Abstract
Formulation development of protein therapeutics using polymeric nanoparticles has found very little success in recent years. Major formulation challenges include rapid denaturation, susceptibility to lose bioactivity in presence of organic solvents and poor encapsulation in polymeric matrix. In the present study, we have prepared hydrophobic ion pairing (HIP) complex of lysozyme, a model protein, using dextran sulfate (DS) as a complexing polymer. We have optimized the process of formation and dissociation of HIP complex between lysozyme and DS. The effect of HIP complexation on enzymatic activity of lysozyme was also studied. Nanoparticles were prepared and characterized using spontaneous emulsion solvent diffusion method. Furthermore, we have also investigated release of lysozyme from nanoparticles along with its enzymatic activity. Results of this study indicate that nanoparticles can sustain the release of lysozyme without compromising its enzymatic activity. HIP complexation using a polymer may also be employed to formulate sustained release dosage forms of other macromolecules with enhanced encapsulation efficiency.
Keywords: Lysozyme, HIP complex, nanoparticles
Introduction
Recently, nanoparticles-based drug delivery systems have been widely studied. Some of major benefits of nanoparticles include achieving sustained/controlled release of the drug at the targeted site, reduced frequency of administration, and ability to overcome biological barrier associated with the parent drug.[1,2]
Delivery of protein-based therapeutics has been a challenging task for scientists in the drug delivery field. Colloidal dosage forms of peptide and protein-based molecules have been investigated to overcome absorption and stability related issues.[3,4] However, the success of this approach is mainly dependent on understanding of formulation parameters. Conventional methods of nanoparticle preparation such as single and double emulsion methods have often failed to provide higher entrapment of peptide and protein molecules in polymeric matrix.[3] Low entrapment is attributed to leaching of these hydrophilic molecules into the external aqueous phase during the encapsulation process. Such hydrophilic macromolecules have a higher affinity for the external aqueous phase relative to hydrophobic polymeric matrices. Use of organic solvents and physical stress such as sonication and homogenization often cause denaturation and irreversible aggregation resulting in loss of biological activity.[5,6] Other challenges include significant burst release and sometimes loss of biological activity following encapsulation within the polymeric matrix. Poly lactic-co-glycolic acid (PLGA) is one of the most widely employed Food and Drug Administration (FDA) approved biocompatible and biodegradable polymer to prepare nanoparticles. Different grades of PLGA polymer are available with varying lactide and glycolide content. However, this polymer has shown a detrimental effect on stability of the entrapped protein molecule.[7–9]
Recently, HIP complexation-based approach has been employed to formulate dosage form for hydrophilic molecules especially for peptide and protein drugs. The solubility of a protein molecule is due to presence of charged amino acids present on the surface. Hydrophobic ion pairing (HIP) complexation is a technique to stoichiometrically complex ionizable functional groups of protein and peptide molecules with oppositely charged functional groups of a complex forming agent.[10] During complex formation, charge of amino acids is neutralized in a reversible manner due to presence of a polymer having oppositely charged functional groups. Hence, ionic complexation dramatically diminishes/reduces aqueous solubility of a water soluble protein molecule.
This approach offers several advantages which are summarized as following: (i) HIP complexation can alter the aqueous solubility of a hydrophilic protein molecule in a reversible manner, (ii) A complex form of hydrophilic/protein molecule possesses higher solubility in the lipid phase which may result in higher permeation across cell membrane, (iii) Encapsulation of hydrophilic/protein molecule in a polymeric matrix is a challenging task, (iv) Literature suggests that loading/encapsulation efficiency of a hydrophilic drug can be also enhanced by employing a complexation-based approach,[11–15] (v) Further, HIP complexation also provides conformation stability to the protein molecule in the presence of organic solvents. Due to above mentioned reasons, HIP complexation-based technique has started to get acceptance in the preparation of colloidal dosage form such as nanoparticles for protein-based therapeutics.[9,10,12]
Traditionally, a surfactant molecule is preferred over a polymer molecule to prepare HIP complex with the protein molecule. However, use of surfactant may cause protein denaturation.[16,17] Further, the toxicity issue associated with surfactant molecule may limit its use via a particular route. In the present study, we have employed dextran sulfate (DS), molecular weight 9000–20000 Da as a model polymer to form HIP complex. DS has been previously employed as a potential adjuvant in various pharmaceutical formulations.[18,19] Based on our literature survey, no other research group has employed a polymer to form HIP complex.
In this study, HIP complexation of lysozyme, a model basic protein, with DS has been investigated. Lysozyme is an enzyme composed of 129 amino acids while DS is a polysaccharide (a polymer of anhydroglucose).[18,19] Basic amino acids in lysozyme can be ionically complexed with sulfate group of DS resulting in HIP complex which can be entrapped in a polymeric matrix. HIP complex of lysozyme with surfactant (e.g sodium oleate) was reported by Yoo et al.[15] These investigators have characterized the HIP complex along with its nanoparticles formulation.
Macromolecules contain complex secondary and tertiary structures stabilized by various covalent and noncovalent forces. Non-covalent forces include electrostatic interactions, hydrogen bonds, hydrophobic interactions, and Van der Waals interactions.[20–22] During HIP complex formation, lysozyme precipitates as a complex when added in specific molar ratios along with DS. Precipitation of lysozyme may impart conformational changes or partial unfolding. One may also argue irreversible aggregation of lysozyme during the precipitation of HIP complex which may not be enzymatically active. Moreover, the aggregated form of a protein molecule may also generate an immune response after administration.[23] Hence, it is very important to characterize the forces involved in the formation of a HIP complex and distinguish HIP complex formation process with aggregation. Due to large size, presence of various functional groups and glucosyl ring structure, DS may interact extensively with the protein molecule via electrostatic interactions, hydrogen bonds, hydrophobic interactions, and Van der Waals interactions.[24,25] Since these forces also play a crucial role in stabilizing the protein molecule, it would be important to understand the effect of HIP complexation on stability of the complexed protein. Contribution from each force during HIP complex formation would depend on factors such as nature of the protein molecule, size and structure of the complexing agent, ionic strength of the medium and pH of aqueous solutions of complexing agent and protein. We have also investigated the effect of complexing agent (DS) on physicochemical properties (solubility and dissociation of protein from HIP complex) of the complex.
In the present study, we have characterized electrostatic interactions of amino groups of lysozyme with the sulfate groups of DS using Fourier transform infrared spectroscopy (FTIR). Moreover, it is also important to understand how lysozyme molecules would dissociate from the complex and generate its native structure. Regeneration of lysozyme in its native form would also eliminate the possibility of its irreversible aggregation during formation of HIP complex. Hence, we also studied enzymatic activity of lysozyme after dissociation from HIP complex and compared it with the enzymatic activity of freshly prepared lysozyme solution.
