Effective vaccination against RSV using a vector incorporating soluble RSV F-protein
Keywords: protection, respiratory syncytial virus, secreted fusion protein, Sendai virus, unconstrained F, vaccine
Abstract
The respiratory syncytial virus (RSV) is responsible for as many as 199000 annual deaths worldwide. Currently, there is no standard treatment for RSV disease and no vaccine. Sendai virus (SeV) is an attractive pediatric vaccine candidate because it elicits robust and long-lasting virus-specific B cell and T cell activities in systemic and mucosal tissues. The virus serves as a gene delivery system as well as a Jennerian vaccine against its close cousin, human parainfluenza virus type 1. Here we describe the testing of a recombinant SeV (SeVRSV-Fs) that expresses an unconstrained, secreted RSV-F protein as a vaccine against RSV in cotton rats. After a single intranasal immunization of cotton rats with SeVRSV-Fs, RSV-specific binding and neutralizing antibodies were generated. These antibodies exhibited cross-reactivity with both RSV A and B isolates. RSV-F-specific IFN-γ-producing T cells were also activated. The SeVRSV-Fs vaccine conferred protection against RSV challenge without enhanced immunopathology. In total, results showed that an SeV recombinant that expresses RSV F in an unconstrained, soluble form can induce humoral and cellular immunity that protects against infection with RSV.
Introduction
Respiratory syncytial virus (RSV) can cause close to 200000 deaths in a single year worldwide (1–6). The most vulnerable individuals are premature infants, children with congenital heart/lung disease and immunocompromized patients. Currently, there is only one form of successful RSV prophylaxis, which involves the injection of preformed anti-RSV antibodies into vulnerable infants (7). However, because of the high cost and logistical difficulty associated with treatments, preformed antibody prophylaxis is rarely available to the children who need it most (8, 9). RSV vaccines have been studied for decades, but there remains no licensed product. Clearly, the development of a successful RSV vaccine is a critical, unmet need in the pediatric healthcare arena (10, 11).
Here we describe the testing of a Sendai virus (SeV)-based vaccine that expresses an unconstrained, soluble, RSV F protein. SeV was chosen as a vector, because it induces rapid and durable B cell and T cell responses in blood and in mucosal tissues of the respiratory tract. The virus has already been tested in adults and 3–6-year-old children, and shown to be immunogenic and well tolerated.
This study addresses a question pertinent to recent literature: can an unconstrained, soluble RSV F protein, truncated to remove transmembrane and intracellular protein regions, suffice as a recombinant vaccine target? The question is based on an understanding that in the absence of its transmembrane region, the prefusion form of RSV F protein is metastable and often converts to a post-fusion form, a low-energy six helix bundle that lacks certain antibody neutralizing determinants (12, 13). Our data show that even though the RSV F protein expressed by SeVRSV-Fs is unconstrained, the vaccine elicits RSV-specific binding and neutralizing antibodies and T cell responses in cotton rats. The vaccine fully protects against RSV challenge without enhanced immunopathology.
Methods
Construct design
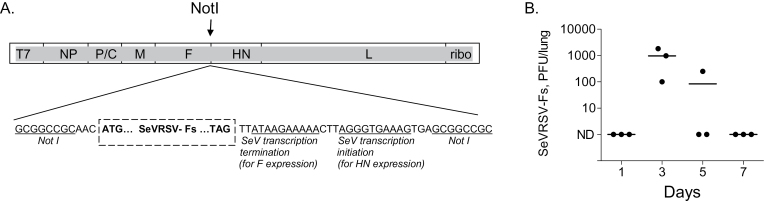
To make SeVRSV-Fs cDNA, we inserted a His tag sequence and a stop codon after the codon for residue 524 in the RSV F gene, using QuickChange Site-directed Mutagenesis Kit (Stratagene). The gene encodes a protein truncated by 50 amino acids at its C-terminus and therefore lacking transmembrane and intracellular regions The mutated gene was inserted into the NotI site of pSV(E), Fig. 1A (14, 15).
Fig. 1.
Design and characterization of recombinant SeV expressing RSV F as a secreted protein. (A) The diagram illustrates the design of SeVRSV-Fs. A unique NotI restriction enzyme site was created in the non-coding region of the HN gene of the full genome SeV cDNA for insertion of the RSV Fs gene. The RSV gene was isolated from RSV-A2 with RT-PCR, digested with NotI and cloned into the NotI site of pSV(E). T7, T7 promoter; ribo, hepatitis delta virus ribozyme sequence. (B) In a preliminary experiment, cotton rats were vaccinated i.n. with 2 x 106 PFU SeVRSV-Fs and examined throughout a one week period for vaccine virus amplification in the lower respiratory tract, by testing homogenized lungs in plaque assays on LLC-MK2 cells. Each symbol represents virus measurements from a different animal.
