NKT cells and IL-4 mediate protective effects of vitamin D against EAE
Keywords: EAE, NKT cells, vitamin D
Abstract
Active vitamin D [1,25-dihydroxyvitamin D3 (1,25D3)] blocks the development of experimental autoimmune diseases. However, the molecular and immunobiological mechanisms underlying 1,25D3’s anti-inflammatory properties are not fully understood. We employed a murine model of experimental autoimmune encephalomyelitis (EAE) in order to determine the role of NKT cells in 1,25D3-mediated protection from EAE. Wild-type (WT) mice or mice lacking all NKT cells (CD1d−/−) or invariant NKT cells (Jα18−/−) were fed control or 1,25D3-supplemented diets. All mice fed with the control diet developed severe EAE. 1,25D3 treatment of WT mice protected them from developing EAE. CD1d−/− and Jα18−/− mice treated with 1,25D3 were not protected to the same extent as WT mice. Myelin oligodendrocyte glycoprotein-specific IL-17 and IFN-γ production was significantly reduced in 1,25D3 WT mice compared with WT but was not decreased in 1,25D3 CD1d−/− mice compared with CD1d−/− mice. IL-4−/− mice were utilized to determine how IL-4 deficiency affects susceptibility to EAE. IL-4−/− mice were not protected from developing EAE by α-galactosylceramide (α-GalCer) or 1,25D3 treatment. Furthermore, 1,25D3 treatment of splenocytes in vitro decreased α-GalCer-induced IL-17 and increased IL-4, IL-5 and IL-10 production. 1,25D3 alters the cytokine profile of invariant NKT cells in vitro. These studies demonstrate that NKT cells are important mediators of 1,25D3-induced protection from EAE in mice and NKT cell-derived IL-4 may be an important factor in providing this protection.
Introduction
Vitamin D ingested in the diet or made in the skin is inactive and is processed twice by the body to form the hormonally active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25D3). 1,25D3 functions by binding to the nuclear vitamin D receptor (VDR) and regulates transcription of targeted genes. The VDR is expressed in immune cells including T cells, B cells and macrophages (1). Previously, we identified an important role for 1,25D3 and the VDR in the development and function of NKT cells. 1,25D3 treatment given in the diet of mice led to increased release of IL-4 and IFN-γ from spleen cells in response to α-galactosylceramide (α-GalCer) stimulation in vitro compared with spleen cells from mice on control diet (2). Furthermore, both VDR-deficient and 1,25D3-deficient mice (Cyp27B1−/−) have fewer NKT cells compared with wild-type (WT) mice (2).
NKT cells are a subset of T cells that expresses NK receptors and semi-invariant CD1d-restricted αβ T-cell receptors (TCRs). NKT cells play an important regulatory role in several models of autoimmunity, including experimental autoimmune encephalomyelitis (EAE) (3). Previous studies have demonstrated that activation of invariant (i)NKT cells with α-GalCer can prevent EAE in WT mice (4, 5), and mice that transgenically over-express iNKT cells (Vα14-Jα28 transgenic) are protected from developing EAE (6). Furthermore, α-GalCer treatment leads to a decreased antigen-specific IL-17 response in both the lymph nodes (LNs) and spleen (7), and the Th17 response is known to be pathogenic in this model (8, 9). iNKT cell activation induces the expansion of myeloid-derived suppressor cells (MDSCs) in the spleen, and disease protection correlates with the infiltration of MDSCs in the central nervous system (CNS) (7).
EAE is a mouse model for multiple sclerosis (MS). MS is an inflammatory demyelinating disease of the CNS that is characterized by a chronic course of relapses followed by periods of stability. Both genes and environmental triggers contribute to susceptibility to MS (10). There are several different models of EAE that have been useful to study various aspects of MS. EAE in the B10PL mouse and the transfer of CNS-specific T cells results in relapsing disease. C57BL/6 mice are relatively resistant to EAE but have the advantage of having CD1d−/− and Jα18−/− for studies of the role of iNKT cells. Previously it has been shown that 1,25D3 can prevent EAE in C57BL/6 and B10.PL mice (11, 12) and block the progression of EAE relapse when started after the first symptoms developed in B10.PL mice (11). Since NKT cells are important for the regulation of EAE, and vitamin D and 1,25D3 regulate NKT cell development and function we hypothesized that vitamin D actions are mediated by NKT cells in EAE. We utilized CD1d−/− and Jα18−/− mice in order to determine the role of NKT cells and iNKT cells in the vitamin D-mediated protection from EAE.
Methods
Mice
8–12 weeks old male and female C57BL/6 WT, CD1d−/− and Jα18−/− (Gift from Dr Sebastian Joyce, Vanderbilt University, Nashville, TN, USA) and IL-4−/− (Jackson Laboratories, Bar Harbor, ME, USA) were produced at Pennsylvania State University. For some experiments, mice were fed synthetic diets that either included 50ng of 1,25D3 per day or did not include 1,25D3 exactly as previously described (13). The diets were fed beginning 1 week before and continuing throughout the experiment. Experimental procedures received approval from the Office of Research Protection Institutional Animal Care and Use Committee at the Pennsylvania State University.