Furthermore, nanoparticles were prepared by spontaneous emulsion solvent diffusion method as published earlier by Yoo et al.[15] Conventional methods of nanoparticle preparation (single or double emulsion methods) involve sonication of lysozyme in presence of organic solvents which may inactivate the enzyme.[26] Moreover, studies have also been reported regarding precipitation of enzymatically inactive lysozyme in the presence of methylene chloride.[26] The spontaneous emulsion solvent diffusion method does not employ sonication or organic solvent.[13] Instead dimethyl sulfoxide (DMSO), a protein compatible solvent may be employed as a common solvent to solubilize HIP complex form of lysozyme. Doelkar et al. have studied the effect of various solvents, non-solvents, polymers, and surfactants on the preparation of nanoparticles using spontaneous emulsion solvent diffusion method.[27] Nanoparticles have been characterized with respect to particle size and surface morphology using scanning electron microscopy (SEM). Moreover the effect of different ratios of PLGA polymer on entrapment of lysozyme was also studied. Finally, release of lysozyme from PLGA nanoparticles was studied and enzymatic activity of the released lysozyme was compared with freshly prepared solution of lysozyme.
Materials and method
Materials
Lysozyme, dextran sulfate sodium salt (molecular weight 9000–20000 Da), poly (DL-lactide-co-glycolide) (PLGA 85:15, molecular weight of 50,000–75,000 Da), bicinchoninic acid (BCA), copper sulfate and lyophilized cells of Micrococcus lysodeikticus were purchased from sigma Aldrich. DMSO and micro BCA protein assay kit were purchased from Thermo scientific. All other solvents and reagents were of analytical grade and purchased from a local supplier and used as such without further purification. Double distilled water (DDW) was used throughout the entire study.
Methods
Effect of different molar ratios of DS to lysozyme on HIP complex formation
Lysozyme is an enzyme composed of 129 amino acids including several basic amino acids. Dextran sulfate contains 2.3 sulfate groups per glucosyl residue. Stock solutions of DS and lysozyme (5mg/mL) were prepared in DDW and Tris buffer pH 9, respectively. HIP complexes were prepared in the different molar ratios of DS/lysozyme such as 0.023, 0.045, 0.068, 0.09, 0.18, and 0.36. Charge ratios corresponding to these molar ratios (of DS:lysozyme) are 0.25:1, 0.5:1, 0.75:1, 1:1, 2:1, and 4:1, respectively. In the other words, at any molar ratio less than 0.09 (charge ratio 1:1), the amount of DS was not sufficient to form complex with all the positively charged amino acids molecules of lysozyme present in the solution. The formed complex solution was vortexed for 3 min followed by centrifugation at 10000 rpm for 10 min at 4°C to separate HIP complex. Uncomplexed lysozyme was estimated in the supernatant using BCA protein assay. Percentage complexation of lysozyme with DS was calculated using the following equation.
Dissociation of lysozyme and DS complex
The dissociation of lysozyme from HIP complex was studied at room temperature to characterize the nature of interactions between lysozyme and DS. Initially, HIP complex of lysozyme and DS were prepared at the molar ratio of 0.09 followed by measurement of uncomplexed lysozyme in the supernatant. Dulbecco’s phosphate buffer saline (DPBS) pH 7.4 was added to the HIP complex having different concentrations of NaCl (10mM, 50 mM, 100 mM, 200 mM, 400 mM, and 750 mM) and redispersed by vortexing. Moreover, HIP complex was also redispersed in the presence of DPBS and DDW for comparison. After 12 hrs of equilibrium, samples were centrifuged and dissociated lysozyme was estimated in the supernatant by BCA protein assay.
FTIR study
FTIR analysis was carried out with an infrared spectrophotometer (Perkin-Elmer, Waltman, MA, USA). Samples (lysozyme, DS, and HIP complex) were kept in intimate contact with the diamond crystal by applying a loading pressure. Samples were cast on diamond crystal top-plate of attenuated total reflectance (ATR) accessory and scanned between 650 and 1800 cm−1. Spectra obtained using this device represents the average of 32 individual scan possessing a spectral resolution of 4 cm−1.
Evaluation of enzymatic activity of lysozyme after dissociation from HIP complex
Initially, HIP complex of lysozyme with DS was prepared as described in the previous section. Then, this complex was incubated with 1 ml of phosphate buffer saline (PBS) buffer (66 mM and pH 6.2) for 12 hrs. This incubation resulted in dissociation of the HIP complex. This solution was further subjected to centrifugation and protein estimation was carried out for supernatant. The enzymatic activity of lysozyme in supernatant was measured and compared with freshly prepared lysozyme solutions in the concentration range of 50, 75, 100, 150, 200, and 250 µg/ml. The enzymatic activity of lysozyme was studied by following an earlier published protocol.[28] Briefly, M. luteus (lysodeikticus) suspension was prepared at a concentration of 0.24 mg/ml in PBS buffer (66 mM) at pH 6.2. Aliquot 50 µl of sample or standard prepared was added in 2.5 ml of M. luteus suspension and incubated for 3 minutes. The activity of lysozyme was determined by measuring the reduction in optical density of a M. luteus suspension at 450 nm. This study was carried out in triplicate at room temperature.
Preparation of nanoparticles
Nanoparticles were prepared using PLGA 85:15 as a polymer. An earlier published process (spontaneous emulsion solvent diffusion method) was adopted with minor modifications for the preparation of nanoparticles.[15] Briefly, 5 mg of complex and PLGA 85:15 were dissolved in 3 ml of DMSO. This solution was added drop wise from a syringe into 25 ml of 1% poloxamer solution under constant stirring. Nanoparticles were formed instantaneously as both solutions were mixed. Prepared nanoparticles were stirred for 1 hr followed by centrifugation. Finally, nanoparticles were washed two times with DDW to remove surface bound lysozyme, poloxamer, and DMSO. Several batches of nanoparticles were prepared with different ratios of lysozyme in HIP complex to PLGA 85:15 (1:5, 1:10, and 1:15). This study was carried out in triplicate. Blank nanoparticles were also prepared by employing only polymer in similar amounts.
Characterization of nanoparticles
Entrapment efficiency of nanoparticles
For entrapment efficiency, 10 ml of DMSO was added to completely dissolve the nanoparticles. Entrapment was calculated based on the absorbance measured at 280 nm with UV spectrophotometer. This absorbance value was corrected for the absorbance of blank nanoparticles prepared using PLGA. This study was performed in triplicate.
Particle size measurement
Mean particle size and polydispersity of nanoparticles were measured by dynamic light scattering technique (DLS) (Brookhaven Inst. Co., Holtsville, NY, USA) at an operating angle of 90° and a temperature of 25°C. A dilute sample of the nanosuspension from each batch was analyzed for the measurement of particle size in triplicate.
