Increased numbers of NKTfh cells follow immunization with α-galactosylceramide
Keywords: CD1d, follicular helper cells, NKT cells
Abstract
NKT follicular helper cells (NKTfh cells) are a recently discovered functional subset of CD1d-restricted NKT cells. Given the potential for NKTfh cells to promote specific antibody responses and germinal center reactions, there is much interest in determining the conditions under which NKTfh cells proliferate and/or differentiate in vivo and in vitro. We confirm that NKTfh cells expressing the canonical semi-invariant Vα14 TCR were CXCR5+/ICOS+/PD-1+/Bcl6+ and increased in number following administration of the CD1d-binding glycolipid α-galactosylceramide (α-GC) to C57Bl/6 mice. We show that the α-GC-stimulated increase in NKTfh cells was CD1d-dependent since the effect was diminished by reduced CD1d expression. In vivo and in vitro treatment with α-GC, singly or in combination with IL-2, showed that NKTfh cells increased in number to a greater extent than total NKT cells, but proliferation was near-identical in both populations. Acquisition of the NKTfh phenotype from an adoptively transferred PD-1-depleted cell population was also evident, showing that peripheral NKT cells differentiated into NKTfh cells. Therefore, the α-GC-stimulated, CD1d-dependent increase in peripheral NKTfh cells is a result of cellular proliferation and differentiation. These findings advance our understanding of the immune response following immunization with CD1d-binding glycolipids.
Introduction
Upon recognition of MHC class II/peptide complexes, some CD4+ T cells differentiate into effector cells known as T follicular helper (Tfh) cells. Tfh cells are readily identified by flow cytometry and have a characteristic CD4+/CD44hi/PD-1+/CXCR5+ cell surface phenotype (reviewed in ref. 1). Tfh cells also express the intracellular transcription factor Bcl6, which is necessary for their differentiation (1). Tfh cells are able to form cognate interactions with antigen-experienced B cells and contribute to their entry into the germinal center (1). It is in the germinal center where B cells undergo class switch recombination, affinity maturation and commitment to the memory B-cell sublineage (2). Furthermore, Tfh cells secrete significant quantities of IL-21, an important cytokine for immunoglobulin class switch (1, 3). Consequently, there is much interest by those delineating the immune response to vaccines in understanding Tfh differentiation, phenotype and function.
Recently, three laboratories independently reported that Tfh cells were not confined to class II-restricted lineages, but could also be found among CD1d-restricted NKT cells (4–6). NKT cells broadly fall into two groups. Type I cells express an invariant Vα14 TCR α-chain and a semi-invariant Vβ chain (Vβ2, 7 or 8) (7, 8). Type I NKT cells can be activated by foreign CD1d-binding ligand (7, 8) and by a pathway dependent on LPS-stimulated alteration of self CD1d ligand presentation (9). Type II NKT cells are also CD1d restricted and exhibit variable Vα, Jα and Vβ usage (10). Type II NKT cells are activated by self CD1d-binding ligands such as sulfatide (11), as well as aluminum salt-containing adjuvant (Alum) (12), and CpG DNA (13). Type I and Type II NKT cells play important roles in immune responses to vaccines, to infection, to cancer and in autoimmunity (7, 8).
The follicular helper subset of NKT cells was designated as NKTfh and was observed following immunization of mice with the CD1d-binding glycolipid ligand α-galactosylceramide (α-GC). Immunization led to the appearance of a population of NKTfh cells that was CD4+/CD44hi/PD-1+/CXCR5+/Bcl6+ and IL-21-secreting, much like classical Tfh cells (4–6).
The observation that α-GC treatment led to emergence of NKTfh cells was significant, because several studies have shown that α-GC has potent adjuvant properties on humoral immunity to T-dependent and T-independent antigens (14–16). Furthermore, the α-GC adjuvant when combined with appropriate vaccine antigens increased protection in vivo against viruses as well as bacterial toxins (14, 17–19). Evidence available thus far highlights the involvement of NKTfh cells during antibody responses to protein (4), lipid (5) and carbohydrate (20) antigens. It is therefore important for researchers to delineate the circumstances and mechanisms by which NKTfh cells increase in number following stimulation. Whether this is a product of proliferation of existing NKTfh cells, differentiation of NKT cells into NKTfh cells, or both mechanisms, has not been addressed in previous studies.
Herein, we use in vivo, in vitro and adoptive transfer approaches to demonstrate that α-GC drives increases in NKTfh numbers in a manner that is dependent on CD1d expression levels and is a result of proliferation and differentiation of the total NKT cell population. These findings advance our understanding of how NKT cells respond to immunization with CD1d-binding glycolipids.
Methods
Mice
Female C57Bl/6 (B6) mice and CD45.1 mice (on a B6 genetic background) were purchased from the National Cancer Institute (Bethesda, MD, USA). Vα14 TCR transgenic mice on a B6 genetic background were purchased from Jackson laboratories (Bar Harbor, ME, USA). CD1d−/− mice were originally provided by Dr M Exley (University of Manchester, Manchester, UK). Vα14 TCR-transgenic mice and CD1d−/− mice were bred in the specific pathogen-free facility at OUHSC (Oklahoma City, OK, USA). CD1d+/− mice were generated by breeding CD1d−/− and C57Bl/6 mice. All procedures were approved by the OUHSC Institutional Animal Care and Use Committee.
Reagents
PBS57-loaded and unloaded CD1d tetramers were provided by the NIAID Tetramer Facility (Emory University, Atlanta, GA, USA). Other reagents were purchased as follows: FITC-conjugated anti-CD1d (1B1), biotin-anti-CXCR5 (2G8), FITC-TCRβ (H57-597), PerCPCy5.5-CD4 (RM4-5) mAbs and PECF594-streptavidin (BD Biosciences, San Jose, CA, USA); PECy7-anti-PD-1 (J43), PE–ICOS (7E.17G9) and PE–Bcl6 (mGL191E) mAbs (eBioscience, San Diego, CA, USA); FITC-anti-CD45.2 (104) mAbs; BV421-streptavidin (Biolegend, San Diego, CA, USA); Anti-PE microbeads (Miltenyi Biotec, Auburn, CA, USA); α-GC (Axorra, Farmingdale, NY, USA); Human IL-2 (PeproTech, Rocky Hill, NJ, USA); Cell-Trace Violet (CTV) (Life technologies, Grand Island, NY, USA).