Reverse genetics rescue was performed as described previously (16). The 293T cell line was infected with a UV-inactivated, T7 RNA polymerase-expressing recombinant vaccinia virus (vTF7.3) for 1h at 37°C. Cells were then cotransfected with plasmids containing recombinant SeV cDNA plasmid and with supporting T7-driven plasmids respectively expressing the NP, P and L genes of SeV (pTF1SVNP, pTF1SVP and pTF1SVL) in the presence of Lipofectamine (Life Technologies, Grand Island, NY, USA). After 40h, cell lysates were prepared and amplified by inoculation into embryonated hen’s eggs. Allantoic fluids were harvested after 3 days and virus was cloned by plaque purification on LLC-MK2 cells. Cloned recombinant virus (termed SeVRSV-Fs) was amplified in hens’ eggs to prepare stocks for further testing.
Purification and testing of RSV F protein
Allantoic fluids were harvested 3 days after the inoculation of eggs with SeVRSV-Fs. Fluids were centrifuged (~300 × g, 10min) to remove debris and filtered (0.45 µM filter). Samples were passed over a PBS-equilibrated Sepharose column bound by the anti-F mAb Palivizumab (Synagis; MedImmune Inc., Gaithersburg, MD, USA). The column was washed with PBS and eluted with 0.2M glycine (pH 2.8), after which pooled protein fractions were dialyzed overnight against PBS. As controls for RSV F protein analysis, RSV-infected cell lysates were used. These were from HEp-2 cells that were infected with RSV-A2 in PBS at room temperature for 1h. After washing, cells were cultured in Dulbecco’s modified eagle’s medium (Cambrex Bio Science Walkersville Inc., Walkersville, MD, USA) supplemented with glutamine, gentamicin and 10% FCS. Three days later, cells were harvested and lysed with NP-40 in buffer [10mM Tris (pH 7.4), 150mM NaCl, 0.5% NP-40, and 1mM EDTA]. Supernatants were clarified by centrifugation (15000 × g, 10min).Western blots were performed with test and control samples by separating proteins on SDS polyacrylamide gels under nonreducing conditions and transferring proteins to an Immobilon membrane (Millipore, Danvers, MA, USA). Membranes were treated with 5% milk/TBST (Tris-buffered saline plus 0.5% Tween), incubated with an RSV F-specific mAb (antibody 1269, kindly provided by Dr. Coelingh, NIAID (17)). Development was with a goat antimouse IgG (H+L) horseradish peroxidase (HRP) conjugate (BioRad, Hercules, CA, USA) followed by a SuperSignal West Pico Chemiluminescent (HRP) Substrate (Pierce, Rockford, IL, USA).
Immunizations and RSV challenges
Immunizations and RSV challenges were conducted as described previously (16, 18–20). Cotton rats (Sigmodon hispidus; Harlan Sprague Dawley, Indianapolis, IN, USA) were grouped (up to five animals per group) to receive SeVRSV-Fs or unmanipulated SeV intranasally (i.n., 2×106 PFU/animal). Additional controls were with PBS alone. After 5 weeks, animals were challenged i.n. with RSV-A2 at a dose of 1.5×106 PFU/cotton rat. Unless described as preliminary, experiments were repeated to ensure reproducibility.
Enzyme-linked immunosorbent assay
The enzyme-linked immunosorbent assay (ELISA) was described previously (16). Briefly, ELISA microtiter plates were coated with 1 ug ml−1 purified F protein for overnight incubation. Plates were blocked with PBS with 3% BSA (Sigma, St Louis, MO, USA), after which serially diluted serum samples from the test and control cotton rats were added and incubated for at least 1h at 37°C. Plates were washed and incubated with rabbit anti-cotton rat antibody (Virion Systems, Rockville, MD, USA) for 30min at room temperature. Plates were developed by incubation with an anti-rabbit IgG-HRP conjugate (diluted in PBS with 1% BSA, Bio-Rad, Cat# 170–6515) for 30min, followed by 2,2′-azino-bis-(3-ethylbenzthiazolinesulfonic acid) (ABTS, Southern Biotechnology Associates Inc., Birmingham, AL, USA). Plates were read at O.D. 405nm.
Neutralization assays
Neutralizing activity was measured using an RSV plaque assay as described previously (16). Serially diluted test and control sera were mixed with RSV (100–150 PFU/well) in EMEM (Cambrex Bio Science Walkersville, Inc.) for 1h at 37°C. The virus-serum mixtures were inoculated onto HEp-2 cell monolayers in 12-well plates, except for virus RSV B1 that was inoculated onto Vero cells. Plates were incubated for 1h (37°C, 5% CO2) and then overlaid with medium supplemented with glutamine, antibiotics, 10% FCS and 0.75% methylcellulose (Fisher Scientific, Fair Lawn, NJ, USA). After culture for 5–6 days (37°C, 5% CO2), the methylcellulose was removed. Cells were treated with formalin phosphate and stained with hematoxylin and eosin. Plaques were counted visually. Inhibition was defined as the percentage reduction of plaques comparing post-vaccine sera with pre-vaccine sera. Viruses included RSV A2 (Laboratory isolate, Type A RSV, American type culture collection, ATCC, Rockville, MD, USA), RSV B1 (Laboratory isolate, Type B RSV, ATCC), VR 1580 (Type B RSV, ATCC), K1014 and K1016 (low passaged isolates, Type A RSV, kindly provided by Dr J. DeVincenzo, Le Bonheur Children’s Hospital, Memphis, TN, USA).
