatively small, limiting the authors’ ability to detect small and moderate effects; however, the findings from this study can be used to obtain more accurate sample size estimations for future investigations. The small sample size also did not allow the authors to evaluate dose-response relationships among heterozygous ε4 carriers and homozygous (ε4, ε4) individuals. Second, the sample was primarily comprised of Caucasian women. The extent to which the results generalize to more diverse populations is unknown. Third, the results indicate that women included in the APOE analysis may be different than those in the AIM study who were not part of the APOE analysis subset. Of little concern are the differences in age and years of education. Although statistically significant, the mean differences in age (X̄ = 59.31, SD = 5.699 years for women in the APOE subset versus X̄ = 60.66, SD = 6.432 years for those not in the subset) and years of education (X̄ = 15.22, SD = 3.157 years for women in the APOE subset versus X̄ = 14.55, SD = 2.66 years for those not in the subset) are most likely not clinically meaningful. In contrast, the differences in mean baseline visual learning and memory and psychomotor efficiency z scores, with women in the APOE analysis subset displaying significantly better performance in both factors, may have implications for the validity and generalizability of results. An additional limitation of this study, inherent to all studies that recruit patients with breast cancer following primary surgery, is the potential effects of surgery and stress of cancer diagnosis on cognitive function. Finally, APOE genotype represents only a single insight by which cognitive changes could be augmented in women with breast cancer; additional genes and mechanisms should be considered in the future. However, the authors also would like to acknowledge this study’s many strengths, including hypothesis-driven gene selection, pre-adjuvant therapy assessment, longitudinal follow-up, inclusion of a healthy control reference group, evaluation of treatment effects (i.e., chemotherapy and anti-estrogen therapy), and control for many known covariates and confounders of cognitive function.
Conclusions and Implications for Practice and Research
Information gained from the current study adds to the base of knowledge regarding the influence of genetic determinants on poorer cognitive performance and cognitive decline experienced by many survivors of early-stage breast cancer. Although not clinically useful at this point in time, the results from this exploratory analysis indicate modification of cognitive function performance and of cognitive changes over time by both APOE genotype and the combination of APOE genotype and prescribed treatment. In particular, performance on tasks of executive function, attention, verbal learning and memory, and visual learning and memory were influenced by APOE genotype.
Additional research is needed on this topic to further elucidate the role of APOE genotype in cognitive function of women with breast cancer, both in terms of vulnerability to and protection from cognitive decline. The results from this study need to be confirmed in a larger, more diverse sample with similarly detailed pretreatment and longitudinal cognitive function and covariate/confounder assessment. Mechanistic structural and functional brain imaging studies should be conducted to evaluate changes and differences in brain morphology and activation patterns by genotype (Vardy et al., 2008). The functions of oxidative stress and antioxidant capacity on cognitive function in women with breast cancer warrant further investigation as well. Information garnered from future studies will permit a greater understanding of the influence of APOE genotype on cognitive function in women with and receiving treatment for breast cancer, provide the basis for development of biomarkers to identify women most at risk for cognitive changes, and inform novel treatments for women experiencing cognitive decline.
Acknowledgments
This research was supported, in part, by the Genomics of Cognitive Function in Breast Cancer grant from the ONS Foundation, the Targeted Research and Academic Training for Nurses in Genomics training program grant (No. T32NR009759), and the Anastrozole Use in Menopausal Women study grant (No. R01CA107408).
References
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nature Reviews Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psycho-Oncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Breast cancer: What are the key statistics about breast cancer? 2013 Retrieved from http://bit.ly/1sicnbs.