EAE induction
To induce EAE, Jα18−/−, CD1d−/−, IL-4−/− and WT C57BL6 mice were injected subcutaneously with 200 μg myelin oligodendrocyte glycoprotein (MOG)35–55 (amino acid sequence, MEVGWYRSPFSRVVHLYRNGK; Anaspec, Fremont, CA, USA) emulsified in Freund’s adjuvant (Difco, Detroit, MI, USA) supplemented with attenuated Mycobacterium tuberculosis H37RA (Difco) to 4mg ml−1. On days 0 and 2 after immunization, the mice were injected intraperitoneally (i.p.) with 200ng pertussis toxin (List Biological Laboratories; Campbell, CA, USA) in 100 μl PBS. Clinical symptoms of EAE were evaluated daily and scored as follows: 0, no clinical signs; 1, loss of tail tonicity; 2, partial hind limb paralysis; 3, total hind limb paralysis; 4, hind and some forelimb paralysis; 5, moribund or dead (11). Mice with EAE score of 5 were sacrificed due to humane reasons. The cumulative disease activity index (CDAI) was calculated by summing all of the EAE scores for the 21 days of the experiment for each mouse.
MOG-specific cytokine production
Mice were immunized subcutaneously with MOG/CFA. 8, 14 or 21 days after immunization, inguinal and axillary LNs were collected and a single-cell suspension was prepared. LN cells (2×106 cells ml−1) were cultured for 72h with MOG peptide (20 μg ml−1). To detect IFN-γ, IL-5, IL-10 and IL-4 in culture supernatants, OptEIA kits were used (BD Pharmingen, San Jose, CA, USA). Detection of IL-17 by ELISA was performed using anti-mouse IL-17 and biotin-anti-mouse IL-17 from BD Pharmingen. The limits of detection were 31 pg ml−1 IFN-γ, 15 pg ml−1 IL-5, 31 pg ml−1 IL-10, 31 pg ml−1 IL-4 and 31 pg ml−1 IL-17.
α-GalCer stimulation
α-GalCer (Axxora, San Diego, CA, USA) was dissolved in PBS containing 0.5% Tween 20 (2). Mice were given 4.4 μg of α-GalCer dissolved in PBS or vehicle through i.p. injection at the time of EAE induction (day 0) (5). For examining iNKT cell responses, mice were i.p. injected with 2 μg α-GalCer or vehicle. Blood was collected from the retro-orbital plexus for serum isolation. For in vitro stimulations, splenocytes were isolated and 2×106 cells per well were incubated for 72h with α-GalCer (200ng ml−1).
Flow cytometry
To examine the LN population, inguinal and axillary LN cells were harvested at the indicated times after immunization with MOG/CFA. Single-cell suspensions were stained with PE-labeled CD1d-α-GalCer tetramers and empty tetramer controls (gift of the National Institutes of Health Tetramer Facility, Atlanta, GA, USA), PE-Cy7-labeled anti-NK1.1 (PK136) and PE-Cy5-labeled anti-TCRβ (H57-597). Cells were analyzed on FC500 (Beckman Coulter, Indianapolis, IN, USA), and analysis was performed using Flow Jo software (Tree Star, Ashland, OR, USA). NKT cells were gated as NK1.1+TCRβ+ cells and iNKT cells were gated as CD1d-α-GalCer tetramer+TCRβ+ cells.
Real-time PCR analysis
Mouse Hprt and Vα14 mRNA were quantified by real-time PCR. In brief, the RNA samples (1 μg) were subjected to reverse transcription analysis using AMV reverse transcriptase (Promega, Madison, WI, USA) according to the manufacturer’s instructions and quantified using the iQ5 multicolor real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA) with iQ5 software V2.0 and LightCycler FastStart DNA Master SYBR Green I. Gene expression was determined as relative expression on a linear curve based on a gel-extracted standard and was normalized to Hprt amplified from the same cDNA mix. Results were expressed as gene of interest/Hprt ratio.
Statistical analysis
Statistical analyses were performed by using PRISM software (GraphPad). Clinical scores were compared by two-way analysis of variance (ANOVA) followed by Bonferroni post-test. Other data were analyzed by ANOVA followed by Tukey post-hoc test. The incidence ratio was compared by the two-tailed Fisher exact probability test. P values <0.05 were considered significant. Experiments were repeated multiple times and results from independent experiments were pooled when possible. For experiments where there was too much variability one representative of multiple experiments is shown. Data are expressed as mean ± SEM.
