Abstract
Background
Low dose X-irradiation (IR) from computer tomography (CT) can generate free radicals, which can damage biologically relevant molecules and ultimately lead to cancer. These effects are especially concerning for children owing to their higher radiosensitivity and longer life expectancy than adults. The lipid phase micronutrients (LPM) coenzyme Q10, carotenoids, E vitamers, and vitamin A are potent radical scavengers that can act as intracellular antioxidants.
Methods
We investigated changes in circulating levels of these LPM in 17 children (0.25–6 y) undergoing medically indicated CT scans involving relatively low IR doses. Blood was drawn before and 1 h after CT scans and analyzed using HPLC with electrochemical and UV/VIS detection.
Results
We found significant decreases (p < 0.05) in post-CT plasma levels in several LPM which suggests that these LPM can serve as biodosimeters and may protect against damage from IR during clinical procedures such as CT. The strongest predictors for pre- to post-CT changes for many LPM were their baseline levels.
Conclusion
Future larger studies are warranted to confirm our findings and to test whether high circulating antioxidant levels protect against IR damage in vivo with an ultimate goal of establishing prophylactic modalities for CT-induced IR damage.
Keywords: Computer tomography, Children, Coenzyme Q10, Carotenoids, Tocopherols, Retinol
Introduction
Computed tomography (CT)1 is an essential tool of modern medicine as it allows rapid, painless, and accurate imaging of most organ systems. The application of CT, however, involves significant exposure to ionizing X-irradiation (IR), which can elicit detrimental cellular effects, such as DNA lesions and protein cross-links, either directly through the ionization of DNA or indirectly through radicals such as reactive oxygen species (ROS) [1]. These effects can significantly increase the risk of developing cancer [2,3], especially in children owing to their higher radiosensitivity from rapidly dividing cells, higher risk of cumulative exposure, and longer remaining lifespan compared to adults [4–6]. Current estimations suggest that CT scans lead to an increased lifetime cancer risk of up to 3% with the highest risk for very young children [2,7].
CT use has risen substantially over the past few decades, particularly in children [2]. This increase has been attributed largely to technological advances in CT, such as improved resolution, faster image acquisition times, and the reduced need for sedation [8,9]. While some investigators have reported a decrease in rise in CT use in recent years [10,11], the incidence of pediatric CT exams is presently still very high, which raises special concerns about the long-term risks associated with diagnostic IR-based techniques in children.
Coenzyme Q10 (Q10), carotenoids, tocopherols, and retinol are lipid phase micronutrients (LPM) known to function as important antioxidant agents that help to mitigate the damaging effects of oxidative and other injuries. Q10 occurs in humans primarily through cellular biosynthesis with limited dietary exposure [12] and functions in cells as electron/proton carriers during cellular respiration [13–15]. Ubiquinol-10 (UL10), the chemically reduced form of Q10, is considered a free radical scavenger that protects cells against oxidative injury and stress [15] and minimizes damage to low-density lipoproteins (LDL) in vitro by dehydrogenating to ubiquinone-10 (UN10) [16–25]. Thus, the UL10/UN10 and UN10/total Q10 (TQ10) ratios have been postulated to serve as useful measures of oxidative damage [25–28], whereas TQ10 may represent general physiological events such as cell death as a result of dying cells releasing Q10 into the circulation [17,29–31].
Pro vitamin A and other carotenoids, tocopherols, and retinol are LPM that function as important antioxidants via neutralizing reactive oxygen species (ROS) and reactive nitrogen species thereby reducing oxidative stress and/or preventing oxidative damage [25,32–37]. Evidence from epidemiological and clinical reports support a central role for these LPM in protecting against a wide array of chronic conditions [38–42] through a variety of protective mechanisms [34,43,44]. However, the effect of CT-induced IR changes in the levels of LPM in children is unknown.
In this pilot study, our aim was to determine whether the plasma levels of LPM change in young children after they received medically indicated CT exams involving relatively low IR doses between 0.78 and 11.30 millisieverts (mSv).
Methods
Patient recruitment
Seventeen pediatric patients (0.25–6 years old) undergoing medically indicated CT scans in the emergency department (ED) of a hospital participated in this study after signed consents from their parent or legal guardian were obtained. These children were recruited for the study either through direct admission to the ED or through private physicians in the radiology department. Exclusion criteria included children with immediate risk of decompensation, children weighing less than 9 lbs, and children with complex medical problems such as cancer. Information regarding the child’s age, birth history, past medical history, medication use, ethnicity, overall health condition, allergies, height and weight, vitamin intake, and a detailed radiological history was obtained from interviews with the parent or legal guardian and also through retrieval of hospital records. Blood draw times, CT scan times, and CT doses (in mSv) were also documented. This study was approved by the Western Institutional Review Board and the University of Hawaii Committee on Human Services and was performed in accordance with the ethical standards laid down in the Declaration of Helsinki.
Sample collection and processing
Peripheral whole blood (2.5–4.0 ml) was drawn by venipuncture into sodium heparin vacutainer® tubes from each child immediately before (‘pre-CT’) and one hour after (‘post-CT’) their scheduled CT exams. When time allowed, ELMA cream was used to minimize pain during venipuncture. If a normal saline IV lock was in place for medical reasons, ca. 2.5 mL of blood was withdrawn then discarded before obtaining blood for the study. For children younger than and up to 2 years of age, ca. 2.5 mL of blood was collected both pre- and post-CT; for all other children the collected volumes for both collections were ca. 4 mL. After the CT exams, both pre- and post-CT tubes were transferred immediately to the University of Hawaii Cancer Center (UHCC) laboratory in a sealed leak proof bag in a biohazard cooler on ice and protected from light. Upon arrival to the laboratory, blood tubes were immediately centrifuged at 1050×g for 20 min at 4 °C. After centrifugation, plasma was aliquoted into cryotubes then stored at −80 °C until analysis. All procedures at the UHCC were conducted under dimmed or yellow lighting to avoid analyte photodegradation.