- Amin KA, Mohamed BM, El-Wakil MAM, Ibrahem SO. Impact of breast cancer and combination chemotherapy on oxidative stress, hepatic and cardiac markers. Journal of Breast Cancer. 2012;15:306–312. doi: 10.4048/jbc.2012.15.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A. Oxidative stress: A dead end or a laboratory hypothesis? Biochemical and Biophysical Research Communications. 2007;362:230–232. doi: 10.1016/j.bbrc.2007.07.124. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory–II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Bender CM, Sereika SM, Berga SL, Vogel VG, Brufsky AM, Paraska KK, Ryan CM. Cognitive impairment associated with adjuvant therapy in breast cancer. Psycho-Oncology. 2006;15:422–430. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- Bender CM, Sereika SM, Houze MP, Brufsky AM, Berga SL, Richey SM, Ryan CM. Deterioration in cognitive function with anastrozole therapy in women with breast cancer. Presented at the International Cognition in Cancer Task Force Meeting; New York, NY. 2010. [Google Scholar]
- Bender CM, Sereika SM, Ryan CM, Brufsky AM, Puhalla S, Berga SL. Does lifetime exposure to hormones predict pretreatment cognitive function in women before adjuvant therapy for breast cancer? Menopause. 2013;20(9):1–8. doi: 10.1097/gme.0b013e3182843eff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasiak J, Arabski M, Krupa R, Wozniak K, Rykala J, Kolacinska A, Zadrozny M. Basal, oxidative and alkylative DNA damage, DNA repair efficacy and mutagen sensitivity in breast cancer. Mutation Research. 2004;554(1–2):139–148. doi: 10.1016/j.mrfmmm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, Smith MA. Oxidative stress in Alzheimer disease: A possibility for prevention. Neuropharmacology. 2010;59(4–5):290–294. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: An in-depth look at survivors’ reports of impact on work, social networks, and health care response. Journal of Cancer Survivorship: Research and Practice. 2009;3:223–232. doi: 10.1007/s11764-009-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Locke DEC, Sabbagh MN, Ahern GL, Rapcsak SZ, Reiman EM. Cerebrovascular risk factors and preclinical memory decline in healthy APOE ε4 homozygotes. Neurology. 2011;76:1078–1084. doi: 10.1212/WNL.0b013e318211c3ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS. Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Advances in pain research and therapy. Vol. 12. New York, NY: Raven Press; 1989. pp. 391–403. [Google Scholar]
- Conroy SK, McDonald BC, Smith DJ, Moser LR, West JD, Kamendulis LM, Saykin AJ. Alterations in brain structure and function in breast cancer survivors: Effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Research and Treatment. 2012;137:493–502. doi: 10.1007/s10549-012-2385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan (D-KEFS) executive function system, examiners manual. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- DNA Genotek. Laboratory protocol for manual purification of DNA from whole sample. 2012 Retrieved from http://www.dnagenotek.com/US/support/protocols-oragene-discover.html.
- Downie FP, Mar Fan HG, Houédé-Tchen N, Yi Q, Tannock IF. Cognitive function, fatigue, and menopausal symptoms in breast cancer patients receiving adjuvant chemotherapy: Evaluation with patient interview after formal assessment. Psycho-Oncology. 2006;15:921–930. doi: 10.1002/pon.1035. [DOI] [PubMed] [Google Scholar]
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips KA. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: A meta-analysis of the current literature. Brain and Cognition. 2005;59:60–70. doi: 10.1016/j.bandc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Farrer L, Cupples L, Haines J, Hyman B, Kukull W, Mayeux R, Van Duijn C. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. JAMA. 1997;278:1349–1356. doi: 10.1001/jama.1997.03550160069041. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Flory JD, Manuck SB, Ferrell RE, Ryan CM, Muldoon MF. Memory performance and the apolipoprotein E polymorphism in a community sample of middle-aged adults. American Journal of Medical Genetics. 2000;96:707–711. doi: 10.1002/1096-8628(20001204)96:6<707. [DOI] [PubMed] [Google Scholar]
- Hamed EA, Zakhary MM, Maximous DW. Apoptosis, angiogenesis, inflammation, and oxidative stress: Basic interactions in patients with early and metastatic breast cancer. Journal of Cancer Research and Clinical Oncology. 2012;138:999–1009. doi: 10.1007/s00432-012-1176-4. [DOI] [PubMed] [Google Scholar]
- Han SD, Bondi MW. Revision of the apolipoprotein E compensatory mechanism recruitment hypothesis. Alzheimer’s and Dementia. 2008;4:251–254. doi: 10.1016/j.jalz.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Hayek T, Oiknine J, Brook JG, Aviram M. Increased plasma and lipoprotein lipid peroxidation in Apo E-deficient mice. Biochemical and Biophysical Research Communications. 1994;201:1567–1574. doi: 10.1006/bbrc.1994.1883. [DOI] [PubMed] [Google Scholar]