Peptide synthesis and IFN-γ enzyme-linked immunospot (ELISPOT) assays
The T cell assays were conducted as described previously (16). Briefly, overlapping F peptides (derived from the RSV A2 F sequence) were prepared by the Hartwell Center for Bioinformatics and Biotechnology at St. Jude Children’s Research Hospital. Peptides were generally 15 amino acids in length. Peptide sequences were initiated at 5 amino acid intervals to cover the entire length of the RSV F protein. Twenty-two peptide pools were made for T cell testing.
Multiscreen-hemagglutinin filtration plates (Millipore, Bedford, MA, USA) were coated with 3.3 μg ml−1 goat anti-cotton rat IFN-γ antibody (R & D Systems, Minneapolis, MN, USA) and incubated overnight at 4°C. Plates were washed and incubated for at least 1h at 37°C with complete tumor medium (CTM (21, 22), a modified Eagle’s medium (Invitrogen, Grand Island, NY, USA) supplemented with 10% FCS, dextrose (500 µg m−1), glutamine (2mM), 2-mercaptoethanol (3×10–5 M), essential and nonessential amino acids, sodium pyruvate, sodium bicarbonate and antibiotics. Mediastinal lymph node (MLN) cells were harvested from cotton rats 10 days after vaccination. Cells were suspended in CTM and added to wells (106 cells/well) containing the peptide pools. The final concentration of each peptide was ~10 μM. As positive controls, cells were stimulated with 4 μg ml−1 Con A (Sigma-Aldrich). The plates were incubated for 48h at 37°C. Plates were then washed with PBS followed by PBS wash buffer (PBS with 0.05% Tween 20). Plates were next incubated with biotinylated goat anti-cotton rat IFN-γ antibody (100 μl of 0.5 μg ml−1; R & D Systems) in PBS containing 0.05% Tween 20 and 1% FCS (incubated at 37°C for at least 2h) followed by streptavidin-conjugated alkaline phosphatase (Cat# D0396, DAKO, Copenhagen, Denmark) diluted 1:500 in PBS wash buffer. After 1h, plates were rinsed with wash buffer and water and spots were developed with 5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium alkaline phosphatase substrate (Sigma-Aldrich). Spots were counted with an Axioplan 2 microscope and software (Carl Zeiss, Munich-Hallbergmoos, Germany).
Histopathology studies
Five days after RSV challenge, lungs were prepared for histological analyses as previously described (16, 18, 20, 23). Briefly, lungs were inflated via the trachea with 10% neutral buffered formalin. They were then submerged in formalin for overnight fixation. Tissue was embedded in paraffin, sectioned at 4 µm, and stained with hematoxylin and eosin. Lung pathology was scored on the basis of three parameters: peribronchiolitis (inflammatory cells around small airways), alveolitis (inflammatory cells within alveolar spaces) and interstitial pneumonitis (inflammatory cell infiltrates and thickening of alveolar walls). Scoring was performed by a pathologist using a scale of 0 (no inflammation) to 4 (maximum inflammation within the experiment), defined independently for each parameter. The pathologist was blinded to the origin of tissues from test or control groups while scoring.
RSV titration in cotton rat lungs after challenge
RSV measurement in cotton rat lungs has been described previously (16). Three days after i.n. RSV challenge, cotton rats were sacrificed. Lungs were harvested and homogenized on ice with a mechanical Dounce homogenizer (PowerGen125 PCR Tissue Homogenizing kit; Fisher Scientific). Homogenates were centrifuged (~1500 × g, 10min) and supernatants were collected. They were stored frozen prior to testing. For virus titration, serially diluted supernatants were inoculated onto HEp-2 cell monolayers (except for virus B1 that was measured on Vero cells) in 12-well plates in EMEM. After 1h at 37°C, 5% CO2, the wells were overlaid with medium supplemented with glutamine, antibiotics, 10% FCS and 0.75% methylcellulose. Cells were incubated for 5–6 days (37°C, 5% CO2), after which the methylcellulose was removed. Cells were fixed with formalin phosphate and the plates were stained with hematoxylin and eosin for plaque counting. The total pulmonary virus burden was expressed as the plaque count (PFU) per cotton rat.
SeVRSV measurement in cotton rat lungs after vaccination
Replication-competent SeVRSV was monitored in cotton rat lungs by plaque count as described previously (24) after i.n. vaccination with 2×106 PFU virus. Briefly, lungs were homogenized in 5ml PBS, centrifuged briefly to remove debris, and plated using serial 10-fold dilutions on adherent LLC-MK2 cell monolayers in six-well plates. After incubation at 33°C for 1h, the inoculum was removed and replaced with an overlay containing acetylated trypsin (5 μg ml−1). Plates were inverted and cultured for 4–6 days at 33°C. A second overlay was added containing neutral red. Plaques were visualized and counted after an additional incubation of cultures for 1–3 days.