Scanning electron microscopy
Scanning electron microscopy (SEM) images were obtained to study morphology of nanoparticles. A drop of nanosuspension (ratio 1:5) was placed on a nucleopore filter in a hand-pumped vacuum chamber with a permeable membrane supporting the filter. Vacuum was applied to enhance the filtration rate. Filters were mounted on SEM stubs with double-sticky carbon tabs and the edges were coated with colloidal silver for conductivity. The samples were then sputter-coated with platinum for 1 min at 20 mA current. Images were taken on a Hitachi S4700 field-emission SEM at accelerating voltages of 5 and 10 KV.
Release of the lysozyme from nanoparticles and its enzymatic activity
The release of lysozyme from nanoparticles was studied. Initially, 1.5 ml of the nanoparticle suspension (ratio 1:5) in PBS buffer was loaded into an Eppendorf’s tube and stirred at 60 rpm at 37°C. At pre-determined time points, nanoparticles suspension was subjected to centrifugation and the supernatant was collected. Nanoparticles were re-suspended with 1 ml of PBS buffer (containing 3–5% mannitol) and release study was continued for 28 days. Concentration of lysozyme in the release samples was estimated by measuring supernatant using BCA/micro BCA assay. We also intended to study activity of lysozyme after releasing from nanoparticles. Hence, the released samples were also subjected to enzymatic activity assay. Activity of lysozyme was determined by measuring the decrease in optical density of a M. luteus suspension at 450 nm. Standard solutions of lysozyme were also prepared and their enzymatic activity was measured for comparison.
Result and discussion
HIP complexation has been investigated due to its potential to form a hydrophobic reversible complex with small molecules, peptides, and macromolecule using oppositely charged molecules. Basically, HIP complexation lowers the aqueous solubility of a charged molecule and, hence, encapsulation of the molecule can be significantly enhanced in a polymeric matrix. Other major advantages of HIP complexation include restriction of conformational flexibility of macromolecules thereby imparting physical stability. In the present work, we have chosen lysozyme, a model protein, for the study. It is a basic protein (129 amino acids) and contains numerous basic amino acids which can be complexed. Traditionally, a surfactant molecule such as sodium dodecyl sulfate (SDS) has been employed in the preparation of ionic complex.[12–15]
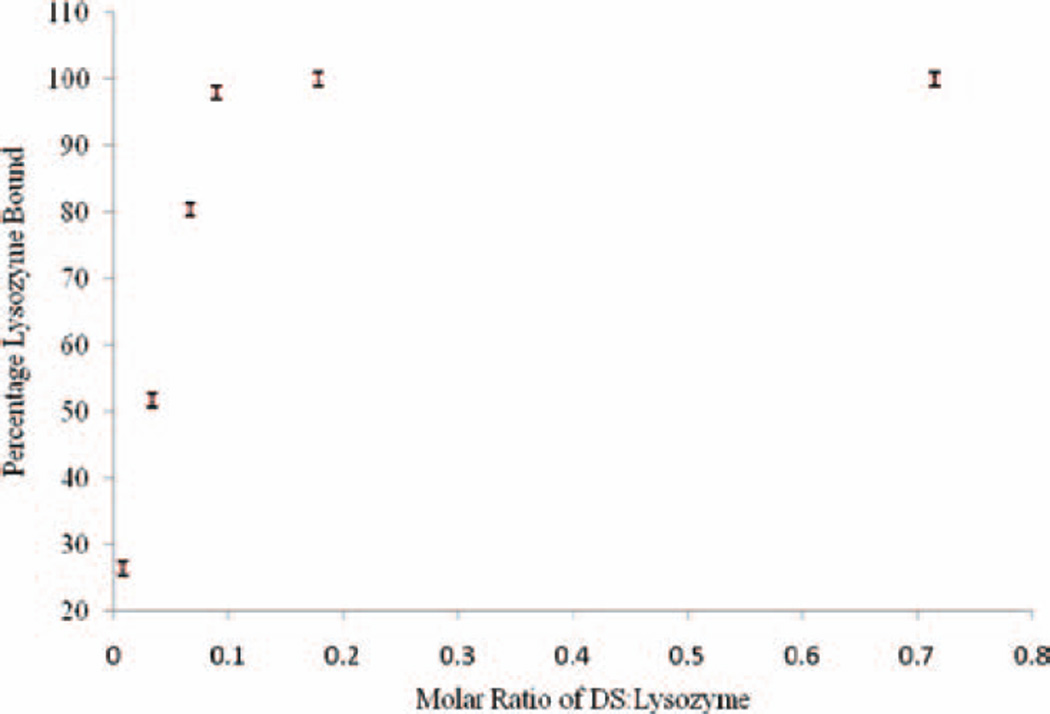
HIP complexes were prepared in the different molar ratios of DS/lysozyme such as 0.023, 0.045, 0.068, 0.09, 0.18, and 0.36. Charge ratios corresponding to these molar ratios (of DS:lysozyme) are 0.25:1, 0.5:1, 0.75:1, 1:1, 2:1, and 4:1, respectively. In other words, at any molar ratio less than 0.09 (charge ratio 1:1), the amount of DS was not sufficient to form complex with all the positively charged amino acids molecules of lysozyme. Results suggest that when molar ratios were 0.023, 0.045, and 0.068, we observed 73%, 48%, and 19.5% of uncomplexed (free) lysozyme molecules in supernatant, respectively (Figure 1). These results are closely matching with the theoretical expected binding of lysozyme with DS. Our results confirm a linear relationship between the amount of DS added and formation of HIP complex (Figure 1). Interestingly, at molar ratio greater than 0.09 (0.18 and 0.36), more than 98% of lysozyme molecules were found in HIP complex form with DS. This result was quite surprising because an excess addition of DS did not alter the formation of HIP complex with lysozyme. This observation was quite different from previously published studies in which the investigators employed a surfactant to form HIP complex of protein molecules.[12] In case of HIP complexation involving a surfactant molecule, investigators observed micellization at higher concentrations of surfactant (beyond critical micellar concentration). Micelle formation results in the solubilization of the HIP complex and eventually the solution turns clear. Our results clearly elucidate the difference in characteristics of HIP complex since a linear polymer (DS) was employed.
Figure 1.
Effect of molar ratios of dextran sulfate:lysozyme on HIP complexation. HIP, hydrophobic ion pairing.
Both DS and SDS have negatively charged sulfate group which primarily forms complex with amino group of protein molecules. However, both DS and SDS molecules have different backbone structure. SDS is a relatively small molecule (Mol wt is 288 Da) while DS used in the study had an average molecular weight of (9–20 KDa). Molecular weight of lysozyme and DS is in the same range, while molecular weight of SDS is ~ 1/50 of the molecular weight of lysozyme. Apart from ionic interaction, DS may extensively interact with protein molecule compared to SDS. This interaction may involve various weak physical forces such as hydrophobic and van der walls interactions.[24,25] HIP complex formation should also be seen as a charge neutralization process. In other words, one would expect maximum complex formation when all the positively charged amino acids of the protein molecule interact with negatively charged functional groups of surfactant/polymer molecules. After this stage, an excess addition of surfactant would lead to micellization causing dissociation of HIP complex. When an excess of DS was added, micellization was not observed. This could be due to several reasons including presence of hydrophobic interactions between DS and lysozyme. Hence, molecular weight and structure of a complex forming agent should be considered as well during a complexation phenomenon.