Chemicals and reagents
Butylated hydroxytoluene (BHT), bis-tris-propane (BTP), lithium acetate dehydrate, and ubiquinone-10 (UN10) were purchased from Sigma Aldrich (St. Louis, MO). Tocol was purchased from Matreya, Inc. (Pleasant Gap, Pa.). Ethanol (EtOH) (100%) was obtained from Pharmco (Brookfield, CT). Acetonitrile (MeCN), dichloromethane (DCM), glacial acetic acid (AA), hexane, and methanol (MeOH) were HPLC grade and purchased from Fisher (Pittsburgh, PA). Hexane and MeOH were kept refrigerated before and during extractions. Ultrapure deionized water (H2O) was used throughout. Retinyl laurate, δ-tocopheryl laurate, n-butyl-β-apo-8′-carotenoate, and butylated UL10 (Bu-UL10) and UN10 (Bu-UN10) were synthesized as previously described [45–47].
Extraction and analyses of UL10 and UN10
For quantification of UL10 and UN10 we followed our previously established procedure [45]. In brief, 200 µL thawed plasma spiked with Bu-UL10 and Bu-UN10 internal standards were extracted with 200 µL chilled hexane immediately after thawing by vortexing for 1.5 min followed by centrifugation at 5042×g for 5 min at 5 °C. Thereafter 2 µL of the hexane layer was injected into an HPLC system (Model Surveyor; ThermoFisher, San Jose, CA, USA) consisting of a Kinetex C18 column (150 mm × 3.0 mm, 2.6 µm; 100A; Phenomenex, Torrance, CA) preceded by a filter cartridge (21 mm, 0.2 µm; Thermo Scientific, Bellefonte, PA). Isocratic elution was performed using a mobile phase of 85:15 A/B (v/v) where A: 9.4 mM lithium acetate dihydrate in MeOH:MeCN:H2O:AA (70/30/1/0.0.5; v/v) and B: DCM, at a flow rate of 0.5 mL/min. Detection was carried out by UV monitoring at 275 and 290 nm followed by coulometric detection using a model 5020 guard cell (ESA, Chelmsford, MA, USA) at +700 mV and a model 5011A analytical cell (ESA) at −700 mV on channel 1 and +800 mV on channel 2. Concentrations of UL10 and UN10 were determined from external calibration curves after adjusting UL10 and UN10 for recovery of Bu-UL10 and Bu-UN10, respectively. TQ10 levels were calculated by summation of UL10 and UN10 levels. Inter-and intra-assay variability of UL10 and UN10 was 8% (820 nM) and 11% (110 nM), respectively.
Extraction and analyses of carotenoids, tocopherols, and retinol
Measurements of carotenoids, tocopherols, and retinol were performed using an established HPLC assay with minor modifications, validated previously in our laboratory, applying photo diode array detection [46]. Briefly, 300 µL freshly thawed plasma was mixed with 300 µL EtOH containing BHT and spiked with the internal standards tocol or δ-tocopheryl laurate (for tocopherols), retinyl laurate (for retinol), and n-butyl-β-apo-8′-carotenoate (for carotenoids) [47,48]. Samples were then extracted with 1.5 mL hexane by vortexing for 1 min. The hexane layer was evaporated in HPLC amber vials at room temperature under a stream of N2 followed by reconstitution in 150 µL mobile phase (see below). Twenty microliters of the reconstituted extract was injected into an RP-HPLC system (Model Surveyor; ThermoFisher, San Jose, CA, USA) consisting of a Spherex C18 analytical column (150 mm × 3.2 mm, 3 µm) coupled to a Spherex C18 pre-column (4 mm × 3.0 mm, 10 µm) (both columns from Phenomenex; Torrance, CA). Isocratic elution was performed using a mobile phase consisting of 1.14 mM BHT in MeOH/DCM/MeCN/0.5M (pH7). BTP (65/25/10/2; v/v) at a flow rate of 0.3 mL/min. All extraction and handling procedures were carried out under yellow light to avoid analyte degradation. Concentrations of carotenoids, tocopherols, and retinol were determined by monitoring at 450 nm, 295 nm, and 325 nm respectively, and by quantitating using peak areas and external calibration curves after adjusting for recovery of the appropriate internal standard.
Assay validation
All HPLC assays were validated by participation in quality assurance programs for fat soluble vitamins organized by the National Institute for Standards and Technology (Gaithersburg, MD).
CT parameters
CT examinations were performed using a LightSpeed VCT Select 64 slices CT scanner. The radiation dose is often expressed in gray/milligrays (mGy) or sieverts/millisieverts (mSv). One gray is the absorption of one joule of energy, in the form of ionizing radiation, per kilogram of matter, e.g. human tissue. The gray measures the absorbed energy of radiation, but the biological effects vary by the type and energy of the radiation and the organ involved. The dose as defined by the biological effects is expressed by the mSv, also called the effective dose, which has the same dimensions as the gray, but is a measure of the potential for damage to human tissue and therefore is the preferred unit for risk estimation [2]. For X-ray radiation, which is the type used in CT scanners the gray is numerically the same value when expressed as sieverts.
The effective organ dose for our patients ranged from 0.78 to 11.30 mSv. The radiation history of each child was expressed as dose in relative numbers according to published data of regulatory and other agencies: each head, chest, and abdominal or pelvic CT is 1.0, 4.0, and 5.0 units, respectively; each X-ray is 0.01 units for a posterior-anterior chest X-ray, 0.02 units for a lateral chest X-ray, and 0.35 units for an abdominal or a pelvic X-ray [49–51].
Statistical analysis
Data analyses were performed using SAS 9.3 statistical software (SAS Institute, Cary, NC). Pre- to post-CT sample comparisons were performed using Student’s paired sample t-test. Stepwise multiple regression with the forward entry method was performed using the Reg procedure in two separate analyses. In the first analysis, the outcome variable was the pre to post-CT change (or delta) and the possible predictors were baseline (pre) values, effective dose in mSv, radiation history, multivitamin use, contrast, CT helical, sex, and age. In the second analysis, the outcome variable was the baseline (pre) value and the possible predictors were radiation history, multivitamin use (yes, no) and contrast (with, without). The significance level was set at p < 0.05.