Results
RSV F protein secreted by SeVRSV-Fs-infected cells
Recombinant SeV particles were designed to express a truncated RSV F protein as described in the Methods section and as illustrated in Fig. 1A. To confirm F protein secretion by infected cells, gel electrophoresis and Western blotting was performed with allantoic fluids from infected eggs. The secreted F protein from SeVRSV-Fs was predictably smaller than the full-length RSV F from RSV particles (data not shown). In a preliminary experiment to ensure vaccine growth, cotton rats received SeVRSV-Fs by the i.n. route and were examined on days 1, 3, 5 and 7 for vaccine amplification. As shown in Fig. 1B, virus peaked on day 3 after inoculation and was cleared by day 7.
Vaccination with SeVRSV-Fs elicits RSV-specific binding and neutralizing antibodies in cotton rats
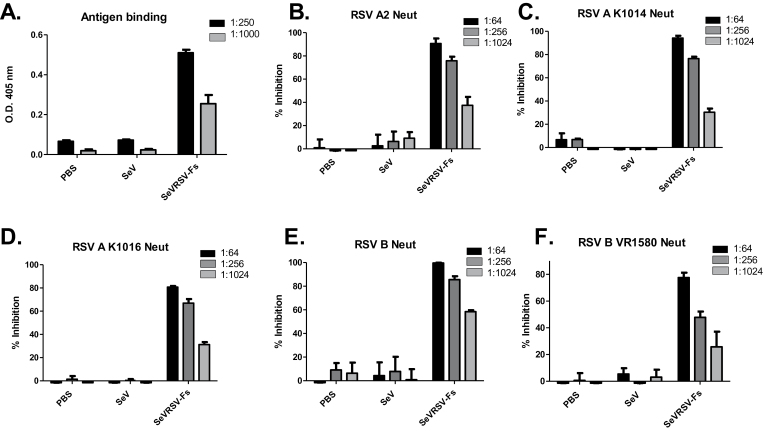
To determine whether the SeVRSV-Fs vaccine induced an antibody response, we inoculated groups of five cotton rats by the i.n. route (2×106 PFU/cotton rat). Animals injected with PBS or unmodified SeV were used as controls to define baseline activities. Blood was collected before and four weeks after vaccination for testing of RSV-specific antibodies. Post-vaccine antibodies bound antigen in an ELISA (Fig. 2A). Pooled serially diluted serum samples were also tested for neutralization by incubating with RSV-A2 prior to infection of cultured cells. Sera were found to be neutralizing against this homologous virus (Fig. 2B). Neutralizing antibodies were also detected against non-homologous viruses, including low passaged isolates of the RSV A subtype (Fig. 2C and D), and two isolates of the RSV B subtype (B1 and VR1580, Fig. 2E and F).
Fig. 2.
Cotton rats primed with SeVRSV-Fs generate RSV-specific neutralizing antibodies. Groups of five cotton rats were inoculated with 2×106 SeVRSV-Fs or unmodified SeV. Sera were collected after four weeks and pooled for each group of animals. (A) Sera were diluted 1:250 and 1:1000 for testing in an ELISA for RSV-F-specific binding antibody. (B–F) Pooled serum samples from cotton rats inoculated with SeVRSV-Fs or unmodified SeV were tested for inhibition of RSV growth in a plaque assay. Results are the mean percentage plaque reduction for post-vaccine test sera compared with pre-vaccine sera from the same animal set. Standard error bars are shown. Neutralization experiments were conducted with different viruses including RSV A2 (laboratory strain type A isolate, panel B), K1014 (low passaged type A isolate, panel C), K1016 (low passaged type A isolate panel D), B1 (laboratory strain type B isolate, panel E) and VR1580 (type B isolate, panel F). Test and control samples were plated in triplicate in ELISAs and in neutralization assays. All ELISAs and neutralization assays revealed statistically significant differences between vaccinated and control samples using unpaired T tests (P < 0.05, GraphPad Prism software, San Diego, CA, USA).
SeVRSV-Fs vaccination elicits RSV-specific T cell responses
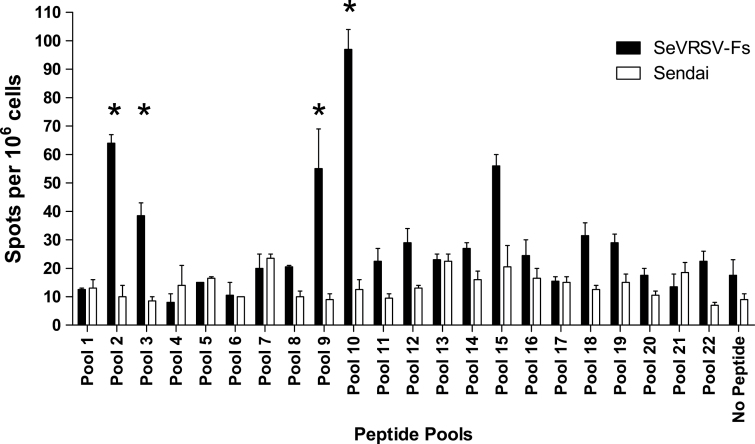
To examine T cell responses, we performed IFNγ-ELISPOT assays. MLN were harvested from cotton rats ten days after vaccination with SeVRSV-Fs or unmanipulated, nonrecombinant virus (Sendai). Cell suspensions prepared from MLN were combined for each experimental group (3 or more animals per group). To examine IFNγ expression, we incubated cell suspensions with pools of overlapping peptides representing the entire RSV F protein (listed in Table 1). As shown in Fig. 3, SeVRSV-Fs induced IFNγ-producing T cells against RSV peptide pools.