Results
1,25D3 regulation of EAE in CD1d−/− mice
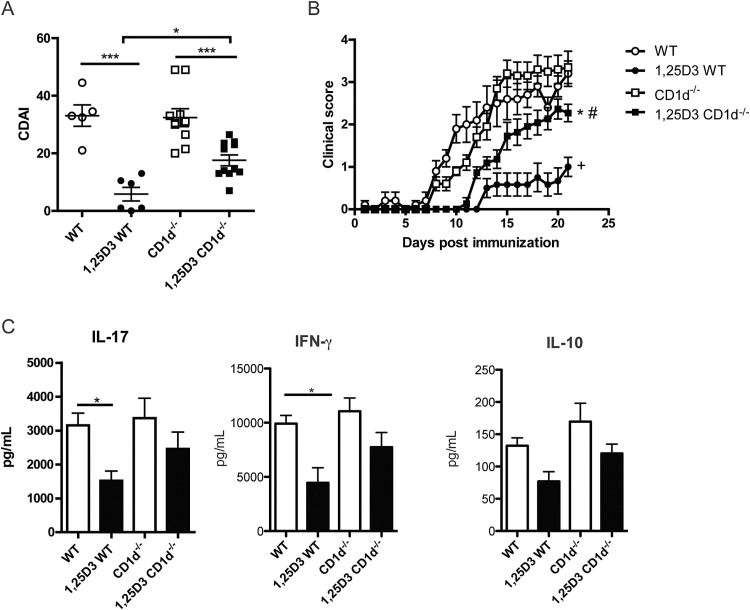
CD1d−/− and WT mice were treated with 1,25D3 (50ng day−1 in the diet) or control-treated for 1 week prior to EAE induction and throughout the course of the experiment. Control-treated WT mice developed EAE on day 12±1 (Table 1), and their CDAI was 33±4 (Fig. 1A). Consistent with previous studies, 1,25D3 effectively prevented EAE in WT mice, (Fig. 1; (11, 12)). None of the 1,25D3-treated (1,25D3) WT mice developed EAE with a score of 2 or greater, and their CDAI was 6±2, which was significantly less than the control-treated WT EAE CDAI scores (P < 0.001).
Table 1.
Summary of EAE scores in WT and CD1d−/− mice
| WT | CD1d−/− | |||
|---|---|---|---|---|
| Control | 1,25D3 | Control | 1,25D3 | |
| Day of onset | 12±1 | — | 13±0* | 16±1 |
| Incidence | 100% (5/5)+++ | 0 (0/5) | 100% (10/10) | 100% (11/11)### |
| Peak severity | 3.3±0.4+++ | 1.1±0.2 | 3.7±0.3* | 2.7±0.2### |
| CDAI | 33±4+++ | 6±2 | 32±3*** | 18±2# |
+++ P < 0.001 WT versus 1,25D3 WT, # P < 0.05; ### P < 0.001 1,25D3 WT versus 1,25D3 CD1d−/−, *P < 0.05; ***P < 0.001 CD1d−/− versus 1,25D3 CD1d−/−.
Fig. 1.
1,25D3 treatment is ineffective to suppress EAE in CD1d−/− mice. (A) Cumulative EAE disease activity index (CDAI) of WT and CD1d−/− mice on control or 1,25D3-supplemented diet. *P < 0.05, ***P < 0.001 by ANOVA-based Tukey test. (B) Daily mean EAE scores of WT and CD1d−/− mice on control or 1,25D3-supplemented diet. +: WT versus 1,25D3 WT P < 0.05; #: 1,25D3 WT versus 1,25D3 CD1d−/− P < 0.05; *: CD1d−/− versus 1,25D3 CD1d−/− P < 0.05 by two-way ANOVA followed by Bonferroni post-test. The clinical data show the mean ± SEM pooled from two independent experiments, n = 5–11 mice in total. (C) Draining LN from WT and CD1d−/− mice were isolated on day 8 of EAE and cultured in vitro with 20 μg ml−1 MOG peptide. IL-17, IFN-γ and IL-10 levels were determined by ELISA. *P < 0.05 by ANOVA-based Tukey test. Values are the mean ± SEM of values from individual animals from three independent experiments, n = 10–13 mice in total.
Control-treated CD1d−/− mice developed EAE with the same severity and kinetics as control-treated WT mice. CD1d−/− mice developed EAE on day 13±0 and their CDAI was 32±3. 1,25D3 treatment did not eliminate EAE symptoms in the CD1d−/− mice, which are deficient in NKT cells (Fig. 1; (14)). Although the CDAI was significantly decreased in 1,25D3 CD1d−/− mice compared with control-treated CD1d−/− mice (1,25D3 CD1d−/− 18±2 versus CD1d−/− 32±3, P < 0.001), 1,25D3 CD1d−/− mice developed significantly worse disease than 1,25D3 WT mice (Fig. 1B, CDAI, 1,25D3 WT: 6±2 versus 1,25D3 CD1d−/−: 18±2, P < 0.05). Furthermore, 100% of 1,25D3 CD1d−/− mice developed EAE while none of the 1,25D3 WT mice developed EAE (Table 1, Fig. 1A, P < 0.01).