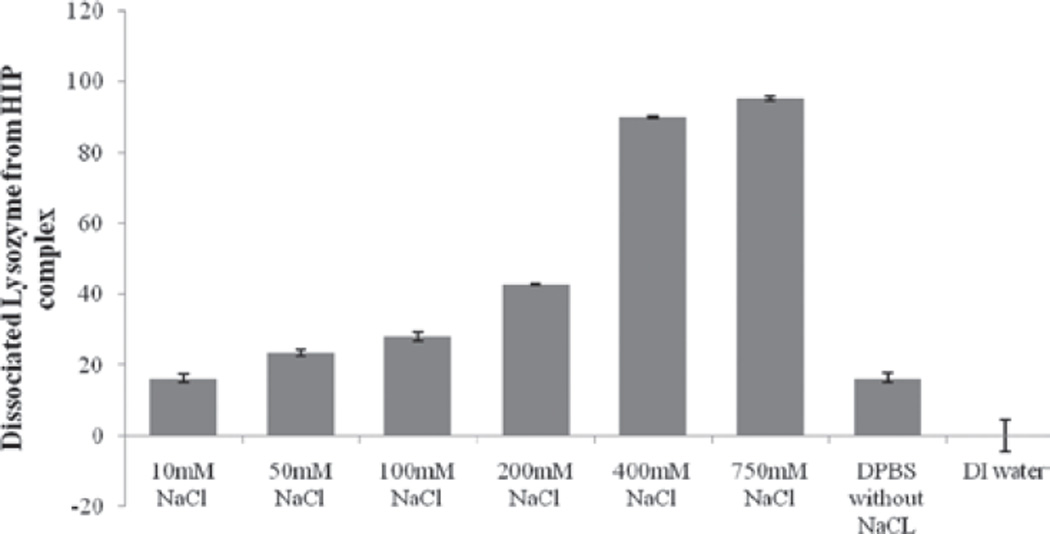
Ideally, HIP complex should dissociate in body fluids which would eventually generate a free form of protein molecule for therapeutic activity. Therefore, we have also studied dissociation of lysozyme from HIP complex by redispersing the complex with DPBS containing various concentrations of NaCl (10 mM to 750 mM). Since ionic interactions play a crucial role in formation of HIP complex, we conceptualized that HIP complex would dissociate in the presence of abundant oppositely charged ions (Cl−). A result of this study is shown in Figure 2 which clearly indicates a linear relationship between addition of NaCl and dissociation of lysozyme from HIP complex. We observed that as concentration of chloride ion increased, these ions replaced the negatively charged sulfate groups resulting in dissociation of HIP complex. This sort of dissociation behavior is different than previously published report. Formation and dissociation of HIP complex depend on various factors. These factors include nature of the protein molecule, size and structure of the complexing agent, ionic strength of the medium and pH of the aqueous solutions of complexing agent and protein. We believe that the discrepancy in the results is mainly due to the choice of the complexing agent. Since HIP complex dissociates in presence of DPBS, it is safe to assume that it would dissociate in physiological conditions in the presence of body fluids. Samples of complexes which were re-dispersed with DDW did not show any measurable dissociation and hence lysozyme was absent in the supernatant. This observation further confirms the presence of ionic interaction between lysozyme and DS in the complex. The similar observation has been reported by other investigators.[15]
Figure 2.
Dissociation of lysozyme from HIP complex in presence of buffers with different NaCl concentrations. HIP, hydrophobic ion pairing.
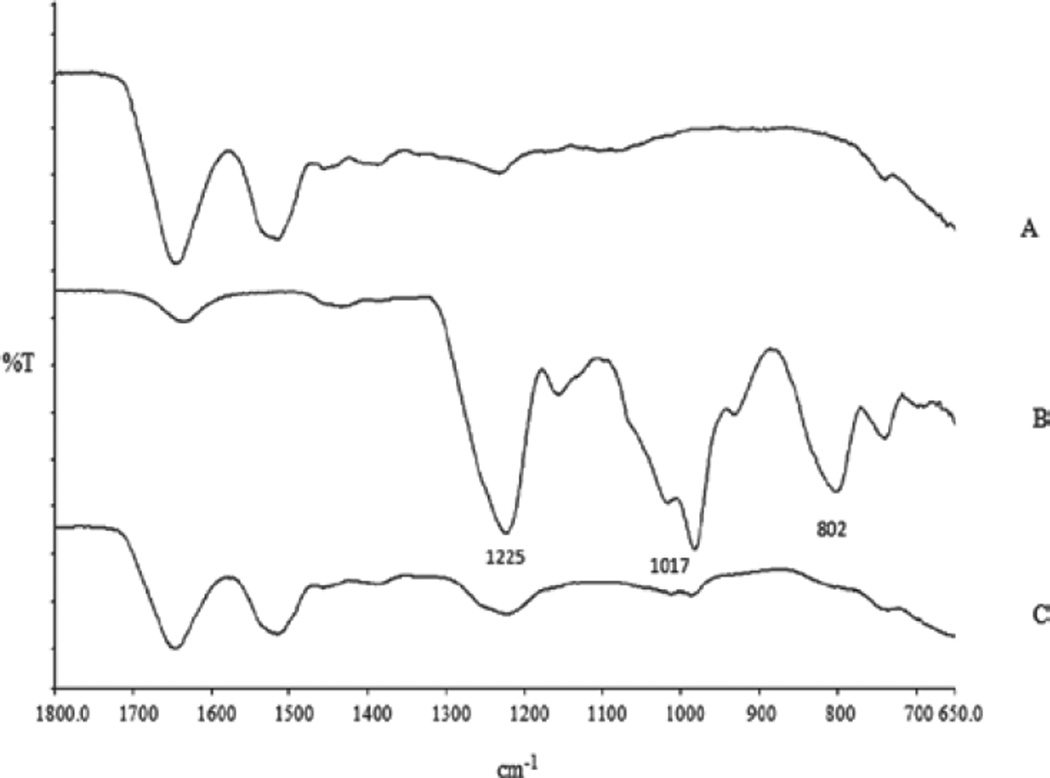
The presence of electrostatic interactions between amino group and sulfate group was further confirmed by FTIR. Previously, FTIR analysis was also performed by other investigators to characterize the interaction between amino group and sulfate group.[14,29–31] Due to complexities of a FTIR spectrum, we could not identify peak shift associated with the amino groups of lysozyme which interacted with sulfate groups of DS. Instead interactions of sulfate groups of DS were followed. Characteristic peaks of sulfate group of DS in the IR region are as follows: [1] 802 cm−1: S-O-S vibration, [2] 1017 cm−1: symmetric SOO− stretching vibration, and [3] 1225 cm−1: asymmetric SOO− stretching vibration (Figure 3). Appearance of these peaks in the IR spectra were close to previously published results.[14,29–31] Intensity of all the characteristic peaks related to sulfate group was significantly reduced in the complex compared to the free form of DS (Figure 3). This result clearly showed that sulfate group of DS was ionically complexed with the amino group of basic amino acids (lysine/arginine).