- Hermelink K, Untch M, Lux MP, Kreienberg R, Beck T, Bauerfeind I, Münzel K. Cognitive function during neoadjuvant chemotherapy for breast cancer: Results of a prospective, multicenter, longitudinal study. Cancer. 2007;109:1905–1913. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Christensen H, Mackinnon AJ, Alisa E, Jorm AF, Henderson AS, Easteal S. Change in cognitive functioning associated with ApoE genotype in a community sample of older adults. Psychology and Aging. 2002;17:194–208. doi: 10.1037/0882-7974.17.2.194. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, Cronin KA. SEER cancer statistics review, 1975–2008 (Vintage 2009 Populations) 2011 Retrieved from http://seer.cancer.gov/csr/1975_2008/
- Hubacek JA, Pitha J, Skodova Z, Adamkova V, Lanska V, Poledne R. A possible role of apolipoprotein E polymorphism in predisposition to higher education. Neuropsychobiology. 2001;43:200–203. doi: 10.1159/000054890. [DOI] [PubMed] [Google Scholar]
- Hurria A, Goldfarb S, Rosen C, Holland J, Zuckerman E, Lachs MS, Hudis C. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient’s perspective. Breast Cancer Research and Treatment. 2006;98:343–348. doi: 10.1007/s10549-006-9171-6. [DOI] [PubMed] [Google Scholar]
- Izaks GJ, Gansevoort RT, Van der Knaap AM, Navis G, Dul-laart RPF, Slaets JPJ. The association of APOE genotype with cognitive function in persons aged 35 years or older. PLOS One. 2011;6:e27415. doi: 10.1371/journal.pone.0027415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Kohil S, Mohile SG, Usuki K, Ahles T, Morrow GR. An update on cancer- and chemotherapy-related cognitive dysfunction. Seminars in Oncology. 2012;38:431–438. doi: 10.1053/j.seminoncol.2011.03.014.AN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, Winstanley J. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. British Journal of Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Molecular Nutrition and Food Research. 2008;52:131–145. doi: 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- Jolivalt C, Leininger-Muller B, Bertrand P, Herber R, Christen Y, Siest G. Differential oxidation of apolipoprotein E isofroms and interaction with phospholipids. Free Radical Biology and Medicine. 2000;28:129–140. doi: 10.1016/S0891-5849(99)00232-4. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Mather KA, Butterwork P, Anstey KJ, Christensen H, Easteal S. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21:1–8. doi: 10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- Joshi G, Sultana R, Tangpong J, Cole MP, St Clair DK, Vore M, Butterfield DA. Free radical mediated oxidative stress and toxic side effects in brain induced by the anti cancer drug adriamycin: Insight into chemobrain. Free Radical Research. 2005;39:1147–1154. doi: 10.1080/10715760500143478. [DOI] [PubMed] [Google Scholar]
- Kasapovic J, Pejic S, Stojiljkovic V, Todorovic A, Radoševic-Jelic L, Saicic ZS, Pajovic SB. Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages after chemotherapy with 5-fluorouracil, doxorubicin and cyclophosphamide. Clinical Biochemistry. 2010;43(16–17):1287–1293. doi: 10.1016/j.clinbiochem.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Mahley RW, Innerarity TL, Rall SC, Weisgraber KH. Plasma lipoproteins: Apolipoprotein structure and function. Journal of Lipid Research. 1984;25:1277–1294. Retrieved from http://www.jlr.org/content/25/12/1277.long. [PubMed] [Google Scholar]
- Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. Journal of Chromatography. 2005;827:65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Matthews CG. Adult neuropsychological test battery. Madison, WI: University of Wisconsin Medical Center; 1964. [Google Scholar]
- McNair D, Lorr M, Droppleman LF. EdITS manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1992. [Google Scholar]
- Mehnert A, Scherwath A, Schirmer L, Schleimer B, Petersen C, Schulz-Kindermann F, Koch U. The association between neuropsychological impairment, self-perceived cognitive deficits, fatigue and health related quality of life in breast cancer survivors following standard adjuvant versus high-dose chemotherapy. Patient Education and Counseling. 2007;66:108–118. doi: 10.1016/j.pec.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research. 1988;16:1215. doi: 10.1093/nar/16.3.1215. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC334765/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidation insults and β-amyloid peptides. Nature Genetics. 1996;14:55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- Mondadori CRA, De Quervain DJF, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, Henke K. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cerebral Cortex. 2007;17:1934–1947. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- Munir F, Burrows J, Yarker J, Kalawsky K, Bains M. Women’s perceptions of chemotherapy-induced cognitive side affects on work ability: A focus group study. Journal of Clinical Nursing. 2010;19(9–10):1362–1370. doi: 10.1111/j.1365-2702.2009.03006.x. [DOI] [PubMed] [Google Scholar]
- Myers JS. Chemotherapy-related cognitive impairment: The breast cancer experience [Online exclusive] Oncology Nursing Forum. 2012;39:E31–E40. doi: 10.1188/12.ONF.E31-E40. [DOI] [PubMed] [Google Scholar]
- Nelson H. Nelson Adult Reading Test (NART) manual. Windsor, Ontario: NFER-Nelson; 1981. [Google Scholar]