In order to determine how 1,25D3 and NKT cells affect the MOG-specific immune response, the draining LNs from mice immunized to develop EAE were collected and re-stimulated in vitro. MOG-specific IL-17, IFN- γ and IL-10 were detected on day 8 post-immunization in all of the mice (Fig. 1C), while IL-4 and IL-5 were undetectable in the LN cultures from all mice at day 8. There was no IL-17, IFN-γ or IL-10 production detected in unstimulated cells. 1,25D3 WT mice produced IL-17 and IFN-γ but the amount of IL-17 and IFN-γ in the LN cultures from 1,25D3 WT mice was less than WT controls (Fig. 1C, P < 0.05). MOG-specific IL-17 and IFN-γ production from LN cultures from CD1d−/− mice was not affected by the 1,25D3 treatment (CD1d−/− same as 1,25D3 CD1d−/−, Fig. 1C). On day 8 of EAE there was no effect of 1,25D3 treatment on MOG-specific IL-10 in either WT or CD1d−/− mice (Fig. 1C). On day 14 of EAE the amounts of MOG-specific IL-17 and IFN-γ in the LNs were not altered by 1,25D3 treatment in WT or CD1d−/− mice (data not shown). 1,25D3 treatment significantly induced IL-10 compared with control treatment in both WT and CD1d−/− mice at day 14 (Supplementary Figure S1, available at International Immunology Online). Early inhibition of IL-17 and IFN-γ by 1,25D3 was associated with greater protection of WT versus CD1d−/− mice from EAE.
1,25D3 or α-GalCer treatments are ineffective at suppressing EAE in IL-4−/− mice
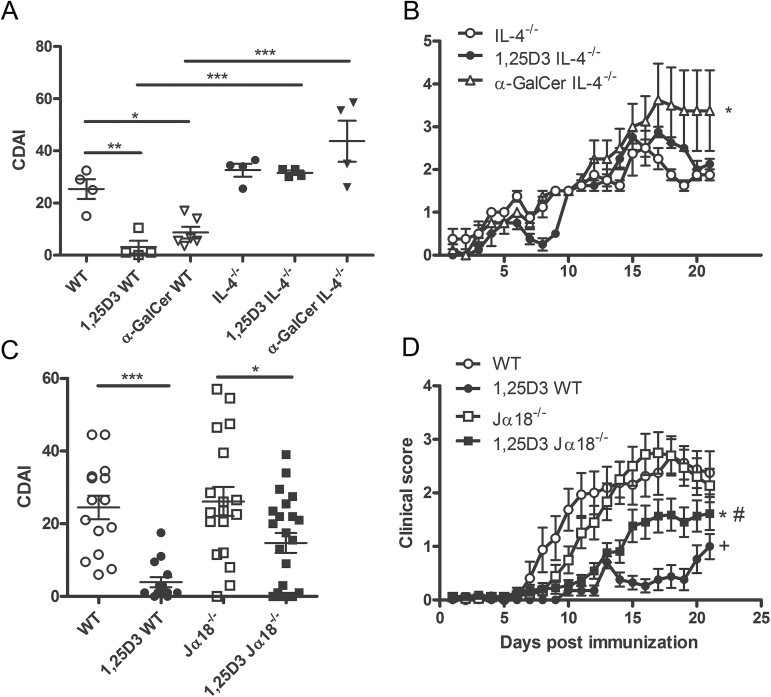
1,25D3 and α-GalCer protected WT mice from the development of EAE (Fig. 2A). Conversely, in IL-4−/− mice both 1,25D3 and α-GalCer treatments were ineffective for suppressing EAE symptoms (Fig. 2A and B, Table 2). CDAI scores were not different in IL-4−/−, 1,25D3 IL-4−/− or α-GalCer IL-4−/− mice (Fig. 2A). In addition, the day of EAE onset, the EAE incidence and the peak EAE severity were not different in IL-4−/− mice that were control, 1,25D3 or α-GalCer treated (Table 2). IL-4 expression is necessary for the effectiveness of 1,25D3 and α-GalCer treatments in EAE.
Fig. 2.
iNKT cells, IL-4 and the effects of 1,25D3 on EAE development. (A) Cumulative EAE scores of WT and IL-4−/− mice on control diet, 1,25D3 diet or α-GalCer treatment. *P < 0.05, **P < 0.01,***P < 0.001 by ANOVA-based Tukey test. (B) Daily mean EAE scores of IL-4−/− mice on control diet, 1,25D3 diet or α-GalCer treatment. *P < 0.05 IL-4−/− versus α-GalCer IL-4−/− by two-way ANOVA followed by Bonferroni post-test. The clinical data show the mean ± SEM, n = 4–6, one representative of two independent experiments. (C) Cumulative EAE disease index (CDAI) of WT and Jα18−/− mice on control or 1,25D3-supplemented diet. *P < 0.05, ***P < 0.001 by ANOVA-based Tukey test. (D) Daily mean EAE scores of WT and Jα18−/− mice on control or 1,25D3-supplemented diet. +: WT versus 1,25D3 WT P < 0.05; #: 1,25D3 WT versus 1,25D3 Jα18−/− P < 0.05; *: Jα18−/− versus 1,25D3 Jα18−/− P < 0.05 by two-way ANOVA followed by Bonferroni post-test. The clinical data show the mean ± SEM pooled from three independent experiments, n = 16–22 mice in total.
Table 2.