Figure 3.
FTIR spectra of lysozyme (A), dextran sulfate (B), and HIP complex (C). FTIR, Fourier transform infrared spectroscopy; HIP, hydrophobic ion pairing.
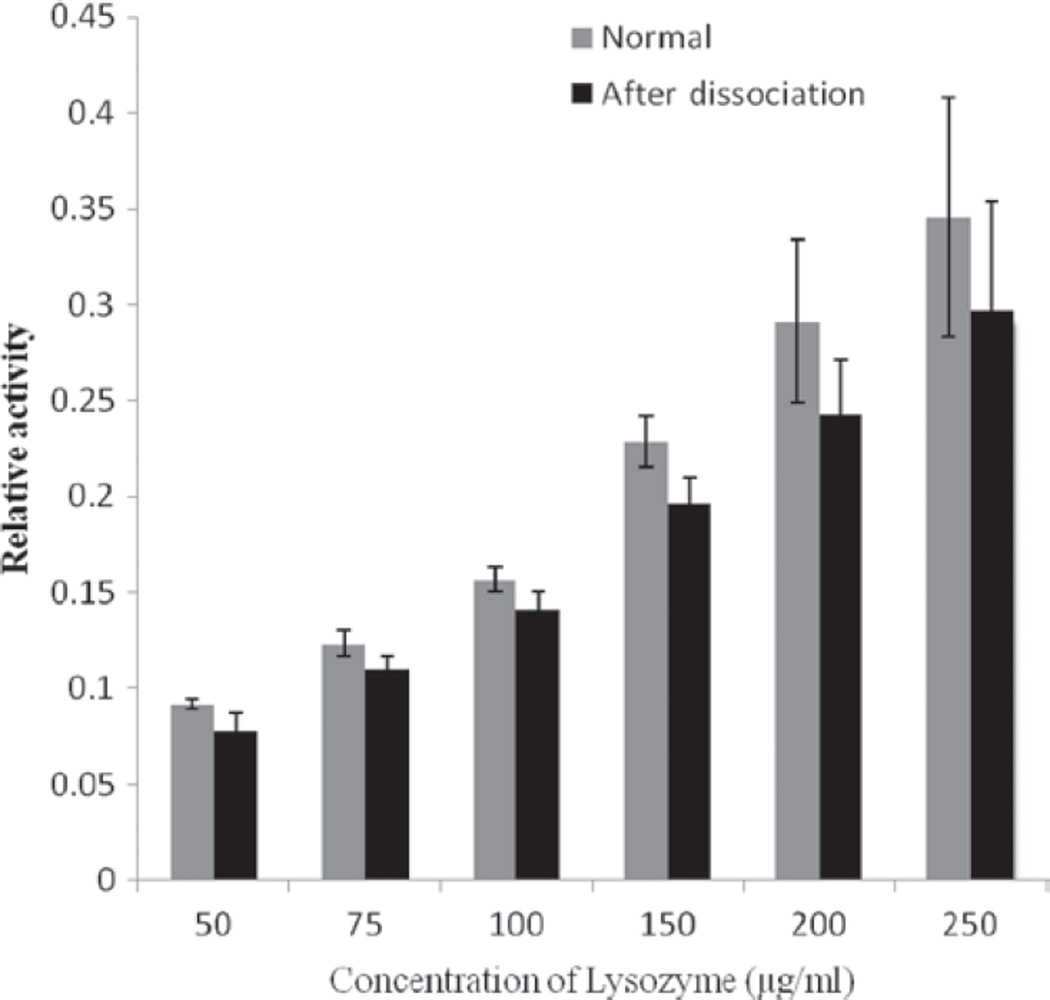
One important aspect of this study was to investigate the effect of HIP complexation on the enzymatic activity of lysozyme. During complexation process, lysozyme-DS complex precipitates from the solution and it is quite possible that during the precipitation lysozyme may lose its enzymatic activity due to irreversible aggregation. Since DS interacts extensively with the protein molecule, probability of protein denaturation and irreversible conformational change is even higher. Therefore, following dissociation of HIP complex, we recovered the lysozyme and measured its enzymatic activity. Furthermore, we compared it with the enzymatic activity of freshly prepared solution of lysozyme. We observed no statistically significant difference in the activity of freshly prepared lysozyme solution and the lysozyme obtained after dissociation from the HIP complex (Figure 4). We believe that during the formation of HIP complex, lysozyme molecule may undergo conformational change. However, after dissociation from the HIP complex, it regains its native conformation and hence its enzymatic activity was not affected. This study further rules out the possibility of irreversible aggregation during formation/precipitation of HIP complex.
Figure 4.
Evaluation of an enzymatic activity of lysozyme after dissociation from HIP complex and comparison with enzymatic activity of freshly prepared solutions of lysozyme. HIP, hydrophobic ion pairing.
Nanoparticles were prepared by spontaneous emulsion solvent diffusion method using PLGA 85:15 as polymer. PLGA 85:15 was chosen as a polymer compared to other grades of PLGA such as PLGA 50:50 and PLGA 65:35. PLGA 85:15 has highest lactide content and a higher lactide content of polymer imparts more hydrophobicity to the polymer. In order to obtain maximum encapsulation of a complexed form of protein molecule, we have employed PLGA 85:15 in our study. We hypothesize that by employing PLGA 85:15 as a polymer, hydrophobic interaction between polymeric matrix and HIP complex could be maximized. DMSO was employed as a solvent which is widely used for protein based molecules including lysozyme.[15] There were three major reasons behind the selection of this method to prepare nanoparticles. First, this method is devoid of organic solvent such as methylene chloride and physical stress such as sonication which often denatures lysozyme.[26] Secondly, DMSO acts as a scavenger of free radicals and hence it enhances the stability of protein molecules. Finally, solubility limitation of HIP complex in organic solvents was also of concern. Solubility of HIP complex in any solvent depends on various parameters including nature of the complexing agent, polarity of the solvent and pH of the buffer containing the macromolecule. Solubility related issues associated with HIP complex may also restrict the application of a particular method to prepare nanoparticles. In our previous publication, we have employed solid in oil in water (S/O/W) emulsion method for the preparation of nanoparticles to overcome solubility related issue of a HIP complex.[11] However, this method still involves treatment of protein molecule with organic solvents and sonication which could denature the protein such as lysozyme.