- Nilsson LG, Nyberg L, Bäckman L. Genetic variation in memory functioning. Neuroscience and Biobehavioral Reviews. 2002;26:841–848. doi: 10.1016/S0149-7634(02)00070-2. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d’une figure complexe. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33:1–24. doi: 10.1016/0028-3932(94)00098-A. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Chan SL, Mattson MP. A mechanism for the neuroprotective effect of apolipoprotein E: Isoform-specific modification by the lipid peroxidation product 4-hydroxynonenal. Journal of Neurochemistry. 2000;74:1426–1433. doi: 10.1046/j.1471-4159.2000.0741426.x. [DOI] [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan neuropsychological test battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Archives in Psychology. 1964;1252:340–382. [Google Scholar]
- Richard F, Amouyel P. Genetic susceptibility factors for Alzheimer’s disease. European Journal of Pharmacology. 2001;412(1):1–12. doi: 10.1016/S0014-2999(00)00903-1. [DOI] [PubMed] [Google Scholar]
- Sadigh-Eteghad S, Talebi M, Farhoudi M. Association of apolipoprotein E epsilon 4 allele with sporadic late onset Alzheimer’s disease. A meta-analysis. Neurosciences. 2012;17:321–326. [PubMed] [Google Scholar]
- Schagen SB, Van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(SICI)1097-0142(19990201)85:3<640::AID-CNCR14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Schilder CM, Eggens PC, Seynaeve C, Linn SC, Boogerd W, Gundy CM, Schagen SB. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: Cross-sectional findings from the neuropsychological TEAM-side study. Acta Oncologica. 2009;48:76–85. doi: 10.1080/02841860802314738. [DOI] [PubMed] [Google Scholar]
- Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, Schagen SB. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: Results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. Journal of Clinical Oncology. 2010;28:1294–1300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- Shilling V, Jenkins V. Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. European Journal of Oncology Nursing. 2007;11:6–15. doi: 10.1016/j.ejon.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Small BJ, Rawson KS, Walsh E, Jim HSL, Hughes TF, Iser L, Jacobsen PB. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117:1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]
- Stilley CS, Bender CM, Dunbar-Jacob J, Sereika S, Ryan CM. The impact of cognitive function on medication management: Three studies. Health Psychology. 2011;29:50–55. doi: 10.1037/a0016940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehlow K, Rotter S, Wassmann S, Adam O, Grohé C, Laufs K, Nickenig G. Modulation of antioxidant enzyme expression and function by estrogen. Circulation Research. 2003;93:170–177. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- Tchen N, Juffs HG, Downie FP, Yi QL, Hu H, Chemerynsky I, Tannock IF. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. Journal of Clinical Oncology. 2003;21:4175–4183. doi: 10.1200/JCO.2003.01.119. [DOI] [PubMed] [Google Scholar]
- Unfer TC, Conterato GM, Da Silva JC, Duarte MM, Emanuelli T. Influence of hormone replacement therapy on blood antioxidant enzymes in menopausal women. Clinica Chimica Acta. 2006;369:73–77. doi: 10.1016/j.cca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: An international perspective from the Venice cognitive workshop. Annals of Oncology. 2008;19:623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- Von Ah D, Habermann B, Carpenter JS, Schneider BL. Impact of perceived cognitive impairment in breast cancer survivors. European Journal of Oncology Nursing. 2013;17:236–241. doi: 10.1016/j.ejon.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Walker CH, Drew BA, Antoon JW, Kalueff AV, Beckman BS. Neurocognitive effects of chemotherapy and endocrine therapies in the treatment of breast cancer: Recent perspectives. Cancer Investigation. 2012;30:135–148. doi: 10.3109/07357907.2011.636116. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. New York, NY: The Psychological Corporation; 1981. [Google Scholar]
- Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA. “Chemobrain” in breast carcinoma?: A prologue. Cancer. 2004;101:466–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- Wehling E, Lundervold AJ, Standnes B, Gjerstad L, Reinvang I. APOE status and its association to learning and memory performance in middle aged and older Norwegians seeking assessment for memory deficits. Behavioral and Brain Functions. 2007;3:57. doi: 10.1186/1744-9081-3-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Baddeley A, Hiorns R. The development and validation of a test battery for detecting and monitoring everyday memory problems. Journal of Clinical and Experimental Neuropsychology. 1989;11:855–870. doi: 10.1080/01688638908400940. [DOI] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, Mcallister TW. Increased brain activation during working memory in cognitively intact adults with the APOE ε4 allele. American Journal of Psychiatry. 2006;163:1603–1610. doi: 10.1176/appi.ajp.163.9.1603. [DOI] [PubMed] [Google Scholar]
- Yu YW, Lin CH, Chen SP, Hong CJ, Tsai SJ. Intelligence and event-related potentials for young female human volunteer apolipoprotein E epsilon4 and non-epsilon4 carriers. Neuroscience Letters. 2000;294:179–181. doi: 10.1016/s0304-3940(00)01569-x. [DOI] [PubMed] [Google Scholar]