Summary of EAE scores in IL-4−/− mice
| IL-4−/− | |||
|---|---|---|---|
| Control | 1,25D3 | α-GalCer | |
| Day of onset | 13±1 | 12±1 | 11±0 |
| Incidence | 75% (3/4) | 100% (4/4) | 75% (3/4) |
| Peak severity | 2.8±0.4 | 3.0±0 | 3.6±0.9 |
| CDAI | 33±2 | 32±2 | 44±8 |
1,25D3 regulation of EAE in Jα18−/− mice
Control-treated Jα18−/− mice developed EAE with the same severity and kinetics as the control-treated WT mice (Fig. 2C and D, Table 3). Jα18−/− mice developed EAE on day 13±1 and their CDAI was 26±4. As seen previously, 1,25D3 WT mice were significantly protected from EAE; however, in this experiment, some 1,25D3 WT mice did develop EAE (18%, Fig. 2C, Table 3). 1,25D3 treatment was less effective at suppressing EAE symptoms in the Jα18−/− mice, which are deficient in iNKT cells (Fig. 2C and D; (15)). The incidence of EAE in 1,25D3 Jα18−/− mice was 59%, which is significantly higher than in 1,25D3 WT mice (Table 3, P < 0.01). Furthermore, the day of EAE onset was significantly earlier in 1,25D3 Jα18−/− mice than in 1,25D3 WT mice (Table 3; P < 0.01). Although the cumulative disease scores (CDAI) were not significantly different between 1,25D3 WT and 1,25D3 Jα18−/− mice (Fig. 2C), the clinical scores over the course of the disease of 1,25D3 Jα18−/− mice were significantly higher than 1,25D3 WT by two-way ANOVA (Fig. 2D, P < 0.05). There was an effect of the 1,25D3 treatment in Jα18−/− mice, since the clinical scores over time were less severe in the 1,25D3 Jα18−/− mice than in control Jα18−/− mice (Fig. 2D, P < 0.001). However, the effect of 1,25D3 on EAE in Jα18−/− mice was less than in WT since 1,25D3 did not significantly change the day of disease onset or incidence of disease compared with control Jα18−/− mice (Table 3).
Table 3.
Summary of EAE scores in WT and Jα18−/− mice
| WT | Jα18−/− | |||
|---|---|---|---|---|
| Control | 1,25D3 | Control | 1,25D3 | |
| Day of onset | 11±1+++ | 19±1 | 13±1 | 15±2## |
| Incidence | 75% (12/16)++ | 18% (3/17) | 78% (14/18) | 59% (13/22)## |
| Peak severity | 2.9±0.3++ | 1.2±0.2 | 3.0±0.3 | 2.1±0.3 |
| CDAI | 24±3+++ | 4±1 | 26±4* | 15±3 |
++ P < 0.01; +++ P < 0.001 WT versus 1,25D3 WT, ## P < 0.01 1,25D3 WT versus 1,25D3 Jα18−/−, *P < 0.05 Jα18−/− versus 1,25D3 Jα18−/−.
1,25D3 effects on iNKT cells during EAE
Since 1,25D3 treatment is less effective for suppressing EAE in Jα18−/− mice and α-GalCer treatments protect WT mice from EAE, we investigated the effects of 1,25D3 on iNKT cells during EAE. The expression of transcripts for the Vα14 TCR of iNKT cells was measured in the CNS of WT mice without EAE (day 0) or at day 21 post-EAE in WT and 1,25D3 WT mice. WT mice immunized with MOG for 21 days had higher expression of Vα14 than WT mice at day 0 (Fig. 3). Vα14 expression was significantly lower in 1,25D3 WT mice than control WT mice on day 21 (Fig. 3). CNS samples from α-GalCer-treated WT mice had the same number of Vα14 transcripts as the untreated WT mice with EAE (Fig. 3). It should be noted that measurement of Vα14 transcripts could reflect the activation-induced down-regulation of iNKT cells, as has been reported to occur with α-GalCer treatments in vivo (16). We examined iNKT cell numbers in the draining LNs by flow cytometry on day 8, 14 and 23 of EAE and also in the spleen on day 14 of EAE. We found no significant differences in iNKT cell numbers between WT and 1,25D3 WT mice (data not shown; Supplementary Figure S2, available at International Immunology Online). We further examined iNKT cell function by stimulating draining LN cells from day 8 and 14 of EAE with α-GalCer ex vivo. α-GalCer stimulation induced IL-17, IFN-γ, IL-10 and IL-4 cytokine secretion similarly in control versus 1,25D3-treated mice (Supplementary Figure S3, available at International Immunology Online). IL-5 secretion at day 8 (but not at day 14) post-EAE induction was lower in the α-GalCer re-stimulated cultures from 1,25D3 treated versus controls (Supplementary Figure S3, available at International Immunology Online). Injection of 2 μg α-GalCer on day 8 of EAE did not induce significantly different levels of IL-4 in the serum of control versus 1,25D3-treated mice (data not shown). Peripheral iNKT cell responses were not different in the spleen, LNs and blood of WT and 1,25D3-treated WT mice after EAE induction.
Fig. 3.
1,25D3 or α-GalCer treatment affects iNKT cell infiltration into the CNS during EAE. Reverse transcription–PCR analysis of Vα14 TCR expression in spinal cord homogenates isolated from WT mice at baseline or mice treated with control, 1,25D3 or α-GalCer from day 21 of EAE. *P < 0.05 by ANOVA-based Tukey test. The data show the mean ± SEM pooled from two independent experiments, n = 5–8 mice in total.