This article describes preparation of nanoparticles using different ratios of PLGA 85:15. Interestingly, we observed significant increase in the size of nanoparticles, as we employed higher amounts of PLGA to prepare nanoparticles. Sizes of the nanoparticles were 220 ± 18 nm; 427.6 ± 28.3 nm and 541.6 ± 63.1 nm when the ratios of HIP complex to PLGA 85:15 were 1:5, 1:10 and 1:15, respectively (Table 1). At the ratio of 1:20, we could not prepare any nanoparticles. Once we started infusing DMSO solution into the nonsolvent (1% poloxamer solution), polymer precipitated instantaneously resulting in large aggregates. This phenomenon has been reported by other investigators during nanoparticle preparation.[27] The possible reason for formation of larger aggregate could be a higher intrinsic viscosity of DMSO solution containing a significantly higher amounts of polymer which resulted in aggregation.
Table 1.
Particle size and encapsulation efficiency of lysozyme in nanoparticles.
| Ratio of protien: PLGA 85:15 |
% Entrapment efficiency |
Particle size (nm) |
|
|---|---|---|---|
| 1 | 1:5 | 38.9% ± 9.05 | 220 ± 18 |
| 2 | 1:10 | 72.9% ± 7.90 | 427.6 ± 28.3 |
| 3 | 1:15 | 96.5% ± 10.2 | 541.6 ± 63.1 |
PLGA, poly lactic-co-glycolic acid.
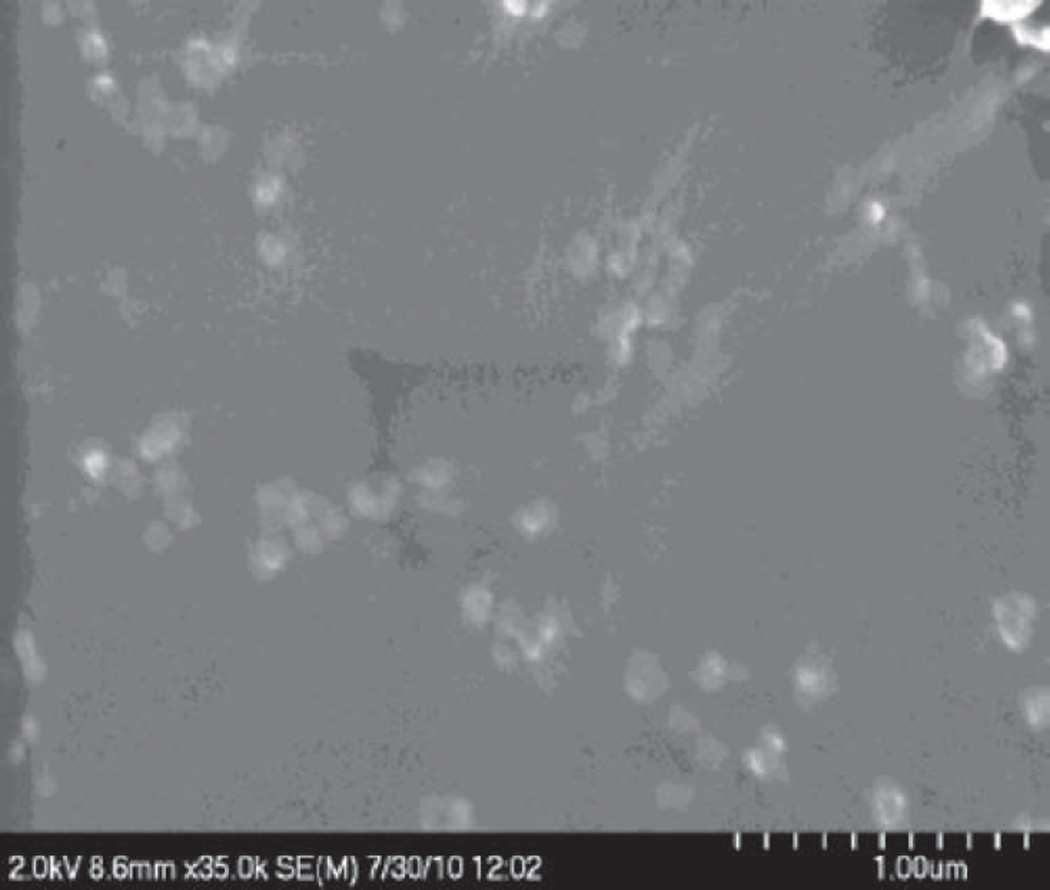
This work also studied the entrapment efficiency of lysozyme in different batches of nanoparticles. We observed the higher entrapment of the lysozyme as we increased the amount of PLGA used in the preparation of nanoparticles. When the ratios of HIP complex to polymer (PLGA 85:15) were 1:5, 1:10, and 1:15, entrapment efficiencies of lysozyme in nanoparticles were 38.8 ± 9.1, 72.81 ± 7.90, and 96.52 ± 10.23 % respectively (Table 1). It is likely that with higher polymer content enhanced hydrophobic interactions with HIP complex was achieved. This could be the reason for higher entrapment of lysozyme in nanoparticles. We also performed SEM analysis to study morphology of nanoparticles. The results clearly show that nanoparticles did not have a clear round shape and smooth surface (Figure 5). Instead they reveal irregular and asymmetric morphology. This could be due to lack of sonication during preparation of nanoparticles. This sort of morphology of nanoparticles was also observed by other investigators previously who have prepared nanoparticles without using sonication.[32]
Figure 5.
Scanning electron microscopic image of polymeric nanoparticles encapsulating lysozyme (lysozyme:PLGA, 1:5). PLGA, poly lactic-co-glycolic acid.