Results
In this pilot study blood samples from 17 children were obtained immediately before and 1 h after undergoing medically indicated CT scans. Characteristics of the participants are presented in Table 1. The children ranged in age from 3 months to 6 years. Twelve children received CT scans of the head region while the remaining children received CT scans of either the abdomen (n = 3), neck (n = 1) or chest (n = 1) region. The CT and effective doses ranged from 92.46 to 525.55 mGy-cm, equivalent to 0.78 to 11.30 mSv, respectively.
Table 1.
Demographics of study participants.
| Patient ID |
Gender | Height (cm) |
Weight (kg) |
Age (m) |
Effective dose (mSv) |
CT location | CT type |
Contrast use |
Multivitamin intake |
Radiation history (dose in relative numbers)a |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 88 | 11.9 | 24 | 2.50 | Head | Axial | No | Yes | 0.02 |
| 2 | M | 84.0 | 9.8 | 14 | 2.50 | Head | Axial | No | No | 0.50 |
| 3 | M | 99.0 | 14.5 | 24 | 11.30 | Abdomen | Helical | Yes | No | 1.02 |
| 4 | M | 95.0 | 13.9 | 36 | 4.42 | Abdomen-Pelvis | Helical | Yes | No | 0.01 |
| 5 | M | 118.0 | 28.0 | 60 | 2.08 | Abdomen-Pelvis | Helical | Yes | No | 0.05 |
| 6 | F | 96.0 | 14.0 | 36 | 2.28 | Head | Axial | No | Yes | 0.02 |
| 7 | M | 65.0 | 12.0 | 17 | 2.08 | Head | Axial | No | No | 2.53 |
| 8 | M | 123.0 | 40.6 | 72 | 1.42 | Orbitis | Axial | Yes | No | 2.05 |
| 9 | F | 118.0 | 20.0 | 60 | 0.78 | Orbitis | Axial | Yes | Yes | 0.00 |
| 10 | F | 102.0 | 14.7 | 48 | 2.86 | Head | Axial | Yes | Yes | 4.37 |
| 11 | M | 105.0 | 15.5 | 48 | 2.86 | Head | Axial | No | Yes | 0.02 |
| 12 | M | 102.0 | 18.6 | 72 | 2.10 | Head | Axial | No | No | 14.12 |
| 13 | M | 112.0 | 19.0 | 48 | 6.14 | Mastoid bone | Axial | Yes | No | 1.00 |
| 14 | F | 114.0 | 18.7 | 48 | 1.28 | Chest | Helical | Yes | No | 0.05 |
| 15 | M | 65.0 | 7.1 | 3 | 1.57 | Neck | Helical | Yes | No | 0.03 |
| 16 | M | 92.0 | 12.1 | 21 | 2.86 | Head | Axial | No | Yes | 1.01 |
| 17 | M | 68.0 | 11.2 | 15 | 2.28 | Head | Axial | No | Yes | 0.00 |
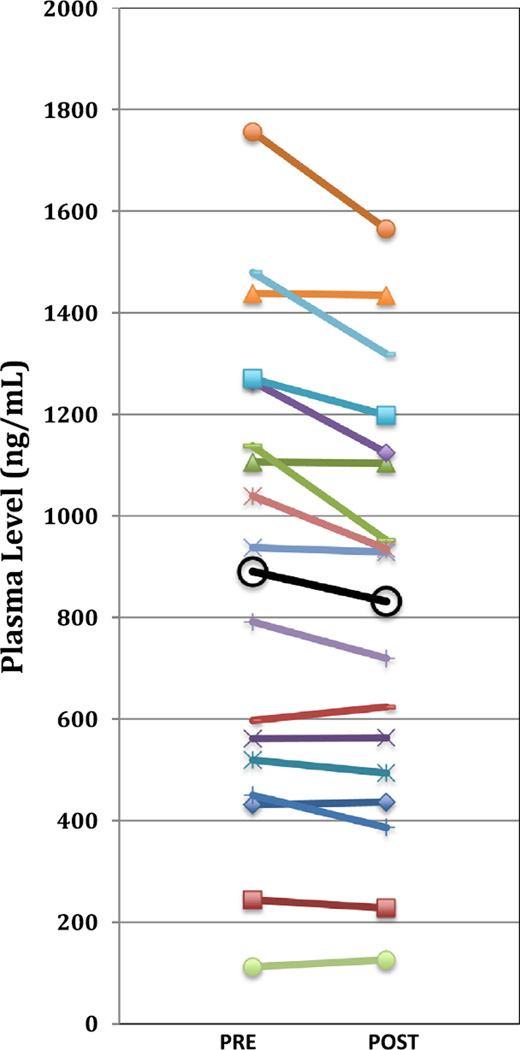
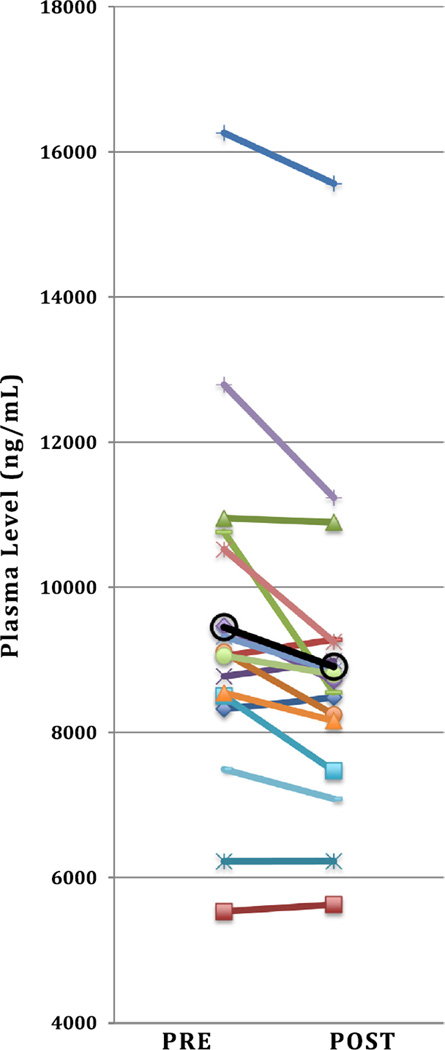
Applying paired t-tests we found significant decreases in mean post- vs. pre-CT plasma levels of many LPM including the carotenoids trans lutein (p = 0.03), total trans lutein/zeaxanthin (p = 0.03), trans anhydro-lutein (p = 0.01), α-cryptoxanthin (p = 0.047), trans β-cryptoxanthin (p = 0.02), total lycopene (p = 0.03), trans lycopene (p = 0.02), 5-cis lycopene (p = 0.03), α-carotene (p = 0.04), trans β-carotene (p = 0.01), total β-carotene (p = 0.01). In addition to total carotenoids (p = 0.004), the E vitamers β– + γ-tocopherol (p = 0.04), α-tocopherol (p = 0.01), and total tocopherols (p = 0.005) were also observed to be lower post CT examination (Table 2). Fig. 1 and 2 illustrate pre-to post-CT differences for total carotenoids and total tocopherols, respectively, for each individual child. In contrast, plasma levels of Q10 compounds UN10 and UL10 were found to increase post-CT (from 62 to 68 nM and 344 to 427 nM, respectively) while the UN10/TQ10 ratio decreased from 17% to 13%, however, these differences were not significant (Table 2).