Since the effects of iNKT cells in EAE occur early during the induction of EAE (4, 5) we determined the effect of 1,25D3 on iNKT cells in vitro from naive animals. α-GalCer stimulation of both WT and IL-4−/− splenocytes induced IFN-γ expression, which was not significantly inhibited by 1,25D3 (Fig. 4). However, α-GalCer stimulation induced IL-17 production, which was inhibited equally by 1,25D3 in both mouse strains (Fig. 4). 1,25D3 increased α-GalCer-induced IL-5 and IL-10 expression in both WT and IL-4−/− mice compared with α-GalCer alone (Fig. 4). 1,25D3 was also able to increase α-GalCer-induced IL-4 expression in WT mice in vitro (Fig. 4). Consistent with these results, we saw a small increase in IL-4+ iNKT cells in the spleens of 1,25D3-treated mice compared with controls on day 8 of EAE (WT: 13.7±1.7% versus 1,25D3 WT: 24.0±2.8%, P < 0.05, n = 4–5). In subsequent experiments differences in IL-4-secreting cells were much smaller, although there was a trend toward increased IL-4+ iNKT cells in 1,25D3-treated WT mice (WT: 10.2±1.4 versus 1,25D3 WT: 14.0±2.8, n = 12; Supplementary Figure S1, available at International Immunology Online). The trend in higher IL-4+ iNKT cells was associated with significantly lower MOG-specific IL-17 (WT: 2190±547 pg ml−1; 1,25D3 WT: 840±152 pg ml−1, P < 0.05, n = 4–5) and IFN-γ production (WT: 17.8±3.2ng ml−1 versus 1,25D3 WT: 2.3±1.0ng ml−1, P < 0.01, n = 4–5) from splenocytes from 1,25D3-treated mice compared with controls. There was not a difference in IL-4+ iNKT cells in draining LNs between 1,25D3- and control-treated mice. 1,25D3 in vitro inhibits α-GalCer-induced IL-17 production from splenocytes while inducing production of IL-10, IL-4 and IL-5. 1,25D3 treatments in vivo reduced MOG-specific IL-17 and IFN-γ responses and induced IL-4 secretion from iNKT cells to a small extent.
Fig. 4.
Effect of 1,25D3 treatment on α-GalCer-induced cytokine levels in vitro. WT or IL-4−/− splenocytes from naive mice were stimulated in vitro with α-GalCer (200ng ml−1) ± 1,25D3 (100nM) for 72h and cytokines in supernatants were assessed by ELISA. *P < 0.05, ***P < 0.001 by ANOVA-based Tukey test, n = 4–5 mice and is one representative of two independent experiments.
Discussion
In the present study, we have investigated the role of NKT and iNKT cells in 1,25D3-mediated protection from EAE in mice. 1,25D3 treatments were less effective at suppressing EAE in both, CD1d−/− and Jα18−/− than in WT mice, indicating that type II NKT cells may not have a large role in this protection. However, some symptoms of EAE were still lower in the 1,25D3 CD1d−/− and 1,25D3 Jα18−/− mice compared with CD1d−/− and Jα18−/− mice, suggesting that in the absence of NKT cells, 1,25D3 still has some suppressive effects in EAE. The data indicate that 1,25D3-mediated protection from EAE is regulated in part by NKT cells.
iNKT cells are important regulators of autoimmune diseases. In particular, studies have shown that patients with MS have decreased iNKT cells or decreased iNKT cell function (17, 18). Furthermore, effective treatment of MS with IFN-β increased iNKT cell numbers and function (19). Our data confirm that activation of iNKT cells with α-GalCer is protective in EAE (5, 6, 20). We have previously shown that mice given a 1,25D3-supplemented diet for 1 week have increased IL-4 and IFN-γ secretion from splenocytes stimulated ex vivo with α-GalCer, demonstrating that 1,25D3 given in the diet can affect iNKT cell function (2). We now demonstrate that CD1d−/− and Jα18−/− mice are not protected from EAE to the same extent as WT mice by treatment with 1,25D3 in the diet. These data provide further evidence for a regulatory role of 1,25D3 on NKT cell function.
NKT cells are able to produce multiple cytokines very rapidly in response to TCR triggering (3). One study demonstrated that IL-4 derived from iNKT cells was required for mediating protective effects of iNKT cells during EAE (21). Furthermore, earlier studies have also shown that IL-4 is required for the α-GalCer-mediated protection from EAE (4, 5). On the basis of the results that showed that IL-4 expression was also required for 1,25D3-mediated protection from EAE, we hypothesized that NKT cell-derived IL-4 might be critical for 1,25D3-mediated effects in EAE. Supporting this finding, we show that 1,25D3 increased α-GalCer-induced IL-4 expression in vitro. In addition, we saw a trend toward increased IL-4+ iNKT cells in the spleens of 1,25D3-treated mice compared with control mice during EAE. NKT cells are unique among lymphocytes in their ability to rapidly release large amounts of IL-4 (22). Consistent with an important role for NKT cell-derived IL-4, NKT cells from MS patients in remission produce more IL-4 than NKT cells from MS patients in relapse (17). However, we have not definitively demonstrated the role of iNKT cell-derived IL-4 using adoptive transfer experiments since 1,25D3, although effective in the Jα18−/− mice, is not completely effective and so the change to be rescued with the transplanted NKT cells would be even smaller. The data suggest that 1,25D3 regulates IL-4 production from NKT cells and other cell types that are important for the 1,25D3-mediated suppression of EAE.