Table 1.
RSV F peptide sequences for T cell testing
| Peptide pool | RSV F sequences |
|---|---|
| 1 | MELLILKANAITTILTAVTFCFASGQNITEEFYQS |
| 2 | QNITEEFYQSTCSAVSKGYLSALRTGWYTSVITIE |
| 3 | GWYTSVITIELSNIKENKCNGTDAKVKLIKQELDK |
| 4 | VKLIKQELDKYKNAVTELQLLMQSTPPTNNRARRE |
| 5 | PPTNNRARRELPRFMNYTLNNAKKTNVTLSKKRKR |
| 6 | NVTLSKKRKRRFLGFLLGVGSAIASGVAVSKVLHL |
| 7 | GVAVSKVLHLEGEVNKIKSALLSTNKAVVSLSNGV |
| 8 | KAVVSLSNGVSVLTSKVLDLKNYIDKQLLPIVNKQ |
| 9 | KQLLPIVNKQSCSISNIETVIEFQQKNNRLLEITR |
| 10 | KNNRLLEITREFSVNAGVTTPVSTYMLTNSELLSL |
| 11 | MLTNSELLSLINDMPITNDQKKLMSNNVQIVRQQS |
| 12 | NNVQIVRQQSYSIMSIIKEEVLAYVVQLPLYGVID |
| 13 | VQLPLYGVIDTPCWKLHTSPLCTTNTKEGSNICLT |
| 14 | TKEGSNICLTRTDRGWYCDNAGSVSFFPQAETCKV |
| 15 | FFPQAETCKVQSNRVFCDTMNSLTLPSEINLCNVD |
| 16 | PSEINLCNVDIFNPKYDCKIMTSKTDVSSSVITSL |
| 17 | DVSSSVITSLGAIVSCYGKTKCTASNKNRGIIKTF |
| 18 | NKNRGIIKTFSNGCDYVSNKGMDTVSVGNTLYYVN |
| 19 | SVGNTLYYVNKQEGKSLYVKGEPIINFYDPLVFPS |
| 20 | NFYDPLVFPSDEFDASISQVNEKINQSLAFIRKSD |
| 21 | QSLAFIRKSDELLHNVNAGKSTTN --------------------- |
| 22 | -------------------------------------------------------------------- |
Overlapping peptides were synthesized. The peptides were generally 15 amino acids in length and were initiated at 5-amino acid intervals to cover the entire length of the RSV F protein. Amino acids represented in each peptide pool are shown. Pools 21 and 22 included amino acids in the transmembrane and cytoplasmic regions of F that were not included in the vaccine (as indicated by dashed lines). Seven peptides were not synthesized due to technical difficulties: ITTILTAVTFCFASG (pool 1); KIKSALLSTNKAVVS (pool 7); NFYDPLVFPSDEFDA (pool 20) and STTNIMITTIIIVII, MITTIIIVIIVILLS, IIVIIVILLSLIAVG and VILLSLIAVGLLLYC (pool 21).
Fig. 3.
RSV-specific T cell responses elicited by cotton rat inoculation with SeVRSV-Fs. Mediastinal lymph nodes (MLN) were isolated from cotton rats 10 days after inoculation with SeVRSV-Fs (Fs) or unmodified SeV (control) to assess T cell function using an IFNγ ELISPOT assay. MLN pooled from each group (≥3 animals per group) were tested against each of 22 peptide pools or against no peptide. Spot-forming cells (SFC) were counted per 106 plated cells. Values are means of duplicate wells with standard errors. Asterisks indicate peptide pools for which mean test results were ≥4× control values. Unpaired T tests were conducted using GraphPad Prism software and showed that results for pools 2, 3 and 10 were all statistically significant (P < 0.05) when vaccinated and control animals were compared.