Table 2.
Pre- vs. post-CT changes in plasma LPM levels.*
| Analyte | Pre-CT | Post-CT | Post- to Pre-CT | ||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean (%) | r | p | |
| UL10 (nM) | 344 ± 232 | 427 ± 221 | 124 | 0.55 | 0.17 |
| UN10 (nM) | 62 ± 49 | 68 ± 53 | 108 | 0.93 | 0.35 |
| TQ10 (nM) | 406 ± 258 | 495 ± 254 | 122 | 0.63 | 0.16 |
| UN10/TQ10 (%) | 17 ± 0 | 13 ± 0 | 76 | 0.56 | 0.18 |
| tr LUT (ng/ml) | 65 ± 33 | 63 ± 31 | 96 | 0.99 | 0.03 |
| tr ZEA (ng/ml) | 21 ± 10 | 21 ± 10 | 97 | 0.99 | 0.09 |
| Tot. tr LUT/ZEA (ng/ml) | 87 ± 42 | 83 ± 40 | 96 | 0.99 | 0.03 |
| Tot. cis LUT/ZEA (ng/ml) | 44 ± 22 | 44 ± 22 | 99 | 0.99 | 0.46 |
| tr AH-LUT (ng/ml) | 29 ± 15 | 27 ± 14 | 95 | 0.99 | 0.01 |
| cis AH-LUT (ng/ml) | 18 ± 10 | 17 ± 9 | 96 | 0.99 | 0.10 |
| αCRX (ng/ml) | 18 ± 8 | 17 ± 8 | 95 | 0.98 | 0.047 |
| tr βCRX (ng/ml) | 103 ± 86 | 96 ± 78 | 92 | 0.99 | 0.02 |
| cis βCRX (ng/ml) | 28 ± 20 | 27 ± 19 | 97 | 0.97 | 0.54 |
| Tot. LYCOP (ng/ml) | 395 ± 308 | 365 ± 273 | 92 | 0.99 | 0.03 |
| tr LYC (ng/ml) | 119 ± 89 | 111 ± 83 | 93 | 0.99 | 0.02 |
| 5 cis lyc (ng/ml) | 183 ± 158 | 167 ± 138 | 92 | 0.99 | 0.03 |
| DHLYC (ng/ml) | 93 ± 72 | 87 ± 63 | 93 | 0.99 | 0.06 |
| αCAR (ng/ml) | 32 ± 32 | 30 ± 31 | 94 | 0.99 | 0.04 |
| tr βCAR (ng/ml) | 126 ± 73 | 116 ± 68 | 92 | 0.99 | 0.01 |
| cis βCAR (ng/ml) | 10 ± 7 | 8 ± 4 | 82 | 0.72 | 0.15 |
| Tot. βCAR (ng/ml) | 136 ± 78 | 124 ± 72 | 92 | 0.98 | 0.01 |
| Tot. CAROT (ng/ml) | 890 ± 473 | 831 ± 428 | 93 | 0.99 | 0.004 |
| δTOC (ng/ml) | 519 ± 35 | 506 ± 38 | 97 | 0.59 | 0.12 |
| β + γTOC (ng/ml) | 1256 ± 533 | 1181 ± 464 | 94 | 0.97 | 0.04 |
| αTOC (ng/ml) | 7675 ± 2334 | 7218 ± 2149 | 94 | 0.97 | 0.01 |
| Tot. TOC (ng/ml) | 9450 ± 2452 | 8904 ± 2217 | 94 | 0.96 | 0.005 |
| tr RET (ng/ml) | 294 ± 88 | 285 ± 93 | 97 | 0.96 | 0.19 |
The bolded values signifies statistical significance (i.e. p < 0.05) between pre-CT and post-CT values.
n = 17 children.
Fig. 1.
Pre- to post-CT changes in total carotenoids for n = 17 children (open circles represent overall mean).
Fig. 2.
Pre- to post-CT changes in total tocopherols for n = 17 children (open circles represent overall mean).
At baseline, the seven patients with reported multivitamin intake had significantly higher levels of trans-β-cryptoxanthin (154 vs. 68 ng/mL; p = 0.036), dihydro-lycopene (134 vs. 65 ng/mL; p = 0.049), and total carotenoids (1161 vs. 700 ng/mL; p = 0.044) compared to patients with no reported multivitamin intake (Table 3). However, in post-CT samples, we found no differences in any of the measured LPM between patients with and without multivitamin intake.