Immunizations
All immunizations were reconstituted in sterile LPS-free PBS in a 200 µl final volume. For all experiments except one, 4 µg of α-GC was administered subcutaneously (s.c.) with doses divided equally over both flanks. If immunization followed NKT cell adoptive transfer (as in Fig. 5), the intra-peritoneal (i.p.) route was used. Induction of NKT anergy typically follows administration of α-GC, when administered via the i.p. route and/or formulated in polysorbate 20 (21, 22). We previously reported that s.c. administration of 4 μg of α-GC per mouse, formulated in PBS and administered by the s.c. route did not cause loss of IL-4 or IFN-γ secretion when re-stimulating NKT cells in vitro 16h after the initial immunization (16). For the current study, we extended that observation by performing in vitro re-stimulation 1 week after immunization. We observed that NKT cells did not lose any capacity for IL-4 or IFN-γ secretion after s.c immunization (data not shown). Human IL-2 (12000U per mouse) in a 100 µl volume of PBS was administered by the i.p. route and given twice per day for 3 days. The i.p. route of administration was used for IL-2 since the standard method of delivery of cytokines in vivo is through the i.p. route, and this method has been used for measuring the effect of IL-2 on Tfh cells (23).
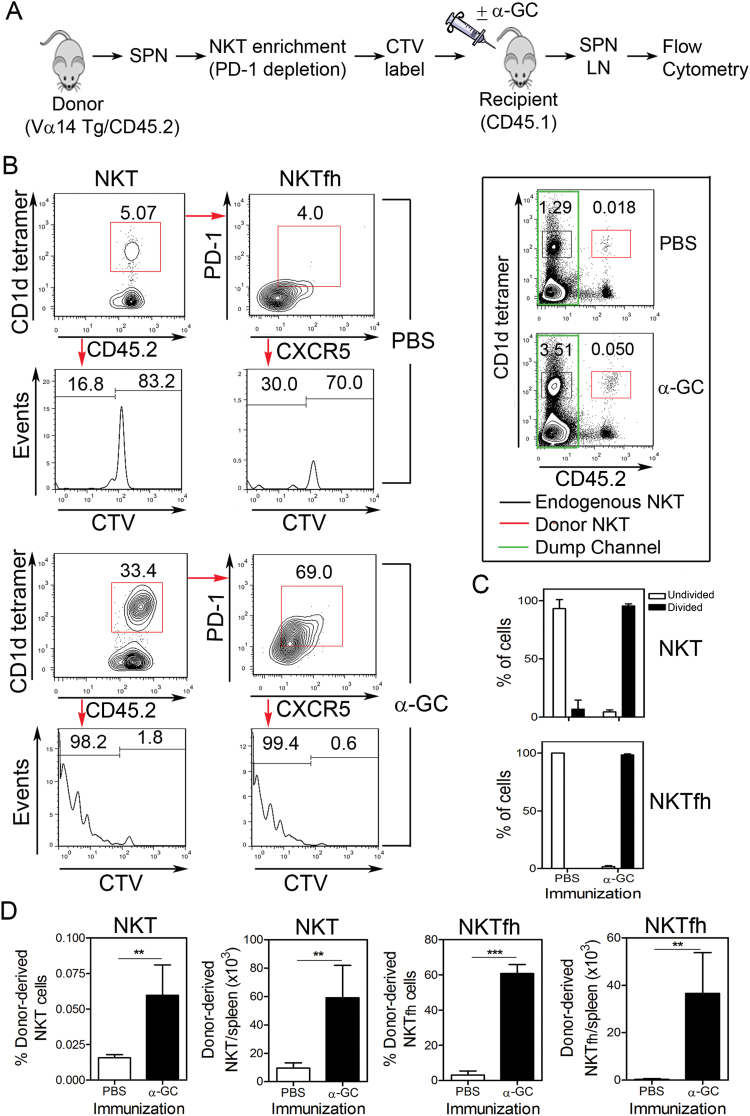
Fig. 5.
NKTfh cells differentiate from non-NKTfh cells in vivo. (A) PD-1-depleted splenocytes were prepared from CD45.2+ Vα14 Tg mice, CTV-labeled and transferred into CD45.1 congenic recipient mice. The recipients were immunized i.p. with PBS or α-GC. After 3 days splenocytes were isolated and examined by flow cytometry. (B) Plots show CD1d Tetramer+/CD45.2+ NKT cells, PD-1-expressing and CXCR5-expressing NKTfh cells and their CTV dye dilution profile after PBS (upper four panels) or α-GC immunization (lower four panels). An electronic dump channel was employed to acquire larger numbers of events and detect donor-derived cells. To do this, only CD45.2+ events corresponding to donor-derived cells were collected. For reference, the framed inset shows CD1d tetramer and CD45.2 staining without the dump channel applied. (C) Graphs depict the mean ± SD percentage of undivided cells (empty bars) and divided cells (filled bars) for five mice per group. (D) Shows frequency and number of donor-derived NKT cells and NKTfh cells. The graph on the far left depicts donor-derived NKT cells as a percentage of all splenocytes recovered from recipients. The graph second from the left depicts the total number of donor-derived NKT cells in recipient spleens. The graph second from the right depicts the percentage of donor-derived NKT cells exhibiting the NKTfh phenotype. The graph on the far right depicts the total number of donor-derived NKTfh cells in recipient spleens. Data are represented as mean ± SD for five mice per group. A two-tailed unpaired t-test was used to determine significance (**P < 0.01, ***P < 0.001). Similar results were obtained in lymph node cells (not depicted) and in an independent experiment that measured NKTfh numbers but not proliferation.
Flow cytometry
Splenocytes were isolated by mechanical disruption and removal of erythrocytes using ammonium chloride-mediated lysis. Cells were then incubated in RPMI640 media, containing 1% v/v fetal bovine serum (FBS), FcR-blocking mAb 2.4G2 at a final concentration of 20 µg ml−1, PBS57-loaded CD1d tetramer at a 1/400 dilution of stock and fluorochrome-conjugated mAbs diluted to the appropriate concentrations. After incubation for 1h at room temperature, cells were washed three times with PBS by centrifugation (200 rcf, 5min and room temperature). Streptavidin was added at a 1/100 dilution of stock, and incubated on ice in the dark for 30min. Cells were then fixed in 1% w/v paraformaldehyde in PBS and analyzed by flow cytometry using a Stratedigm S1200Ex flow cytometer (Stratedigm, San Jose, CA, USA). Data were analyzed using FlowJo software (Tree star Inc., Ashland, OR, USA).