Since we previously demonstrated that 1,25D3 can suppress IL-17 production from CD4+ T cells in vitro (23), we determined whether NKT cells were required for the 1,25D3-mediated suppression of IL-17 production during EAE. We demonstrate that MOG-specific IL-17 production was significantly reduced in 1,25D3 WT mice compared with WT but was not decreased in 1,25D3 CD1d−/− mice compared with CD1d−/− mice. In addition, the IFN-γ response was attenuated in 1,25D3 WT mice compared with WT, but not in CD1d−/− mice. Furthermore, we now demonstrate that 1,25D3 can inhibit α-GalCer-induced IL-17 production from splenocytes in vitro. The reduction in IL-17 is associated with an increase in IL-4, IL-5 and IL-10 from iNKT cells in vitro. However, the data fall short of definitively demonstrating that the in vitro effects of 1,25D3 on iNKT cell cytokine secretion accounts for the suppression of EAE by 1,25D3 in vivo. Collectively, these studies do suggest that iNKT cell-mediated changes in the IL-17 response could be important for ameliorating disease in 1,25D3-treated mice.
1,25D3 treatment still significantly decreased disease severity in both the CD1d−/− and Jα18−/− mice. The VDR is widely expressed in immune cells (1). Previous studies have shown that 1,25D3 treatment of mice with EAE decreased disease severity and increased the number of Mac-1+ cells in the CNS, indicating the 1,25D3-mediated reduction in disease severity may be mediated in part by macrophages (24, 25). In addition, 1,25D3 treatment has rapidly induced apoptosis of T cells (7h after administration) in the CNS when given to mice with established EAE (25). Using Cre-mediated deletion of the VDR in CD4+ T cells showed that VDR function in T cells was required for the maximal effectiveness of 1,25D3 in EAE (12). The VDR would be absent in NKT cells in the CD4-driven deletion of the VDR in T cells, since NKT cells develop from CD4/CD8 double-positive thymocytes (26). Although NKT cells are important for mediating the effects of 1,25D3 in EAE, 1,25D3 also targets other immune cells to suppress EAE.
Both low levels of vitamin D and low levels of iNKT cells are associated with MS. Furthermore, iNKT cell dysfunction is associated with MS (17, 18). Multiple studies have demonstrated that decreased levels of vitamin D are associated with an increased risk for MS and increasing vitamin D levels decreased the risk of relapse in MS patients (27–31). Furthermore, vitamin D is important for iNKT cell development and function (2, 32). Our study now shows that iNKT cells are required for the full protective effects of 1,25D3 during EAE, and IL-4 is important for this protection. Future studies using depletion of iNKT cells rather than genetic deletion will rule out compensatory mechanisms that could occur in knockout mice. Vitamin D represents an attractive target for enhancing or restoring the protective function of NKT cells in MS patients in order to treat disease. Further studies will need to be performed in MS patients to determine whether vitamin D is regulating NKT cell in MS.
Supplementary data
Supplementary data are available at International Immunology Online.
Funding
National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (DK070781); National Institutes of Neurologic and Stroke Grant (NS067563); National Center for Complementary and Alternative Medicine; Office of Dietary Supplements (AT005378).
Supplementary Material
Acknowledgements
We thank Dr Sebastian Joyce (Vanderbilt University) for providing the original CD1d−/− and Ja18−/− breeders. We thank the National Institutes of Health Tetramer Facility (Atlanta, GA, USA) for the CD1d tetramers. We thank Veronika Weaver for animal husbandry. A.W. and M.T.C. designed and performed experiments, analyzed and interpreted data and wrote the manuscript. J.Z. performed experiments and analyzed and interpreted data.
Conflict of Interest statement: The authors declared no conflict of interests.