Table 3.
Pre- vs. post-CT levels of LPM in children with and without vitamin intake.
| Analyte | Vitamin intake | Pre-CT No vitamin intake | Vitamin intake | Post-CT No vitamin intake | ||
|---|---|---|---|---|---|---|
| Mean ± SD (n = 7) | Mean ± SD (n = 10) | pb | Mean ± SD (n = 7) | mean ± SD (n = 10) | pc | |
| UL10 (nM) | 423 ± 313 | 282 ± 132a | 0.24 | 443 ± 260 | 416 ± 203 | 0.81 |
| UN10 (nM) | 73 ± 68 | 54 ± 31a | 0.47 | 78 ± 73 | 60 ± 36 | 0.52 |
| TQ10 (nM) | 496 ± 353 | 336 ± 136a | 0.23 | 521 ± 305 | 477 ± 228 | 0.74 |
| UN10/TQ10 (%) | 15 ± 13 | 18 ± 14a | 0.71 | 14 ± 11 | 12 ± 5 | 0.75 |
| tr LUT (ng/mL) | 63 ± 19 | 67 ± 19 | 0.84 | 61 ± 17 | 63 ± 39 | 0.89 |
| tr ZEA (ng/mL) | 20 ± 7 | 22 ± 12 | 0.77 | 21 ± 7 | 21 ± 12 | 0.95 |
| Tot. tr LUT/ZEA (ng/mL) | 84 ± 26 | 89 ± 52 | 0.82 | 82 ± 24 | 84 ± 50 | 0.90 |
| Tot. cis LUT/ZEA (ng/mL) | 50 ± 20 | 40 ± 23 | 0.36 | 51 ± 20 | 39 ± 24 | 0.29 |
| tr AH-LUT (ng/mL) | 32 ± 10 | 27 ± 18 | 0.50 | 29 ± 10 | 26 ± 17 | 0.61 |
| cis AH-LUT (ng/mL) | 22 ± 10 | 15 ± 10 | 0.15 | 21 ± 9 | 15 ± 9 | 0.17 |
| αCRX (ng/mL) | 22 ± 8 | 16 ± 8 | 0.14 | 21 ± 7 | 15 ± 8 | 0.17 |
| tr βCRX (ng/mL) | 154 ± 84 | 68 ± 70 | 0.036 | 140 ± 76 | 65 ± 66 | 0.05 |
| cis βCRX (ng/mL) | 39 ± 16 | 21 ± 20 | 0.08 | 37 ± 12 | 21 ± 20 | 0.07 |
| Tot. LYCOP (ng/mL) | 544 ± 378 | 290 ± 209 | 0.09 | 474 ± 331 | 289 ± 210 | 0.18 |
| tr LYC (ng/mL) | 144 ± 95 | 102 ± 86 | 0.36 | 126 ± 86 | 100 ± 83 | 0.54 |
| 5-cis LYC (ng/mL) | 266 ± 202 | 124 ± 90 | 0.07 | 229 ± 175 | 124 ± 93 | 0.12 |
| DHLYC (ng/mL) | 134 ± 94 | 65 ± 35 | 0.049 | 118 ± 81 | 65 ± 37 | 0.09 |
| αCAR (ng/mL) | 40 ± 26 | 27 ± 35 | 0.43 | 35 ± 24 | 27 ± 35 | 0.58 |
| tr βCAR (ng/mL) | 162 ± 49 | 101 ± 78 | 0.09 | 145 ± 45 | 96 ± 76 | 0.15 |
| cis βCAR (ng/mL) | 13 ± 8 | 7 ± 5 | 0.12 | 10 ± 3 | 7 ± 5 | 0.15 |
| Tot. βCAR (ng/mL) | 175 ± 55 | 108 ± 82 | 0.08 | 155 ± 48 | 103 ± 80 | 0.15 |
| Tot. CAROT (ng/mL) | 1161 ± 437 | 700 ± 416 | 0.044 | 1044 ± 378 | 682 ± 412 | 0.08 |
| δTOC (ng/mL) | 512 ± 32 | 524 ± 38 | 0.51 | 489 ± 39 | 517 ± 35 | 0.13 |
| β + γTOC (ng/mL) | 1222 ± 764 | 1279 ± 337 | 0.84 | 1068 ± 618 | 1259 ± 335 | 0.42 |
| αTOC (ng/mL) | 7755 ± 1786 | 7619 ± 2748 | 0.91 | 6980 ± 1494 | 7384 ± 2578 | 0.72 |
| Tot. TOC (ng/mL) | 9489 ± 1780 | 9422 ± 2928 | 0.96 | 8537 ± 1332 | 9161 ± 2716 | 0.58 |
| tr RET (ng/mL) | 318 ± 76 | 276 ± 95 | 0.35 | 314 ± 68 | 264 ± 105 | 0.29 |
Bolded if significant (p < 0.05)
n = 9.
p Value from students t-test comparisons of pre-CT vitamin vs. pre-CT No vitamin intake levels.
p Value from students t-test comparisons of post-CT vitamin vs. post-CT No vitamin intake levels.
When radiation exposure was coded as a continuous variable, patients with higher previous exposure to radiation had higher baseline levels of trans lutein (p = 0.03), total trans lutein/zeaxanthin (p = 0.04), and trans lycopene levels (p = 0.01; Table 4). The strongest and most common predictor of pre- to post-CT changes (for analytes trans lutein, cis anhydro-lutein, trans and cis β-cryptoxanthin, 5 cis-lycopene, dihydrolycopene, cis β-carotene, α-tocopherol, total, tocopherol, and the UN10/TQ10 ratio; all p < 0.05) were their pre-CT levels (data not shown). In other words, for these LPM, the higher the pre-CT levels, the greater reduction in post-CT levels. We also found that multivitamin intake was a significant predictor of higher plasma levels of trans β-cryptoxanthin (p = 0.04), dihydrolycopene (p = 0.049), and total carotenoids (p = 0.04; Table 4).