In vitro NKTfh expansion
Five hundred thousand spleen cells from naive mice were cultured in media containing RPMI 1640 with 10% v/v heat-inactivated FBS, 2mM l-glutamine, 0.01M HEPES, 55 μM β-ME, 1mM Na pyruvate, 1mM MEM and anti-bacterial/anti-fungal reagent. CTV (3 μM final concentration) was used to label cells according to the manufacturers’ instructions. Where appropriate α-GC was added to the cultures at a final concentration of 200ng ml−1 and human IL-2 was added at a final concentration of 50U ml−1. Cells were cultured for 3 days before analysis.
NKTfh depletion and adoptive transfer
Splenocytes from CD45.2-expressing Vα14 Tg donor mice were stained with PE-conjugated anti-B220, and -PD-1 mAb. These were incubated for 15min with anti-PE microbeads. After washing with MACS Buffer (1× PBS, pH 7.2 containing 0.5% BSA and 2mM EDTA) cells were subject to negative sorting according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA, USA). Flow cytometry was used to confirm NKT enrichment and PD-1 depletion. The NKTfh-depleted splenocytes were then CTV-labeled, re-suspended in 100 μl sterile PBS, and adoptively transferred into CD45.1-expressing B6 recipient mice by para-orbital injection. After 24h, recipient mice were injected i.p. with vehicle or vehicle containing 4 μg α-GC. Three days after immunization, splenocytes and lymph node cells were isolated and stained for endogenous and donor-derived NKTfh cells.
Statistics
Data were analyzed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). An unpaired t-test was used for experiments with two experimental groups. One-way analysis of variance with the Bonferroni post-test was used for multiple experimental groups (*, **, and *** represent P values of <0.05, <0.01, and <0.001, respectively).
Results
NKTfh expansion in vivo is stimulated by α-GC
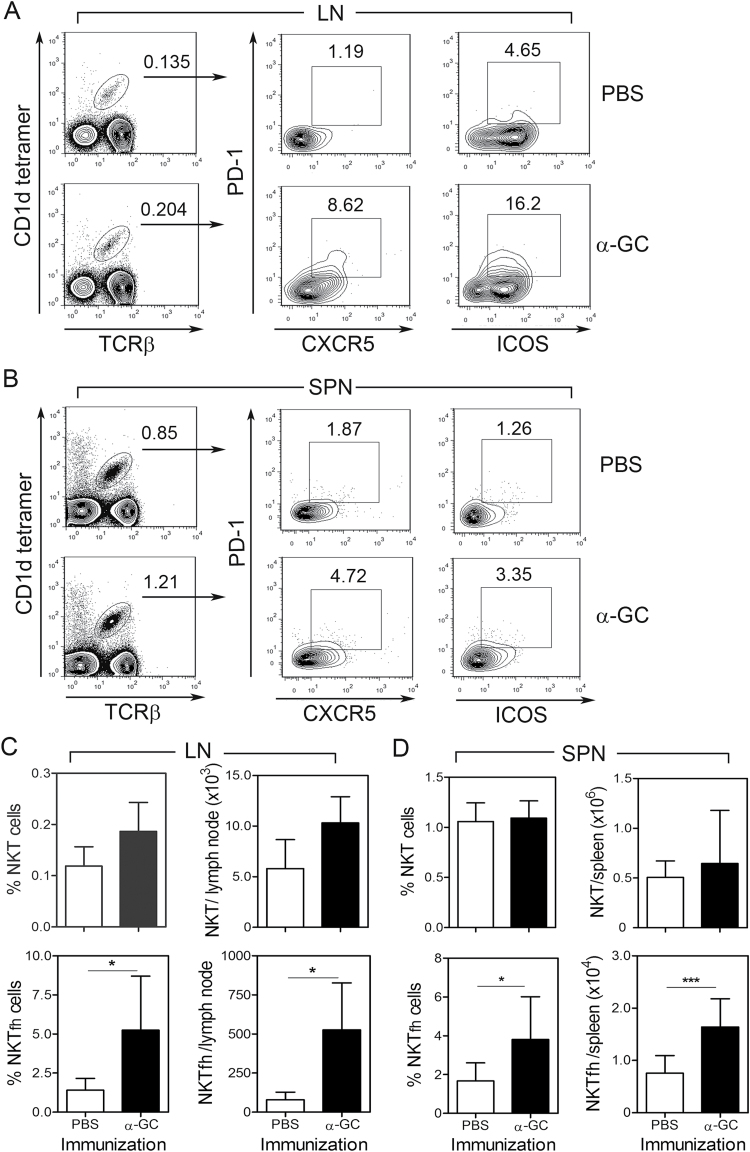
B6 mice were immunized with vehicle (PBS) or α-GC and splenocytes and lymph node cells analyzed by flow cytometry to determine if α-GC affects NKTfh cell numbers (Fig. 1). Following α-GC administration, a CXCR5+/PD1+ and ICOS+/PD-1+ NKTfh population increased in lymph nodes (Fig. 1A) and spleen (Fig. 1B) as compared with vehicle-treated controls. Isotype and fluorescence minus one controls used to delineate the CXCR5+/PD1+ and ICOS+/PD-1+ populations are depicted in Supplementary Figure S1(A and B), available at International Immunology Online. To confirm that the CXCR5+/PD-1+ population observed herein was representative of NKTfh cells, expression of the transcription factor Bcl6 was measured (Supplementary Figure S1C, available at International Immunology Online). Bcl6 expression was detected in all NKT cells, but was significantly higher in the CXCR5+/PD-1+ population than in the CXCR5−/PD-1− population, consistent with previous observations (6).
Fig. 1.