SeVRSV-Fs confers protection against RSV challenge in the cotton rat model
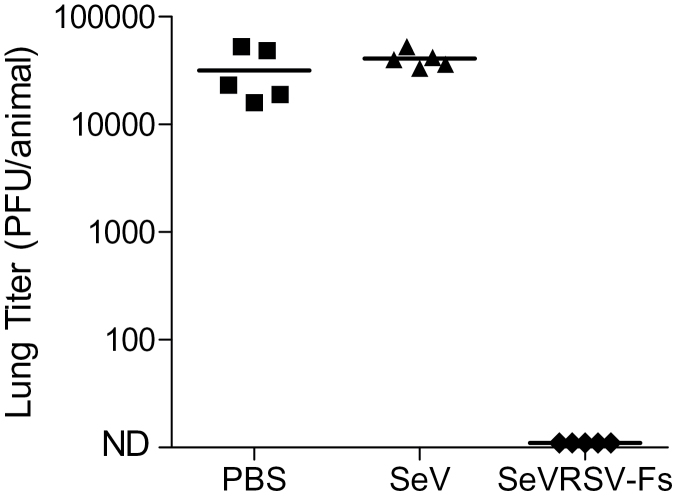
SeVRSV-Fs was next tested for protection of animals from RSV challenge. Five weeks after vaccination, animals were challenged i.n. with RSV-A2. Three days after challenge, animals were sacrificed and lungs were collected for measurement of RSV burden. As shown in Fig. 4, animals vaccinated with a single dose of SeVRSV-Fs were protected from RSV challenge. In contrast, all animals inoculated with control SeV and subsequently challenged with RSV were infected.
Fig. 4.
SeVRSV-Fs confers protection against RSV challenge. Vaccinated animals were rested for 5 weeks and challenged with RSV A2. Virus replication in the lungs was then determined by plaque assay. Each symbol represents virus from an individual cotton rat with five cotton rats per group. A Fisher’s exact test with GraphPad Prism software demonstrated that differences in virus loads between vaccinated and control animals were statistically significant (P < 0.05).
No enhanced immunopathology upon RSV challenge following vaccination with SeVRSV-Fs
Cotton rats inoculated with SeVRSV-Fs or PBS were sacrificed 5 days after challenge with RSV to test for immunopathology. The lungs were harvested, perfused with formalin, sectioned and stained with hematoxylin and eosin for histologic examination. Peribronchiolitis, alveolitis, and interstitial pneumonitis were detected in control cotton rats 5 days after i.n. RSV challenge, and were not enhanced in vaccinated animals. Scores for test and control animals are shown in Table 2.
Table 2.
Histopathology following vaccination and subsequent RSV challenge
| Animal number | Vaccine group | Peribronchiolitis | Alveolitis | Interstitial pneumonitis |
|---|---|---|---|---|
| 1 | SeVRSV-Fs | 2 | 2 | 1 |
| 2 | SeVRSV-Fs | 2 | 1 | 1 |
| 3 | SeVRSV-Fs | 2 | 1 | 1 |
| 4 | SeVRSV-Fs | 0 | 0 | 0 |
| 5 | SeVRSV-Fs | 1 | 1 | 1 |
| 6 | PBS control | 4 | 2 | 2 |
| 7 | PBS control | 4 | 3 | 2 |
| 8 | PBS control | 2 | 3 | 3 |
| 9 | PBS control | 4 | 4 | 4 |
| 10 | PBS control | 3 | 3 | 3 |
Cotton rats were vaccinated with SeVRSV-Fs or PBS and then challenged with RSV. Five days later, cotton rats were scored for immunopathology. The most severe disease in each category within the experiment was scored as ‘4’ and the absence of disease was scored as ‘0’.
Discussion
This report describes the immunization of cotton rats with SeVRSV-Fs, an SeV-based vaccine produced by reverse genetics to deliver a truncated RSV F gene to mammalian cells. Cells infected with SeVRSV-Fs expressed a secreted, unconstrained RSV F protein. Vaccination generated binding and neutralizing antibody responses as well as T cell responses toward RSV. Following a single i.n. inoculation with SeVRSV-F, cotton rats were fully protected from RSV infection after challenge. Immunopathology was not enhanced after RSV challenge in animals that were vaccinated with SeVRSV-Fs compared with controls.
Our previous studies with SeV vaccines have demonstrated the induction of rapid and durable B and T cell responses systemically and at upper respiratory tract and lower respiratory tract mucosal surfaces (25, 26). An unmanipulated SeV was previously demonstrated to confer complete protection against challenge with its cousin, human parainfluenza virus-type 1 (hPIV-1), in African green monkeys (24). Unmanipulated SeV is currently in clinical trials. It has been well tolerated in adults (27) and 3–6-year-old children (data not shown), paving the way for similar studies with recombinant SeV vaccine products.
A recombinant SeV that expresses the full length RSV F has already been tested in cotton rats and has been shown to protect against challenges with both RSV A and B isolates (16). Protection is demonstrated even in the presence of passively transferred RSV-specific and SeV-specific neutralizing antibodies in a cotton rat maternal antibody model (28). The vaccine safely protects against RSV challenge in African green monkeys (29). Sendai viruses are attractive vaccine candidates, in part because of their appealing immunogenicity and safety profiles, and in part because they maintain genetic stability when successively passaged and amplified to high titers in hens’ eggs or mammalian (e.g. Vero) cell cultures (data not shown). The study described here demonstrates that an SeV recombinant expressing a truncated, secreted RSV F protein can serve as a successful vaccine against RSV.