Table 4.
Predictors of baseline micronutrient levels.
| Analyte | Predictor | Baseline direction with increasing predictora | Partial r2 | p |
|---|---|---|---|---|
| trLUT | Radiation history | Higher | 0.28 | 0.03 |
| Tot. tr LUT/ZEA | Radiation history | Higher | 0.24 | 0.04 |
| tr βCRX | Multivitamin intake | Higher | 0.26 | 0.04 |
| tr LYC | Radiation history | Higher | 0.37 | 0.01 |
| DHLYC | Multivitamin intake | Higher | 0.23 | 0.049 |
| Tot. CAROT | Multivitamin intake | Higher | 0.24 | 0.04 |
The bolded values signifies statistical significance (i.e. p < 0.05) between pre-CT and post-CT values.
Using the Reg procedure, a stepwise multiple regression with forward entry method was applied where the outcome variable (baseline analyte level) was regressed against multi viatmin intake (yes, no) or radiation history as possible predictors.
Discussion
In this pilot study, we found marked decreases in the levels of several circulating LPM of young children undergoing CT exams for medical reasons. We hypothesized that the IR exposure from CT scans would lead to changes in plasma LPM levels due to the nature of these compounds to act as radical scavengers [15,18,52,53] and thereby be consumed during this X-ray based procedure. Previous reports have observed no [54] or minor [55] diurnal variations of plasma/serum LPM (retinol, α-tocopherol and α-/β-carotene, lutein/zeaxanthin, cryptoxanthin) in vivo. Even if the diurnal variation was to occur, it would have been to a minor extent [54,55] and would be deemed inconsequential since our measurements and comparisons were made on a relative (post- vs. pre -CT) basis within one hour of each other – a period of time in which our analytes would not change in circulating levels. Therefore, we have reasonable confidence that the marked decreases in the LPM levels observed in our study are due to the CT-induced IR damage and not diurnal variation.
As expected, subjects with reported vitamin intake had higher levels of some pro-vitamin A carotenoids at baseline than nonvitamin users, however, no differences in levels were observed between these two groups post-CT. This could be due to the higher levels of the supplement users being consumed by the CT procedure thereby protecting the subjects from IR damage. However, due to the small number of individuals, this observation requires further confirmation.
Interestingly, both UL10 and UN10 levels were increased post- vs. pre-CT, although not significantly. The increase in UN10 post-CT could not be explained by UL10 oxidation as previously suggested [28] as post-CT UL10 levels were also increased. The higher post-CT Q10 levels could be explained by the higher cell death rate, which would free more Q10 into the circulation. The accurate measurement of UL10 and UN10 requires the use of appropriate internal standards that reflect both the absolute losses and the [inevitable] oxidation of UL10 to UN10 that occurs during sample processing and analysis. We previously confirmed the rate of oxidation of the Q10 internal standard analogs to be exactly the same as the native UL10 and UN10 compounds [48], thus demonstrating that our custom synthesized butylated UL10 and UN10 analogs were ideal internal standards. In general, further studies are needed to explore the reasoning of post-CT level increases in the Q10 compounds with the use of appropriate internal standards.
CT involves doses of X-rays capable of ionizing surrounding atoms and molecules. Since humans are composed mainly of water, hydroxyl free radicals or other ROS are the most common products of IR exposure that react readily with other molecules [2]. These and other reactive species have been well documented to elicit various forms of DNA damage [2] that, if left unrepaired, can lead to mutations and ultimately to the induction of many cancers [2,3]. The damage by free radicals is dependent on the balance between the amount of radicals formed and the cellular antioxidant content at the time of IR exposure [56]. For antioxidants to exert their protective effects, these compounds must be present within the cellular environment at the time of insult because radical species [generally] have a very short lifetime [57,58].
Previous data have suggested a protective effect of antioxidants against several forms of IR-induced DNA damage. However, much of this data has been generated from in vitro studies or animal models reviewed in [52,53,58,59] using only a few analytes whereas in vivo studies have focused exclusively on adults undergoing total-body irradiation prior to bone marrow transplantation, which applied much higher radiation doses than in our study [60,61]. Circulating vitamin C and uric acid levels decreased after TBI [61] and so did plasma α-tocopherol and β-carotene levels after TBI combined with or exclusively with chemotherapy [60].
To our knowledge, this is the first study looking at the effects of CT-induced IR on circulating antioxidants in young children in vivo. The relatively low number of subjects in our study may explain some of our non-significant findings and larger in vivo studies with children are needed to explore this in further detail.
In the clinical setting and particularly the ED, the use of CT will continue to be of tremendous benefit for a variety of situations such as diagnosing and managing children with blunt trauma, seizures, altered mental status, headache and abdominal pain, and others. For that reason, future studies are required to test whether supplementation with vitamins can act as a prophylactic modality for CT-induced IR damage particularly in children who are most vulnerable to IR insults.
Conclusion
In our pilot study we found marked decreases in the levels of several LPM in young children receiving medically indicated CT scans involving relatively low IR doses. To our knowledge, this is the first study looking at the effects of physiologically relevant IR doses in young children in vivo. The dietary intake of antioxidants has been associated with a lower incidence of cancer owing to their presumed protective effect against oxidative and other stress; however, the role of LPM in minimizing CT-induced IR induced damage remains elusive and needs to be further researched. Young children are especially vulnerable to IR due to their higher radiosensitivity, higher risk of cumulative exposure, and longer life expectancy compared to adults and are therefore at higher risk to experience IR related long-term damage. There is a paradox of CT use in that the same modality used to help diagnose and treat cancer can also contribute to cancer. Thus, because of the indispensable nature of CT studies in modern medicine, future and larger in vivo studies are needed to test whether increasing circulating lipid-phase micronutrient levels can alter the cellular antioxidant defense system and protect against IR damage with the ultimate goal to establish prophylactic modalities for CT-induced IR damage.