Increase in NKTfh cell numbers in vivo is stimulated by α-GC. Splenocytes and lymph node cells were harvested from B6 mice 7 days after immunization with PBS or α-GC (s.c). Cells were then stained and analyzed by flow cytometry. (A and B) Shows TCRβ+/CD1d tetramer+ NKT cells (left panels) which were further examined for expression of PD-1 and CXCR5 (middle panels) and PD-1 and ICOS (right panels). (A) Depicts lymph node cells and (B) shows splenocytes. (C) Shows mean ± SD frequency and absolute numbers of total NKT and NKTfh cells in lymph node. (D) Shows mean ± SD frequency and absolute numbers of total NKT and NKTfh cells in spleen. Data shown in A–D are each representative of two independent experiments using five mice per group. Statistically significant differences between experimental groups were determined using a two-tailed unpaired t-test (*P < 0.05, ***P < 0.001).
While the increase in PD-1 and CXCR5 expression was evident in splenocytes, the effect was most striking in the lymph nodes, perhaps under the influence of the s.c. immunization route. The PD-1/CXCR5 combination detected fewer cells than the PD-1/ICOS combination, in lymph node and to a lesser extent in spleen. This suggests the presence of additional ICOS-bearing NKT cells that are not true NKTfh cells. As expected, α-GC administration did not stimulate significant increases in total NKT cell frequency and number in lymph nodes (Fig. 1C) and spleen (Fig. 1D). The frequency and number of NKTfh cells increased dramatically and significantly following α-GC administration and was evident in lymph nodes (Fig. 1C) and spleen (Fig. 1D). The degree of increase in NKTfh numbers was noticeably greater than that of total NKT cells. Total NKT cells and NKTfh cells are overwhelmingly CD4+ and CD44hi (4–6). This was also observed in our experiments (data not shown).
CD1d expression regulates α-GC-induced NKTfh generation
CD1d−/− mice do not develop any NKT cells (24). Therefore, determining the effect of CD1d expression on NKTfh induction was achieved by breeding CD1d+/+ B6 controls to CD1d−/− B6 mice to generate CD1d+/− B6 mice (Fig. 2A). As reported by us previously (25), CD1d+/− mice displayed lower CD1d cell surface expression (58%) than B6 controls, but had comparable numbers of NKT cells (Fig. 2A and C). In that study, NKT cells from CD1d+/− and CD1d+/+ mice were shown to have comparable intrinsic function ex vivo, but NKT cells from CD1d+/− mice had ‘weaker’ NKT activation in vivo, attributable to reduced CD1d expression.
Fig. 2.
CD1d expression regulates α-GC-induced NKTfh increases. (A) Shows CD1d expression (histograms on left) in CD1d+/+ and CD1d+/− mice. The bar graph on the right depicts mean ± SD CD1d expression (CD1d+/+ n = 4, CD1d+/− n = 5). (B) Splenocytes were harvested from B6 and CD1d+/− mice 7 days after s.c. immunization with PBS or α-GC. Contour plots depict PD1+/CXCR5+ NKTfh cells (pre-gated on TCRβ+/CD1d tetramer+ NKT cells). (C) Bar graphs show mean ± SD frequency and absolute numbers of total NKT cells (left two panels) and of NKTfh cells (right two panels) (CD1d+/+ n = 5–6, CD1d+/− n = 12). Statistically significant differences between groups were determined by an unpaired t-test (*P < 0.05). Data in C show pooled results from two independent experiments.
B6 and CD1d+/− mice were therefore immunized with PBS or α-GC (Fig. 2B and C). Flow cytometry analyses showed that α-GC led to significant increases in NKTfh cells but not total NKT cells in B6 controls. The response was attenuated in CD1d+/− mice. This shows that CD1d expression levels regulate α-GC-induced increases in the NKTfh subset.
Exogenous IL-2 does not promote or suppress NKTfh expansion in vivo
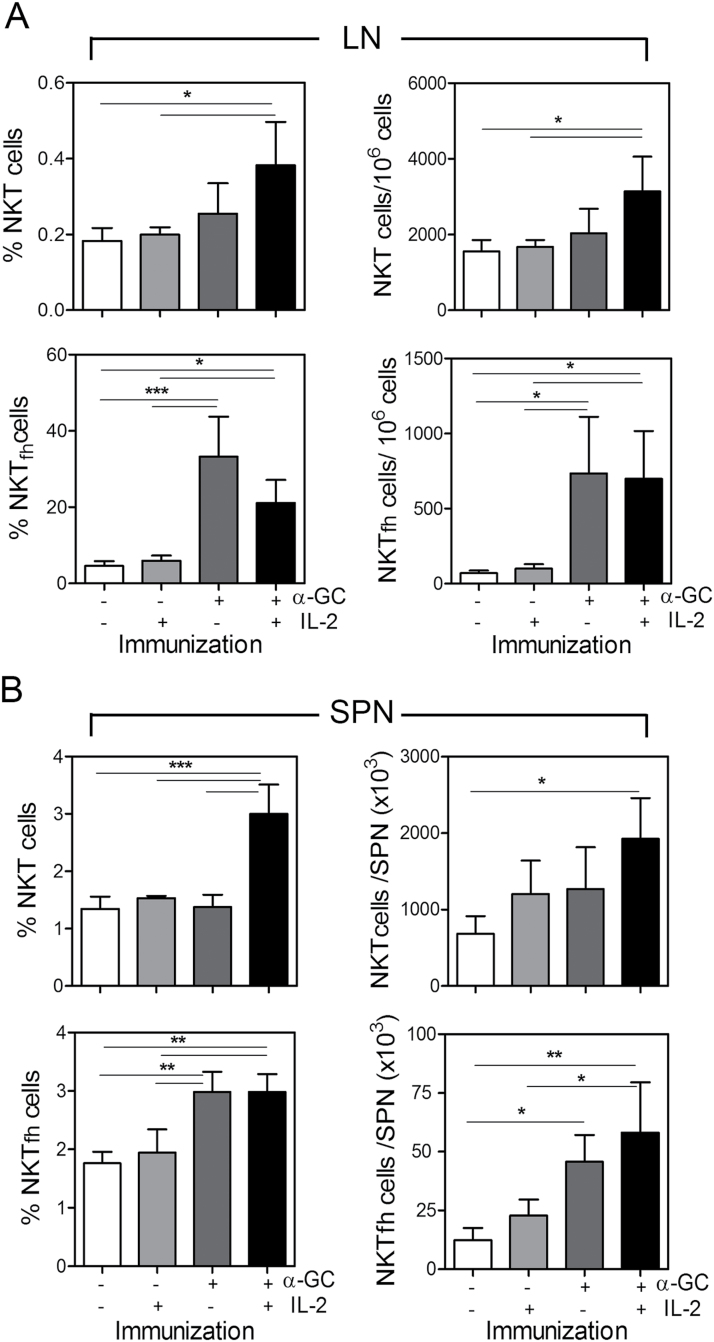
Since IL-2 is known to suppress Tfh differentiation, we included IL-2 treatments in experiments to determine if similar effects on NKTfh cells could be observed. B6 mice were immunized s.c with PBS or α-GC on day 0 and followed 3 days later by repeated injections of PBS or IL-2. On day 7, the total splenic and lymph node CD1d-tetramer+/TCRβ+ NKT cells and PD-1+/CXCR5+ NKTfh cells were enumerated by flow cytometry (Fig. 3). IL-2 administered alone did not significantly change the frequency or number of total NKT cells or NKTfh cells in lymph nodes (Fig. 3A). As shown in other experiments, α-GC significantly increased the frequency and number of NKTfh cells, but had no significant effect on total NKT cell frequency and number. When IL-2 and α-GC were both administered, significant increases in the NKTfh subset and in total NKT cells were observed, but to a greater degree in NKTfh cells. IL-2 plus α-GC did not lead to further increases in NKTfh frequency or numbers as compared with α-GC alone.