Our results address a recent question in the RSV field that concerns the configuration of RSV F protein in a vaccine. Some researchers suggest that RSV F should be constrained in its pre-fusion form, on the basis of arguments that: (i) there are important antibody determinants (e.g. D25, AM22, 5C4) that exist only within the pre-fusion form of F and (ii) the majority of neutralizing antibodies in RSV immune human sera bind to the pre-fusion F molecule (12, 13). Opposing arguments are that: (i) several important antigenic determinants including 101F exist on post-fusion F proteins (30, 31), (ii) the Palivizumab neutralizing mAb binds postfusion F and provides excellent prophylaxis against RSV A and B isolates in clinical practice (7, 9), (iii) antibodies clear viruses by a variety of mechanisms that function both before and after the fusion of virus to its target cell (32–34) and (iv) the artifactual manipulations required to constrain soluble F protein in its pre-fusion form may inadvertently alter important native antigenic epitopes, a situation that was associated with significant morbidity and mortality when a formalin-treated RSV vaccine was tested in children (11, 35). Our results support the argument that RSV F constraint is not required, consistent with data from Swanson et al. (36) who tested a subunit F protein as an RSV vaccine in mice.
It is important to consider that antibodies protect against virus infections using sophisticated mechanisms that function before and after virus fusion. In the context of HIV-1 infection, for example, antibodies can inhibit infection well after virus has penetrated the cell. This is in part due to the shuttling of virus particles to proteasomes when antibodies bind viral antigens and TRIM-21 (32). Antibodies are also well known for their support of antibody-dependent cell-mediated cytotoxicity (ADCC), a mechanism by which cytotoxic cells (NK or CD8+ T cells) are linked to their virus-infected targets (37, 38). Moreover, antibody-antigen complexes and free antibodies modulate inflammation by up- and down-regulation of innate and adaptive immune cells (39–41). Together, there are a plethora of antibody-mediated mechanisms that counter virus infection and disease, and that are clearly not dependent on the pre-fusion structure of RSV F. To date, the most clinically relevant prophylactic regimen against RSV infections is with an antibody that binds the post-fusion form of RSV F (8).
In conclusion, we have demonstrated that SeVRSV-Fs confers protection against RSV challenge in a cotton rat model and that constraint of the secreted F protein in its pre-fusion form is not required. Clearly, a number of vaccine products that present soluble F in an unconstrained form remain viable vaccine candidates for the protection of humans from RSV infection.
Funding
NIH (NIAID P01 AI054955, NIAID R01 AI088729, NIAID R01 A1081779, NCI P30-CA21765); American-Lebanese Syrian Associated Charities.
Acknowledgements
The authors thank Virion Systems for providing cotton rat antibody reagents. The authors thank Dr J. DeVincenzo, Le Bonheur Children’s Hospital, Memphis, TN, for kindly providing primary RSV isolates for neutralization assays. T.T., K.S., C.J.R., A.P., and J.L.H. are named as inventors on applications pending in the U.S. and Europe directed to a modified Sendai virus vaccine vector.
References
- 1. McCormick J., Tubman R. 2002. Readmission with respiratory syncytial virus (RSV) infection among graduates from a neonatal intensive care unit. Pediatr. Pulmonol. 34:262. [DOI] [PubMed] [Google Scholar]
- 2. Anderson L. J., Parker R. A., Strikas R. L. 1990. Association between respiratory syncytial virus outbreaks and lower respiratory tract deaths of infants and young children. J. Infect. Dis. 161:640. [DOI] [PubMed] [Google Scholar]
- 3. Groothuis J. R., Gutierrez K. M., Lauer B. A. 1988. Respiratory syncytial virus infection in children with bronchopulmonary dysplasia. Pediatrics 82:199. [PubMed] [Google Scholar]
- 4. Madhi S. A., Schoub B., Simmank K., Blackburn N., Klugman K. P. 2000. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1. J. Pediatr. 137:78. [DOI] [PubMed] [Google Scholar]
- 5. Nair H., Nokes D. J., Gessner B. D., et al. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buckingham S. C., Quasney M. W., Bush A. J., DeVincenzo J. P. 2001. Respiratory syncytial virus infections in the pediatric intensive care unit: clinical characteristics and risk factors for adverse outcomes. Pediatr. Crit. Care Med. 2:318. [DOI] [PubMed] [Google Scholar]
- 7. Fernández P., Trenholme A., Abarca K., et al. 2010. A phase 2, randomized, double-blind safety and pharmacokinetic assessment of respiratory syncytial virus (RSV) prophylaxis with motavizumab and palivizumab administered in the same season. BMC Pediatr. 10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeVincenzo J. 2008. Passive antibody prophylaxis for RSV. Pediatr. Infect. Dis. J. 27:69. [DOI] [PubMed] [Google Scholar]