Acknowledgments
Source of funding
University of Hawai’i Cancer Center Developmental Fund, National Cancer Institute P30 CA71789.
Footnotes
Abbreviations used: CT, computer tomography; LPM, lipid phase micronutrients; ROS, reactive oxygen species; UL10, ubiquinol-10; ED, emergency department; BHT, butylated hydroxytoluene; BTP, bis-tris-propane; UN10, ubiquinone-10; DCM, dichloromethane; EtOH, ethanol; MeOH, methanol; TQ10, Total coenzyme Q10; UN10/TQ10, ubiquinol-10/ubiquinone-10 ratio; tr LUT, trans lutein; tr ZEA, trans zeaxanthin; Tot tr LUT/ZEA, total trans lutein+zeaxanthin; Tot cis LUT/ZEA, total cis lutein + zeaxanthin; tr AH-LUT, trans anhydrolutein; cis AH-LUT cis anhydrolutein; αCRX, α-cryptoxanthin; tr βCRX, trans β-cryptoxanthin; cis βCRX, cis β-cryptoxanthin; Tot LYCOP, total lycopenes; tr LYC, trans lycopene; 5cis LYC, 5-cis lycopene; DHLYC, dihydrolycopene; αCAR, α-carotene; tr βCAR, trans β-carotene; cis βCAR, cis β-carotene; TOT βCAR, total β-carotene; Tot CAROT, all carotenoids; δTOC, δ-tocopherol; β + γTOC, β– + γ-tocopherol; αTOC, α-tocopherol; Tot TOC, total tocopherol; RET, retinol.
Financial disclosure
None declared.
Conflict of interest
None declared.
References
- 1.Borek C. J. Nutr. 2004;134(11):3207S–3209S. doi: 10.1093/jn/134.11.3207S. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DJ, Hall EJ. N. Engl. J. Med. 2007;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 3.Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, et al. Proc. Natl. Acad. Sci. U.S.A. 2003;100(24):13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner D, Elliston C, Hall E, Berdon W. AJR – Am. J. Roentgenol. 2001;176(2):289–296. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 5.Kleinerman RA. Pediatr. Radiol. 2006;36(Suppl. 2):121–125. doi: 10.1007/s00247-006-0191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNSCEAR. United Nations Scientific Committee on the Effects of Atomic Radiation: Sources, Effects and Risks of Ionizing Radiation. New York: 2013. [Google Scholar]
- 7.Berrington de Gonzalez A, Mahesh M, Kim K-P, Bhargavan M, Lewis R, Mettler F, et al. Arch. Intern. Med. 2009;169(22):2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White KS. Pediatr. Radiol. 1996;26(1):5–14. doi: 10.1007/BF01403695. [DOI] [PubMed] [Google Scholar]
- 9.Frush DP, Donnelly LF. Radiology. 1998;209(1):37–48. doi: 10.1148/radiology.209.1.9769810. [DOI] [PubMed] [Google Scholar]
- 10.Menoch MJ, Hirsh DA, Khan NS, Simon HK, Sturm JJ. Pediatrics. 2012;129(3):e690–e697. doi: 10.1542/peds.2011-2548. [DOI] [PubMed] [Google Scholar]
- 11.Health Devices. 2012;41(10):332–333. [PubMed] [Google Scholar]
- 12.Mattila P, Kumpulainen J. J. Food Compos. Anal. 2001;14:409–417. [Google Scholar]
- 13.Barshop BA, Gangoiti JA. Mitochondrion. 2007;7(Suppl.):S89–S93. doi: 10.1016/j.mito.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Nohl H, Gille L, Kozlov AV. Biofactors. 1999;9(2–4):155–161. doi: 10.1002/biof.5520090210. [DOI] [PubMed] [Google Scholar]
- 15.Ernster L, Dallner G. Biochim. Biophys. Acta. 1995;1271(1):195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 16.Laguna TA, Sontag MK, Osberg I, Wagener JS, Accurso FJ, Sokol RJ. Pediatr. J. 2008;153(3):402–407. doi: 10.1016/j.jpeds.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Tang PH, Miles MV, DeGrauw A, Hershey A, Pesce A. Clin. Chem. 2001;47(2):256–265. [PubMed] [Google Scholar]
- 18.Crane FL. J. Am. Coll. Nutr. 2001;20(6):591–598. doi: 10.1080/07315724.2001.10719063. [DOI] [PubMed] [Google Scholar]
- 19.Lass A, Sohal RS. Arch. Biochem. Biophys. 1998;352(2):229–236. doi: 10.1006/abbi.1997.0606. [DOI] [PubMed] [Google Scholar]
- 20.Kagan VE, Tyurina YY, Witt E. Subcell. Biochem. 1998;30:491–507. doi: 10.1007/978-1-4899-1789-8_20. [DOI] [PubMed] [Google Scholar]
- 21.Forsmark-Andree P, Dallner G, Ernster L. Free Radic. Biol. Med. 1995;19(6):749–757. doi: 10.1016/0891-5849(95)00076-a. [DOI] [PubMed] [Google Scholar]
- 22.Turrens JF, Alexandre A, Lehninger AL. Arch. Biochem. Biophys. 1985;237(2):408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 23.Boveris A. Methods Enzymol. 1984;105:429–435. doi: 10.1016/s0076-6879(84)05060-6. [DOI] [PubMed] [Google Scholar]
- 24.Kagan V, Serbinova E, Packer L. Biochem. Biophys. Res. Commun. 1990;169(3):851–857. doi: 10.1016/0006-291x(90)91971-t. [DOI] [PubMed] [Google Scholar]
- 25.Stocker R, Bowry VW, Frei B. Proc. Natl. Acad. Sci. U.S.A. 1991;88(5):1646–1650. doi: 10.1073/pnas.88.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber C. Int. J. Vitam. Nutr. Res. 1994;64(4):311–315. [PubMed] [Google Scholar]