Fig. 3.
IL-2 and α-GC differentially stimulate NKT and NKTfh increases in vivo. B6 mice were s.c immunized with PBS or α-GC on day 0. Three days later, PBS or 12000U of IL-2 in PBS were delivered i.p. twice per day for 3 days before isolation of lymphocytes and examination by flow cytometry on day 7. (A) Shows mean ± SD frequency and number of total NKT cells (upper panels) and of NKTfh cells (lower panels) in lymph nodes. (B) Shows mean ± SD frequency and number of total NKT cells (upper panels) and of NKTfh cells (lower panels) in spleen. Data are representative of two independent experiments using four mice per group. One-way analysis of variance (ANOVA) with Bonferroni’s post-test was used to determine significant differences between untreated controls and treatment groups (*P < 0.05, **P < 0.01, ***P < 0.001).
A similar result to that observed in lymph nodes was observed in splenocytes (Fig. 3B). IL-2 administered alone did not increase NKTfh frequency or numbers. As expected, and serving as a control for IL-2 function, treatment with IL-2 led to a reduction in the percentage of TCRβ+/CD4+/CD44hiCXCR5+/PD-1+ Tfh cells (Supplementary Figure S2, available at International Immunology Online). The magnitude of the effect of α-GC alone was less in the spleen than in the lymph nodes. However, α-GC significantly increased the frequency and number of NKTfh cells (numbers increased 3.7-fold as compared with untreated control). In contrast, α-GC had no significant effect on total NKT cell frequency and number (numbers increased 1.85-fold as compared with untreated control) (Fig. 3B). This confirms that NKTfh expansion was greater than total NKT expansion. Administration of IL-2 and α-GC led to a greater increase in frequency and numbers of splenic NKT cells than administration of α-GC alone. Administration of IL-2 plus α-GC did not increase NKTfh cells more than administration of α-GC alone.
These data show that exogenously introduced IL-2 did not suppress or promote differentiation of NKTfh cells either alone or in combination with α-GC. Furthermore, the results suggest that the α-GC-driven increase in NKTfh cells was not solely attributable to a general increase in NKT cells, but to a selective increase in NKTfh cells. This selective increase in NKTfh cells could have potentially arisen from proliferation of existing NKTfh cells and/or differentiation from NKT cells.
NKTfh expansion is a result of differentiation and proliferation
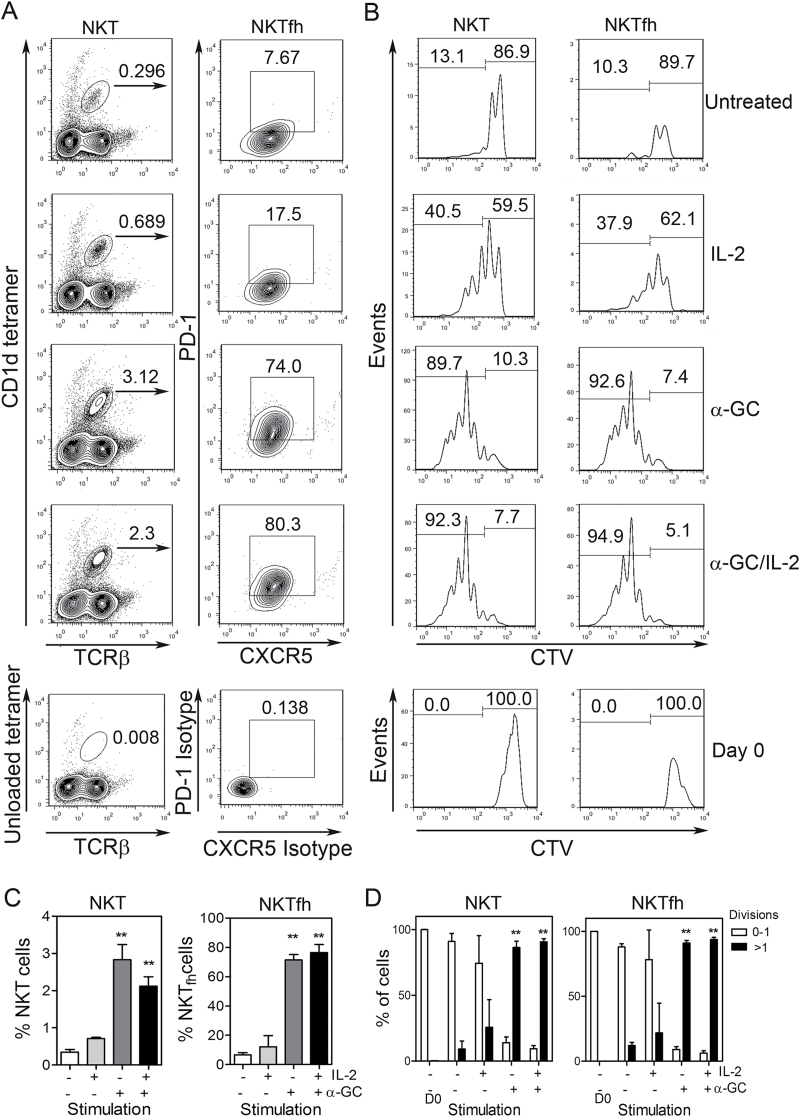
To determine whether NKTfh cells were a product of cellular proliferation and/or differentiation, splenocytes were labeled with CTV before culturing with media, IL-2, α-GC or IL-2 plus α-GC for 3 days (Fig. 4). Flow cytometry was then used to measure total CD1d-tetramer+/TCRβ+ NKT cells and PD-1+/CXCR5+ NKTfh cells (Fig. 4A and C), while CTV dye dilution was used to monitor cell proliferation (Fig. 4B and D).