- 9. Boivin G., Caouette G., Frenette L., Carbonneau J., Ouakki M., De Serres G. 2008. Human respiratory syncytial virus and other viral infections in infants receiving palivizumab. J. Clin. Virol. 42:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collins P. L., Melero J. A. 2011. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 162:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rudraraju R., Jones B. G., Sealy R., Surman S. L., Hurwitz J. L. 2013. Respiratory syncytial virus: current progress in vaccine development. Viruses 5:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magro M., Mas V., Chappell K., et al. 2012. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc. Natl Acad. Sci. USA 109:3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McLellan J. S., Chen M., Leung S., et al. 2013. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 340:1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bousse T., Matrosovich T., Portner A., Kato A., Nagai Y., Takimoto T. 2002. The long noncoding region of the human parainfluenza virus type 1 f gene contributes to the read-through transcription at the m-f gene junction. J. Virol. 76:8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhan X., Slobod K. S., Krishnamurthy S., et al. 2008. Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections. Vaccine. 26:3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhan X., Hurwitz J. L., Krishnamurthy S., et al. 2007. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine. 25:8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beeler J. A., van Wyke Coelingh K. 1989. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J. Virol. 63:2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prince G. A., Jenson A. B., Hemming V. G., et al. 1986. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactivated virus. J. Virol. 57:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prince G. A., Curtis S. J., Yim K. C., Porter D. D. 2001. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J. Gen. Virol. 82(Pt 12):2881. [DOI] [PubMed] [Google Scholar]
- 20. Prince G. A., Prieels J. P., Slaoui M., Porter D. D. 1999. Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus). Lab. Invest. 79:1385. [PubMed] [Google Scholar]
- 21. Woodland D., Happ M. P., Bill J., Palmer E. 1990. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive T cells. Science 247:964. [DOI] [PubMed] [Google Scholar]
- 22. Kappler J. W., Skidmore B., White J., Marrack P. 1981. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J. Exp. Med. 153:1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takimoto T., Hurwitz J. L., Coleclough C., et al. 2004. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J. Virol. 78:6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hurwitz J. L., Soike K. F., Sangster M. Y., et al. 1997. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine 15:533. [DOI] [PubMed] [Google Scholar]
- 25. Sealy R., Jones B. G., Surman S. L., Hurwitz J. L. 2010. Robust IgA and IgG-producing antibody forming cells in the diffuse-NALT and lungs of Sendai virus-vaccinated cotton rats associate with rapid protection against human parainfluenza virus-type 1. Vaccine 28:6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rudraraju R., Surman S., Jones B., Sealy R., Woodland D. L., Hurwitz J. L. 2011. Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8+ T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology 410:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Slobod K. S., Shenep J. L., Luján-Zilbermann J., et al. 2004. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine 22:3182. [DOI] [PubMed] [Google Scholar]
- 28. Jones B. G., Sealy R. E., Surman S. L., et al. 2014. Sendai virus-based RSV vaccine protects against RSV challenge in an in vivo maternal antibody model. Vaccine 32:3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones B. G., Sealy R. E., Rudraraju R., et al. 2012. Sendai virus-based RSV vaccine protects African green monkeys from RSV infection. Vaccine 30:959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McLellan J. S., Chen M., Chang J. S., et al. 2010. Structure of a major antigenic site on the respiratory syncytial virus fusion glycoprotein in complex with neutralizing antibody 101F. J. Virol. 84:12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McLellan J. S., Yang Y., Graham B. S., Kwong P. D. 2011. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J. Virol. 85:7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mallery D. L., McEwan W. A., Bidgood S. R., Towers G. J., Johnson C. M., James L. C. 2010. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc. Natl Acad. Sci. USA 107:19985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McEwan W. A., Tam J. C., Watkinson R. E., Bidgood S. R., Mallery D. L., James L. C. 2013. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat. Immunol. 14:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scott R., de Landazuri M. O., Gardner P. S., Owen J. J. 1977. Human antibody-dependent cell-mediated cytotoxicity against target cells infected with respiratory syncytial virus. Clin. Exp. Immunol. 28:19. [PMC free article] [PubMed] [Google Scholar]
- 35. Chin J., Magoffin R. L., Shearer L. A., Schieble J. H., Lennette E. H. 1969. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 89:449. [DOI] [PubMed] [Google Scholar]
- 36. Swanson K. A., Settembre E. C., Shaw C. A., et al. 2011. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc. Natl Acad. Sci. USA 108:9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koff W. C., Caplan F. R., Case S., Halstead S. B. 1983. Cell-mediated immune response to respiratory syncytial virus infection in owl monkeys. Clin. Exp. Immunol. 53:272. [PMC free article] [PubMed] [Google Scholar]
- 38. Meguro H., Kervina M., Wright P. F. 1979. Antibody-dependent cell-mediated cytotoxicity against cells infected with respiratory syncytial virus: characterization of in vitro and in vivo properties. J. Immunol. 122:2521. [PubMed] [Google Scholar]
- 39. Corthésy B. 2013. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 4:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wolf H. M., Hauber I., Gulle H., et al. 1996. Anti-inflammatory properties of human serum IgA: induction of IL-1 receptor antagonist and Fc alpha R (CD89)-mediated down-regulation of tumour necrosis factor-alpha (TNF-alpha) and IL-6 in human monocytes. Clin. Exp. Immunol. 105:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wolf H. M., Fischer M. B., Pühringer H., Samstag A., Vogel E., Eibl M. M. 1994. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood 83:1278. [PubMed] [Google Scholar]