- 27.Bowry VW, Stanley KK, Stocker R. Proc. Natl. Acad. Sci. U.S.A. 1992;89(21):10316–10320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagendijk J, Ubbink JB, Vermaak WJ. J. Lipid Res. 1996;37(1):67–75. [PubMed] [Google Scholar]
- 29.Menke T, Niklowitz P, Wiesel T, Andler W. Pediatr. Diabetes. 2008;9(6):540–545. doi: 10.1111/j.1399-5448.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto Y, Yamashita S, Fujisawa A, Kokura S, Yoshikawa T. Biochem. Biophys. Res. Commun. 1998;247(1):166–170. doi: 10.1006/bbrc.1998.8752. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto Y, Yamashita S. Mol. Aspects Med. 1997;18(Suppl.):S79–S84. doi: 10.1016/s0098-2997(97)00007-1. [DOI] [PubMed] [Google Scholar]
- 32.Palace VP, Khaper N, Qin Q, Singal PK. Free Radic. Biol. Med. 1999;26(5–6):746–761. doi: 10.1016/s0891-5849(98)00266-4. [DOI] [PubMed] [Google Scholar]
- 33.Lin AM, Chen KB, Chao PL. Ann. N. Y. Acad. Sci. 2005;1053:319–329. doi: 10.1196/annals.1344.028. [DOI] [PubMed] [Google Scholar]
- 34.Krinsky NI, Johnson EJ. Mol. Aspects Med. 2005;26(6):459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Packer L. Ann. N. Y. Acad. Sci. 1993;691:48–60. doi: 10.1111/j.1749-6632.1993.tb26156.x. [DOI] [PubMed] [Google Scholar]
- 36.Wiseman H. FEBS Lett. 1993;326(1–3):285–288. doi: 10.1016/0014-5793(93)81809-e. [DOI] [PubMed] [Google Scholar]
- 37.Gille L, Rosenau T, Kozlov AV, Gregor W. Biochem. Pharmacol. 2008;76(3):289–302. doi: 10.1016/j.bcp.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Halliwell B. Nutr. Rev. 1999;57:104–113. doi: 10.1111/j.1753-4887.1999.tb06933.x. [DOI] [PubMed] [Google Scholar]
- 39.Cooper DA, Eldridge AL, Peters JC. Nutr. Rev. 1999;57(7):201–214. doi: 10.1111/j.1753-4887.1999.tb06944.x. [DOI] [PubMed] [Google Scholar]
- 40.Rock CL, Jacob RA, Bowen PE. J. Am. Diet. Assoc. 1996;96(7):693–702. doi: 10.1016/S0002-8223(96)00190-3. (quiz 703-694). [DOI] [PubMed] [Google Scholar]
- 41.Ames BN. Ann. N. Y. Acad. Sci. 1999;889:87–106. doi: 10.1111/j.1749-6632.1999.tb08727.x. [DOI] [PubMed] [Google Scholar]
- 42.Halliwell B. Nutr. Rev. 1997;55(1 Pt 2):S44–S49. doi: 10.1111/j.1753-4887.1997.tb06100.x. (discussion S49–52). [DOI] [PubMed] [Google Scholar]
- 43.Debier C, Larondelle Y. Br. J. Nutr. 2005;93(2):153–174. doi: 10.1079/bjn20041308. [DOI] [PubMed] [Google Scholar]
- 44.Rao AV, Rao LG. Pharmacol. Res. 2007;55(3):207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Franke AA, Morrison C. Free Radic. Biol. Med. 2010;48:1610–1617. doi: 10.1016/j.freeradbiomed.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franke AA, Custer LJ, Cooney RV. J. Chromatogr. B. 1993;614:43–57. doi: 10.1016/0378-4347(93)80222-p. [DOI] [PubMed] [Google Scholar]
- 47.Franke A, Morrison C, Custer L, Li X, Lai JF. J. Chromatogr. A. 2013;2013:1–9. doi: 10.1016/j.chroma.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franke AA, Lai JF, Morrison CM, Pagano I, Li X, Halm BM, et al. Free Radic. Res. 2013;47(9):757–768. doi: 10.3109/10715762.2013.822495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Radiology. 2008;248(1):254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 50.Valentin J. Ann. ICRP. 2007;37(1):1–79. doi: 10.1016/j.icrp.2007.09.001. (iii). [DOI] [PubMed] [Google Scholar]
- 51.A.N. Society. [accessed 2.06.2008];Radiation dose chart. 2012 < http://www.ans.org/pi/resources/dosechart/>. [Google Scholar]
- 52.Fang YZ, Yang S, Wu G. Nutrition. 2002;18(10):872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 53.Weiss JF, Landauer MR. Ann. N. Y. Acad. Sci. 2000;899:44–60. [PubMed] [Google Scholar]
- 54.Nierenberg DW, Stukel T. Am. J. Med. Sci. 1987;294(3):187–190. doi: 10.1097/00000441-198709000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Cantilena LR, Stukel TA, Greenberg ER, Nann S, Nierenberg DW. Am. J. Clin. Nutr. 1992;55(3):659–663. doi: 10.1093/ajcn/55.3.659. [DOI] [PubMed] [Google Scholar]
- 56.Turner ND, Braby LA, Ford J, Lupton JR. Nutrition. 2002;18(10):904–912. doi: 10.1016/s0899-9007(02)00945-0. [DOI] [PubMed] [Google Scholar]
- 57.Pryor WA. Annu. Rev. Physiol. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]
- 58.Hosseinimehr SJ. Drug Discov. Today. 2007;12(19–20):794–805. doi: 10.1016/j.drudis.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 59.Weiss J, Landauer M. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 60.Clemens MR, Ladner C, Ehninger G, Einsele H, Renn W, Buhler E, et al. Am. J. Clin. Nutr. 1990;51(2):216–219. doi: 10.1093/ajcn/51.2.216. [DOI] [PubMed] [Google Scholar]
- 61.Chevion S, Or R, Berry EM. Biochem. Mol. Biol. Int. 1999;47(6):1019–1027. doi: 10.1080/15216549900202143. [DOI] [PubMed] [Google Scholar]