Fig. 4.
NKTfh cells are a product of proliferation and differentiation in vitro. Splenocytes were labeled with CTV as described in Methods before culturing for 3 days with the treatment conditions indicated. (A) Representative contour plots show CD1d tetramer+/TCRβ+ cells (left panels) and PD-1+/CXCR5+ NKTfh cells (right panels) for each treatment condition. Lowermost panels show the unloaded tetramer control (left) and isotype controls for PD-1 and CXCR5 (right). (B) Panels on left show CTV dye dilution profiles for CD1d tetramer+/TCRβ+ cells (total NKT). Panels on right show the profiles for PD-1+/CXCR5+ NKT cells (NKTfh cells). Lowermost panels show that NKT and NKTfh cells had taken up CTV before the cultures were started. (C) Shows mean ± SD percent NKT cells (left panel) and NKTfh cells (right panels) for two mice per group. One-way analysis of variance (ANOVA) with Bonferroni’s post-test was used to determine significant differences between untreated controls and treatment groups (**P < 0.01). (D) Graphs indicate the mean ± SD percentage of cells with 0–1 divisions (empty bars) and with >1 division (filled bars) cells for two mice per group. NKT cells are depicted in the left panel and NKTfh cells in the right panel. Data are representative of two similar experiments.
In untreated cell cultures, NKT cells were observed at a low frequency, with a similarly low frequency of CXCR5+/PD-1+ NKTfh cells and low PD-1 expression on those cells. Eighty-seven percent of NKT cells had either not divided or divided once, which is consistent with previous studies (21). These cells were designated as ‘0–1 divisions’ (right side of the marker). Thirteen percent of cells had undergone more than one round of division and these were designated as ‘>1 division’ (left side of the marker) (Fig. 4A and B). All other conditions were compared with this baseline.
Addition of IL-2 alone increased the frequency of total NKT cells ~2- to 3-fold, with a corresponding change in the proportion of CXCR5+/PD-1+ cells. An increase in proliferation of cells compared with untreated controls was evident (~41% of NKT cells and ~38% of NKTfh cells), and the proliferation profiles were comparable between NKT cells and NKTfh cells (Fig. 4A and B). This shows that IL-2 alone did not did not selectively expand NKTfh cells as compared with NKT cells and had a modest effect on cellular proliferation (as compared with untreated cultures). Consistent with other experiments, IL-2 alone did not significantly promote NKTfh differentiation.
Addition of α-GC alone led to a significant increase in total NKT cells and the acquisition of the NKTfh phenotype by the majority (~74%) of those cells. Proliferation of NKT cells and NKTfh cells was increased as compared with untreated cells or those cultured with IL-2 alone and the dye-dilution profiles were near-identical in both populations. Addition of α-GC alone led to ~90% of cells undergoing division, with most having undergone more than one round of division (Fig. 4B). However, it should be noted that complete dilution of the CTV dye was not observed and therefore that α-GC-induced proliferation was not maximal. In a previous study, supplementation of α-GC-stimulated NKT cultures with IL-2 resulted in more rounds of division per NKT cell even though most cells were already dividing in response to α-GC (21). It was thus possible to determine whether addition of α-GC plus IL-2 to the cultures had any further effect on the number of divisions per cell, as opposed to the proportion of cells dividing. As shown in Fig. 4(B), the combination of IL-2 and α-GC and did not cause further rounds of CTV dilution as compared with α-GC alone. The representative data shown in Fig. 4(A and B) are graphically represented in Fig. 4(C and D). Analysis of cells after 42h in culture showed minimal cellular proliferation or NKTfh differentiation, suggesting that these events took place in the latter part of the 72h time course shown in Fig. 4 (data not shown).
Therefore, neither IL-2 nor α-GC selectively promoted proliferation of NKTfh cells relative to total NKT cells. However, the proportion of NKT cells that were NKTfh cells was much higher with α-GC or with α-GC plus IL-2 (~80%) when compared with media alone (<18%). These data show that increases in NKTfh frequencies were attributable to both differentiation and proliferation.
NKTfh cells can differentiate from non-NKTfh cells
To further demonstrate that NKTfh cells were a product of differentiation and proliferation, splenocytes from Vα14 Tg mice were simultaneously enriched for NKT cells while removing NKTfh cells (Fig. 5A). This was achieved by depleting PD-1- and B220-bearing cells, the efficiency of which was such that no NKT cells were detected among the residual PD-1- or B220-expressing cells following depletion (Supplementary Figure S3, available at International Immunology Online).
The PD-1-depleted cells were CTV-labeled and adoptively transferred into CD45.1 congenic recipient mice before i.p. immunization with PBS or α-GC. The splenocytes were isolated 3 days after immunization and analyzed by flow cytometry (Fig. 5B). It was observed that the donor-derived total NKT population constituted a greater percentage of the total donor-derived cells in α-GC-treated recipients than in PBS-treated recipients (Fig. 5B). Enumeration of total donor-derived NKT cells recovered from recipient mice also demonstrated their expansion (Fig. 5D). Examination of the CTV dye-dilution profiles showed that donor-derived total NKT cells underwent proliferation following α-GC treatment and that this was not observed in the PBS-treated group (Fig. 5B). Quantification of divided versus nondivided cells confirmed the stark difference in dye-dilution profiles of donor-derived total NKT cells following PBS or α-GC immunization (Fig. 5C).