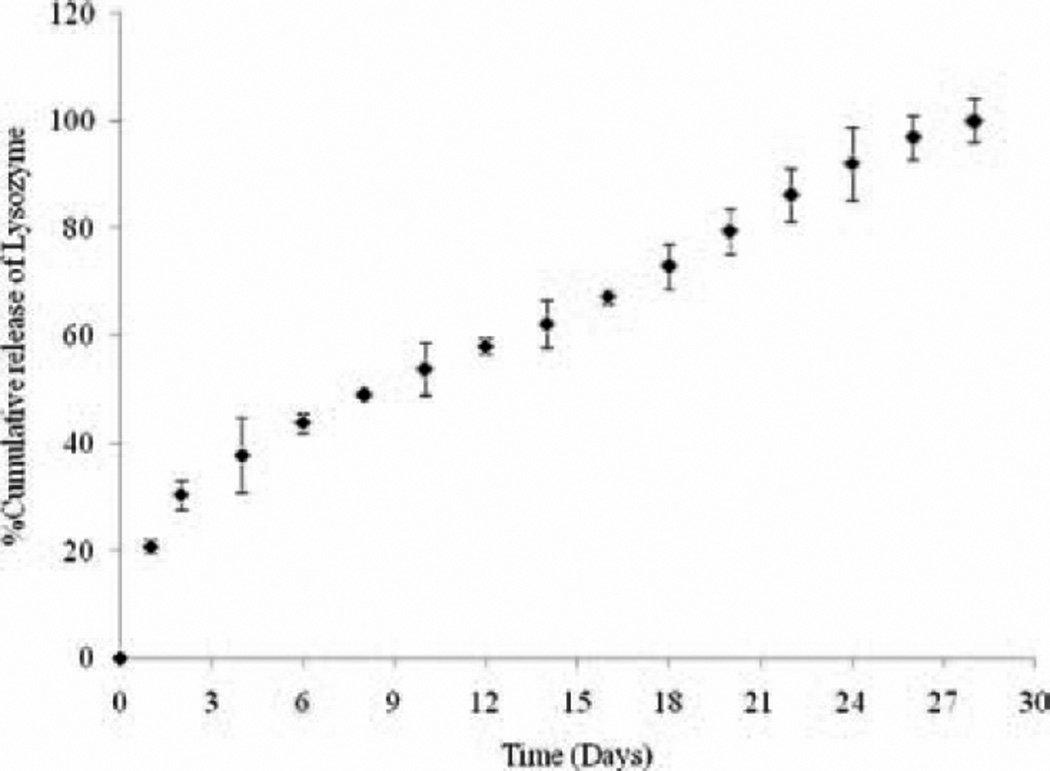
Finally, we studied the release of lysozyme from nanoparticles (ratio of lysozyme:polymer 1:5) (Figure 6). We performed release study of lysozyme from nanoparticles to evaluate if nanoparticles can sustain its release. Further, we also carried out an activity assay to ensure stability of lysozyme in polymeric matrix of nanoparticles. Sustained release of lysozyme from nanoparticles was observed over 28 days with minimal burst effect (≈ 20% in first 2 days) (Figure 7). Releases of a drug molecule from PLGA nanoparticles involve various phases. Initially, drug adsorbed on the surface of nanoparticles releases via burst effect. This phase is followed by a slower diffusion phase. Variation in drug release is mainly due to different rate of diffusion of HIP complex from nanoparticles. Since, particles are not of uniform round shape; this sort of release behavior was expected.
Figure 6.
In vitro release of lysozyme from PLGA nanoparticles (lysozyme:PLGA, 1:5). PLGA, poly lactic-co-glycolic acid.
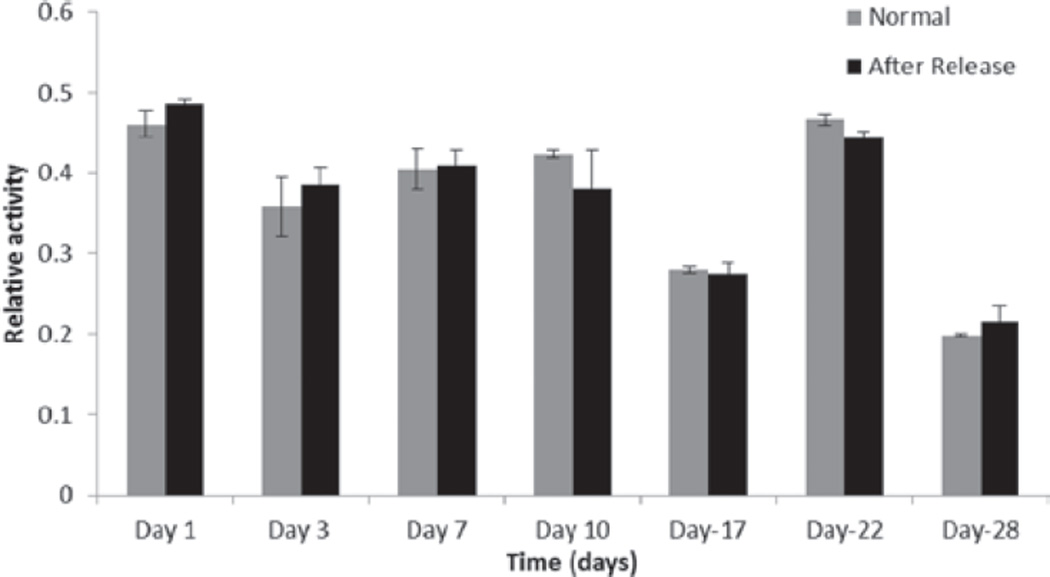
Figure 7.
Evaluation of an enzymatic activity of lysozyme after released from nanoparticles and comparison with enzymatic activity of freshly prepared solutions of lysozyme.
We hypothesize several mechanisms for sustained release behavior of lysozyme from nanoparticles such as slow degradation of PLGA 85:15 and strong hydrophobic interactions of HIP complex with polymeric matrix. Moreover, dissociation of lysozyme from the HIP complex was a slow process. As mentioned earlier, lysozyme-DS complex was entrapped in polymeric matrix and dissociation of lysozyme from the HIP complex was mainly dependent on the ionic strength of the surrounding medium. PBS buffer used in this study contains NaCl concentration of 137 mM. Hence dissociation of HIP complex was slow in the presence of PBS buffer. Finally, diffusion of the complex would occur slowly compared to free lysozyme. Hence, this approach may provide significant advantages in terms of sustaining the release of a protein molecule from the polymeric matrix of nanoparticles. Currently, we are studying the HIP complexation of lysozyme with different molecular weights of DS. We are also investigating the effect of different molecular weights of DS on the release of lysozyme from polymeric matrix (data not included). Finally, enzymatic activity of all release samples were measured and compared with freshly prepared solutions of lysozyme. Results of this experiment are shown in Figure 7. The data clearly indicate that enzymatic activity of lysozyme was not affected during encapsulation and release from PLGA polymer.
Conclusion
We have successfully prepared and characterized HIP complex of lysozyme using, dextran sulfate, a sulfated polysaccharide as a complexing agent. We observed a linear relationship between amounts of dextran sulfate added and formation of HIP complex. Electrostatic interactions seem to play a major role in the formation of HIP complex. Presence of electrostatic interactions was confirmed by rapid dissociation of lysozyme from HIP complex and also by FTIR studies. Nanoparticles were successfully prepared using spontaneous emulsion solvent diffusion method and characterized with respect to size and surface morphology. Entrapment of lysozyme was found to be higher as we increased the amount of PLGA in the preparation of nanoparticles. Nanoparticles have shown to sustain the release of lysozyme without significant burst release. Enzymatic activity of lysozyme was neither affected due to HIP complexation nor following release from polymeric matrix of nanoparticles. HIP complexation using a polymer may also be employed to formulate sustained release dosage forms for larger protein molecules such as antibodies.