References
- 1. Cantorna M. T., Zhu Y., Froicu M., Wittke A. 2004. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am. J. Clin. Nutr. 80(6 Suppl):1717S. [DOI] [PubMed] [Google Scholar]
- 2. Yu S., Cantorna M. T. 2008. The vitamin D receptor is required for iNKT cell development. Proc. Natl. Acad. Sci. USA 105:5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bendelac A., Savage P. B., Teyton L. 2007. The biology of NKT cells. Annu. Rev. Immunol. 25:297. [DOI] [PubMed] [Google Scholar]
- 4. Singh A. K., Wilson M. T., Hong S., et al. 2001. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J. Exp. Med. 194:1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jahng A. W., Maricic I., Pedersen B., et al. 2001. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J. Exp. Med. 194:1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mars L. T., Laloux V., Goude K., et al. 2002. Cutting edge: V alpha 14-J alpha 281 NKT cells naturally regulate experimental autoimmune encephalomyelitis in nonobese diabetic mice. J. Immunol. 168:6007. [DOI] [PubMed] [Google Scholar]
- 7. Parekh V. V., Wu L., Olivares-Villagómez D., Wilson K. T., Van Kaer L. 2013. Activated invariant NKT cells control central nervous system autoimmunity in a mechanism that involves myeloid-derived suppressor cells. J. Immunol. 190:1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hofstetter H. H., Karulin A. Y., Forsthuber T. G., Ott P. A., Tary-Lehmann M., Lehmann P. V. 2005. The cytokine signature of MOG-specific CD4 cells in the EAE of C57BL/6 mice. J. Neuroimmunol. 170:105. [DOI] [PubMed] [Google Scholar]
- 9. Komiyama Y., Nakae S., Matsuki T., et al. 2006. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177:566. [DOI] [PubMed] [Google Scholar]
- 10. Hauser S. L., Chan J. R., Oksenberg J. R. 2013. Multiple sclerosis: Prospects and promise. Ann. Neurol. 74:317. [DOI] [PubMed] [Google Scholar]
- 11. Cantorna M. T., Hayes C. E., DeLuca H. F. 1996. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 93:7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mayne C. G., Spanier J. A., Relland L. M., Williams C. B., Hayes C. E. 2011. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur. J. Immunol. 41:822. [DOI] [PubMed] [Google Scholar]
- 13. Cantorna M. T., Hayes C. E., DeLuca H. F. 1998. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J. Nutr. 128:68. [DOI] [PubMed] [Google Scholar]
- 14. Nakai Y., Iwabuchi K., Fujii S., et al. 2004. Natural killer T cells accelerate atherogenesis in mice. Blood 104:2051. [DOI] [PubMed] [Google Scholar]
- 15. Cui J., Shin T., Kawano T., et al. 1997. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science 278:1623. [DOI] [PubMed] [Google Scholar]
- 16. Subleski J. J., Hall V. L., Wolfe T. B., et al. 2011. TCR-dependent and -independent activation underlie liver-specific regulation of NKT cells. J. Immunol. 186:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Araki M., Kondo T., Gumperz J. E., Brenner M. B., Miyake S., Yamamura T. 2003. Th2 bias of CD4+ NKT cells derived from multiple sclerosis in remission. Int. Immunol. 15:279. [DOI] [PubMed] [Google Scholar]
- 18. O’Keeffe J., Gately C. M., Counihan T., et al. 2008. T-cells expressing natural killer (NK) receptors are altered in multiple sclerosis and responses to alpha-galactosylceramide are impaired. J. Neurol. Sci. 275:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gigli G., Caielli S., Cutuli D., Falcone M. 2007. Innate immunity modulates autoimmunity: type 1 interferon-beta treatment in multiple sclerosis promotes growth and function of regulatory invariant natural killer T cells through dendritic cell maturation. Immunology 122:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Furlan R., Bergami A., Cantarella D., et al. 2003. Activation of invariant NKT cells by alphaGalCer administration protects mice from MOG35-55-induced EAE: critical roles for administration route and IFN-gamma. Eur. J. Immunol. 33:1830. [DOI] [PubMed] [Google Scholar]
- 21. Oh S. J., Chung D. H. 2011. Invariant NKT cells producing IL-4 or IL-10, but not IFN-gamma, inhibit the Th1 response in experimental autoimmune encephalomyelitis, whereas none of these cells inhibits the Th17 response. J. Immunol. 186:6815. [DOI] [PubMed] [Google Scholar]
- 22. Yoshimoto T., Paul W. E. 1994. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J. Exp. Med. 179:1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bruce D., Yu S., Ooi J. H., Cantorna M. T. 2011. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int. Immunol. 23:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nashold F. E., Miller D. J., Hayes C. E. 2000. 1,25-Dihydroxyvitamin D3 treatment decreases macrophage accumulation in the CNS of mice with experimental autoimmune encephalomyelitis. J. Neuroimmunol. 103:171. [DOI] [PubMed] [Google Scholar]
- 25. Pedersen L. B., Nashold F. E., Spach K. M., Hayes C. E. 2007. 1,25-Dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J. Neurosci. Res. 85:2480. [DOI] [PubMed] [Google Scholar]
- 26. Gapin L., Matsuda J. L., Surh C. D., Kronenberg M. 2001. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat. Immunol. 2:971. [DOI] [PubMed] [Google Scholar]
- 27. van der Mei I. A., Ponsonby A. L., Dwyer T., et al. 2007. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J. Neurol. 254:581. [DOI] [PubMed] [Google Scholar]
- 28. Kampman M. T., Wilsgaard T., Mellgren S. I. 2007. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J. Neurol. 254:471. [DOI] [PubMed] [Google Scholar]
- 29. Islam T., Gauderman W. J., Cozen W., Mack T. M. 2007. Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology 69:381. [DOI] [PubMed] [Google Scholar]
- 30. Pierrot-Deseilligny C., Rivaud-Péchoux S., Clerson P., de Paz R., Souberbielle J. C. 2012. Relationship between 25-OH-D serum level and relapse rate in multiple sclerosis patients before and after vitamin D supplementation. Ther. Adv. Neurol. Disord. 5:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simpson S., Jr, Taylor B., Blizzard L., et al. 2010. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann. Neurol. 68:193. [DOI] [PubMed] [Google Scholar]
- 32. Yu S., Cantorna M. T. 2011. Epigenetic reduction in invariant NKT cells following in utero vitamin D deficiency in mice. J. Immunol. 186:1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.