Donor-derived NKT cells were also examined for the presence of CXCR5+ and PD-1+ NKTfh cells. Less than 10% of donor-derived NKT cells exhibited the NKTfh phenotype in the PBS-immunized group (Fig. 5B). Notably, the expression of PD-1 was very low in the cells detected. In contrast, immunization with α-GC led to approximately half of the donor-derived NKT cells exhibiting the NKTfh phenotype and the PD-1 expression was considerably higher than in the PBS-treated group (Fig. 5B and D). Examination of the dye-dilution profiles revealed that the donor-derived NKTfh cells have a near-identical degree of proliferation to the total donor-derived NKT cells (Fig. 5B), which was also reflected in quantification of divided versus nondivided NKTfh cells (Fig. 5C).
An important internal control was employed in this experiment, whereby endogenous NKT cells expanded in response to α-GC immunization (Fig. 5B, inset panel). The degree of increase in endogenous NKT cells was greater than observed in previous experiments, which is attributable to the i.p. route of immunization used. The i.p. immunization was required to ensure stimulation of transferred NKT cells. Consequently, NKT increases in the lymph nodes were less impressive following i.p. immunization than the s.c. immunizations employed earlier in the study. However, despite the low numbers of donor-derived NKT cells that were detected in the lymph nodes, it was observed that total donor-derived NKT cells and NKTfh cells yielded similar results to those observed after examination of recipient mouse spleens (data not shown). The data therefore show that some donor-derived peripheral NKT cells (devoid of NKTfh cells) had the capacity to differentiate into NKTfh cells following treatment with α-GC. Indeed, while donor-derived total NKT cells expanded 6.16-fold following α-GC treatment, NKTfh cells expanded 102.6-fold.
Treatment with α-GC did not increase NKTfh cell numbers in the thymus
In a further experiment, we examined the thymus for NKT cells expressing the NKTfh markers PD-1 and CXCR5 (Supplementary Figure S4A, available at International Immunology Online). Expression of PD-1 was lower than that observed for CXCR5+/PD-1+ cells in the periphery. The frequency and numbers of thymic CXCR5+/PD-1lo NKT cells did not increase following immunization with α-GC (Supplementary Figure S4B, available at International Immunology Online).
Discussion
The recent discovery of NKTfh cells was coupled to observations that they had the potential to interact with B cells and influence humoral immunity (4–6). However, very little is known regarding how increases in their numbers following immunization are regulated. In this study, we demonstrated that increases induced by α-GC were the product of proliferation of NKT cells and differentiation into NKTfh cells. We observed that treatment with α-GC led to a modest yet significant expansion in the spleen and a profound expansion in the lymph nodes following s.c. injection. I.p. injections caused greater NKTfh expansion in the spleen in our adoptive transfer experiments. While the study was not designed to compare immunization routes, it does seem that this is a factor in the magnitude of NKTfh expansion observed. It was also observed that lowering CD1d expression in vivo, which leads to attenuated NKT activation (25), resulted in attenuation of the α-GC-induced increase in NKTfh cells. This demonstrates that changes in TCR engagement by CD1d/glycolipid complexes alter NKTfh expansion. In a previous study (4), PD-1- and CXCR5-associated signals were brighter than reported herein. As well as different instrumentation and settings, the higher fluorescence intensity in that study is likely attributable to PE-conjugated anti-PD-1 and a three-step detection of CXCR5.
It has been shown that IL-2 inhibits the generation of conventional Tfh cells (23), thus we tested the effect of IL-2 on NKTfh expansion. Our in vivo and in vitro studies show that IL-2 did not suppress the α-GC-induced increase in NKTfh cells. NKT cells develop in the thymus from CD1d-expressing immature thymocytes and the key steps in their development have been delineated (reviewed in ref. 26). One remaining issue however, concerns whether diverse functional NKT subsets (Th1, Th2 and Th17) represent plasticity in the periphery, lineage commitment or both. Numerous studies show that the α-GC stimulates NKT cells to simultaneously secrete IL-4 and IFN-γ supporting arguments for plasticity (reviewed in ref. 27). Indeed, different versions of the α-GC molecule have been synthesized such that NKT-derived cytokine profiles are skewed toward Th1 or Th2 (28). While different CD1d ligands could differentially expand a pre-committed Th1-, Th2- or Th17 NKT cell, they could also differentially direct cell fate in the periphery. A recent study by the Hogquist laboratory showed that in BALB/c mice, Th2-skewed NKT cells could not be driven to the Th1 phenotype, arguing for distinct lineage commitment in the thymus (29). Furthermore, subsetting thymic NKT cells by profiling transcription factors T-bet, GATA-3 and RORγT supported the hypothesis that there are distinct NKT lineages (29). The same study referred to an unpublished observation by the authors that they did not detect substantial numbers of NKTfh cells in the thymus. Our observation of thymic CXCR5+/PD-1lo NKT cells appears consistent with the observation of the Hogquist group. It seems at this stage that available data neither precludes nor supports the possibility of NKTfh lineage commitment in the thymus and therefore the presence of a population that on export to the periphery could expand following stimulation.
We analyzed the effects of IL-2 and α-GC on NKTfh expansion relative to the total Type I NKT population. Through in vivo and in vitro assays, we deduced that increases in the NKTfh population following α-GC treatment were at least in part attributable to differentiation of NKT cells into NKTfh cells. This is because the proliferation profiles of NKTfh cells matched those of total NKT cells, but there were larger increases in representation by the NKTfh subset.
Adoptive transfer of PD-1-depleted NKT cells into congenic recipients was used to demonstrate that cells could acquire the NKTfh phenotype and that this effect could be further boosted by α-GC administration to the recipient mouse. It is possible that in vivo homeostasis maintains a ‘baseline’ frequency of NKTfh cells as is observed in untreated mice. This suggests that other factors or a self CD1d ligand could be responsible for this. Stimulation of α-GC could therefore further expand a population that is committed to NKTfh differentiation. Arguably, the PD-1-depleted cells that were adoptively transferred could contain NKT cells committed to the NKTfh differentiation program but not displaying the PD-1+ phenotype. Most NKT cells display a low level of CXCR5 expression and some of them could be NKTfh-committed cells. However, flow cytometry did not detect any contaminating NKTfh cells in the donor NKT population used for adoptive transfer (Fig. 5 and Supplementary Figure S3, available at International Immunology Online). If the flow cytometry analysis had failed to detect one or two NKTfh rare events, then every contaminating cell would still need to divide at least 14–15 times to account for the ~50000 donor-derived NKTfh cells detected in just one location. In 3 days, this is unlikely and was not reflected in the data obtained because 3–5 divisions were evident for most NKTfh cells. Furthermore the fold change in NKTfh expansion outstripped that of the total donor-derived NKT expansion. Therefore, the evidence is consistent with a model in which α-GC leads to both the proliferation of peripheral NKT cells and further differentiation into NKTfh cells. It should be noted that the necessary i.p. immunization following NKT adoptive transfer could lead to anergy which is avoided in the majority of our experiments using s.c. immunizations. The possibility of NKTfh cell anergy is one that warrants exploration in future studies.
Our results therefore show that NKTfh cells are a product of peripheral NKT cell differentiation and proliferation. Future studies will determine whether multiple NKT lineages can differentiate into multiple subsets of NKTfh cells or if a single thymus-derived, lineage-committed precursor is required for NKTfh expansion in the periphery.
Supplementary data
Supplementary data are available at International Immunology Online.
Funding
National Institutes of Health (USA) grant (AI078993); Oklahoma Center for Advancement of Science and Technology grant (HR012-005); American Association of Immunologists Careers in Immunology Fellowship.
Supplementary Material
Acknowledgements
We thank the NIAID Tetramer Facility (Emory University, Atlanta, GA, USA) for providing CD1d tetramers. We thank Dr Noah Butler (University of Oklahoma Health Sciences Center) for reading the manuscript and providing helpful comments. We are also grateful to Mrs Gillian Lang and Mr Scott Devera for technical assistance.
Conflict of interest statement: The authors declare no commercial or financial conflict of interest.
References
- 1. Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29:621. [DOI] [PubMed] [Google Scholar]
- 2. McHeyzer-Williams L. J., McHeyzer-Williams M. G. 2005. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23:487. [DOI] [PubMed] [Google Scholar]
- 3. Spolski R., Leonard W. J. 2010. IL-21 and T follicular helper cells. Int. Immunol. 22:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang P. P., Barral P., Fitch J., et al. 2012. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat. Immunol. 13:35. [DOI] [PubMed] [Google Scholar]
- 5. King I. L., Fortier A., Tighe M., et al. 2012. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat. Immunol. 13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tonti E., Fedeli M., Napolitano A., et al. 2012. Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4(+) T cell help. J. Immunol. 188:3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taniguchi M., Seino K., Nakayama T. 2003. The NKT cell system: bridging innate and acquired immunity. Nat. Immunol. 4:1164. [DOI] [PubMed] [Google Scholar]
- 8. Kronenberg M. 2005. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 23:877. [DOI] [PubMed] [Google Scholar]
- 9. Brigl M., Tatituri R. V., Watts G. F., et al. 2011. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med. 208:1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rhost S., Sedimbi S., Kadri N., Cardell S. L. 2012. Immunomodulatory type II natural killer T lymphocytes in health and disease. Scand. J. Immunol. 76:246. [DOI] [PubMed] [Google Scholar]
- 11. Jahng A., Maricic I., Aguilera C., Cardell S., Halder R. C., Kumar V. 2004. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 199:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah H. B., Devera T. S., Rampuria P., Lang G. A., Lang M. L. 2012. Type II NKT cells facilitate Alum-sensing and humoral immunity. J. Leukoc. Biol. 92:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao J., Weng X., Bagchi S., Wang C. R. 2014. Polyclonal type II natural killer T cells require PLZF and SAP for their development and contribute to CpG-mediated antitumor response. Proc. Natl Acad. Sci. USA 111:2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galli G., Pittoni P., Tonti E., et al. 2007. Invariant NKT cells sustain specific B cell responses and memory. Proc. Natl Acad. Sci. USA 104:3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lang G. A., Exley M. A., Lang M. L. 2006. The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology 119:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Devera T. S., Shah H. B., Lang G. A., Lang M. L. 2008. Glycolipid-activated NKT cells support the induction of persistent plasma cell responses and antibody titers. Eur. J. Immunol. 38:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Devera T. S., Aye L. M., Lang G. A., Joshi S. K., Ballard J. D., Lang M. L. 2010. CD1d-dependent B-cell help by NK-like T cells leads to enhanced and sustained production of Bacillus anthracis lethal toxin-neutralizing antibodies. Infect. Immun. 78:1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Devera T. S., Joshi S. K., Aye L. M., Lang G. A., Ballard J. D., Lang M. L. 2011. Regulation of anthrax toxin-specific antibody titers by natural killer T cell-derived IL-4 and IFNγ. PLoS One 6:e23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ko S. Y., Ko H. J., Chang W. S., Park S. H., Kweon M. N., Kang C. Y. 2005. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J. Immunol. 175:3309. [DOI] [PubMed] [Google Scholar]
- 20. Bai L., Deng S., Reboulet R., et al. 2013. Natural killer T (NKT)-B-cell interactions promote prolonged antibody responses and long-term memory to pneumococcal capsular polysaccharides. Proc. Natl Acad. Sci. USA 110:16097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uldrich A. P., Crowe N. Y., Kyparissoudis K., et al. 2005. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J. Immunol. 175:3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parekh V. V., Wilson M. T., Olivares-Villagómez D., et al. 2005. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J. Clin. Invest. 115:2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ballesteros-Tato A., León B., Graf B. A., et al. 2012. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 36:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Exley M. A., Bigley N. J., Cheng O., et al. 2003. Innate immune response to encephalomyocarditis virus infection mediated by CD1d. Immunology 110:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lang G. A., Johnson A. M., Devera T. S., Joshi S. K., Lang M. L. 2011. Reduction of CD1d expression in vivo minimally affects NKT-enhanced antibody production but boosts B-cell memory. Int. Immunol. 23:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Godfrey D. I., Stankovic S., Baxter A. G. 2010. Raising the NKT cell family. Nat. Immunol. 11:197. [DOI] [PubMed] [Google Scholar]
- 27. Taniguchi M., Harada M., Kojo S., Nakayama T., Wakao H. 2003. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21:483. [DOI] [PubMed] [Google Scholar]
- 28. Venkataswamy M. M., Porcelli S. A. 2010. Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Semin. Immunol. 22:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee Y. J., Holzapfel K. L., Zhu J., Jameson S. C., Hogquist K. A. 2013. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 14:1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.