Acknowledgments
The authors are thankful to Dr. Elisabet Kostoryz, School of Dentistry for helping us in dynamic light scattering study.
This work was supported by the National Institutes of Health grants R01 EY 09171-16 and R01 EY 10659-14.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- 1.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 2.Gelperina S, Kisich K, Iseman MD, Heifets L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med. 2005;172:1487–1490. doi: 10.1164/rccm.200504-613PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang S, Yuan W, Jin T. Formulating protein therapeutics into particulate forms. Expert Opin Drug Deliv. 2009;6:1123–1133. doi: 10.1517/17425240903156374. [DOI] [PubMed] [Google Scholar]
- 4.Martins S, Sarmento B, Ferreira DC, Souto EB. Lipid-based colloidal carriers for peptide and protein delivery–liposomes versus lipid nanoparticles. Int J Nanomedicine. 2007;2:595–607. [PMC free article] [PubMed] [Google Scholar]
- 5.van der Walle CF, Sharma G, Ravi Kumar M. Current approaches to stabilising and analysing proteins during microencapsulation in PLGA. Expert Opin Drug Deliv. 2009;6:177–186. doi: 10.1517/17425240802680169. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen L, Moeller EH, van de Weert M, Nielsen HM, Frokjaer S. Preparing and evaluating delivery systems for proteins. Eur J Pharm Sci. 2006;29:174–182. doi: 10.1016/j.ejps.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Houchin ML, Topp EM. Chemical degradation of peptides and proteins in PLGA: a review of reactions and mechanisms. J Pharm Sci. 2008;97:2395–2404. doi: 10.1002/jps.21176. [DOI] [PubMed] [Google Scholar]
- 8.Schwendeman SP. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit Rev Ther Drug Carrier Syst. 2002;19:73–98. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- 9.van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm Res. 2000;17:1159–1167. doi: 10.1023/a:1026498209874. [DOI] [PubMed] [Google Scholar]
- 10.Meyer JD, Manning MC. Hydrophobic ion pairing: altering the solubility properties of biomolecules. Pharm Res. 1998;15:188–193. doi: 10.1023/a:1011998014474. [DOI] [PubMed] [Google Scholar]
- 11.Gaudana R, Khurana V, Parenky A, Mitra AK. Encapsulation of Protein-Polysaccharide HIP Complex in Polymeric Nanoparticles. J Drug Deliv. 2011;2011:458128. doi: 10.1155/2011/458128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Cui F, Shi K, Cun D, Wang R. Design of high payload PLGA nanoparticles containing melittin/sodium dodecyl sulfate complex by the hydrophobic ion-pairing technique. Drug Dev Ind Pharm. 2009;35:959–968. doi: 10.1080/03639040902718039. [DOI] [PubMed] [Google Scholar]
- 13.Yuan H, Jiang SP, Du YZ, Miao J, Zhang XG, Hu FQ. Strategic approaches for improving entrapment of hydrophilic peptide drugs by lipid nanoparticles. Colloids Surf B Biointerfaces. 2009;70:248–253. doi: 10.1016/j.colsurfb.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Dai WG, Dong LC. Characterization of physiochemical and biological properties of an insulin/lauryl sulfate complex formed by hydrophobic ion pairing. Int J Pharm. 2007;336:58–66. doi: 10.1016/j.ijpharm.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Yoo HS, Choi HK, Park TG. Protein-fatty acid complex for enhanced loading and stability within biodegradable nanoparticles. J Pharm Sci. 2001;90:194–201. doi: 10.1002/1520-6017(200102)90:2<194::aid-jps10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T, Itagaki H, Fukuda T, Tamura U, Sato Y, Suzuki Y. Hemoglobin denaturation caused by surfactants. Biol Pharm Bull. 1995;18:540–543. doi: 10.1248/bpb.18.540. [DOI] [PubMed] [Google Scholar]
- 17.DeTrana C, Hurwitz RM. Painful purpura: an adverse effect to a thrombolysin. Arch Dermatol. 1990;126:690–691. [PubMed] [Google Scholar]
- 18.Dalwadi G, Sunderland B. An ion pairing approach to increase the loading of hydrophilic and lipophilic drugs into PEGylated PLGA nanoparticles. Eur J Pharm Biopharm. 2009;71:231–242. doi: 10.1016/j.ejpb.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Siddalingappa B, Chan PH, Benson HA. Development of a chitosan-based nanoparticle formulation for delivery of a hydrophilic hexapeptide, dalargin. Biopolymers. 2008;90:663–670. doi: 10.1002/bip.21055. [DOI] [PubMed] [Google Scholar]
- 20.Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS. Stability of protein pharmaceuticals: an update. Pharm Res. 2010;27:544–575. doi: 10.1007/s11095-009-0045-6. [DOI] [PubMed] [Google Scholar]
- 21.Gao G, Yan Y, Pispas S, Yao P. Sustained and extended release with structural and activity recovery of lysozyme from complexes with sodium (sulfamate carboxylate) isoprene/ethylene oxide block copolymer. Macromol Biosci. 2010;10:139–146. doi: 10.1002/mabi.200900186. [DOI] [PubMed] [Google Scholar]
- 22.Stigter D, Dill KA. Charge effects on folded and unfolded proteins. Biochemistry. 1990;29:1262–1271. doi: 10.1021/bi00457a023. [DOI] [PubMed] [Google Scholar]
- 23.Frokjaer S, Otzen DE. Protein drug stability: a formulation challenge. Nat Rev Drug Discov. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 24.McClements DJ. Non-covalent interactions between proteins and polysaccharides. Biotechnol Adv. 2006;24:621–625. doi: 10.1016/j.biotechadv.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Sacco D, Dellacherie E. Interaction of a macromolecular polyanion, dextran sulfate, with human hemoglobin. FEBS Lett. 1986;199:254–258. doi: 10.1016/0014-5793(86)80490-2. [DOI] [PubMed] [Google Scholar]
- 26.Krishnamurthy R, Lumpkin JA, Sridhar R. Inactivation of lysozyme by sonication under conditions relevant to microencapsulation. Int J Pharm. 2000;205:23–34. doi: 10.1016/s0378-5173(00)00473-7. [DOI] [PubMed] [Google Scholar]
- 27.Bilati U, Allémann E, Doelker E. Nanoprecipitation versus emulsion-based techniques for the encapsulation of proteins into biodegradable nanoparticles and process-related stability issues. AAPS PharmSciTech. 2005;6:E594–E604. doi: 10.1208/pt060474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai C, Bakowsky U, Rytting E, Schaper AK, Kissel T. Charged nanoparticles as protein delivery systems: a feasibility study using lysozyme as model protein. Eur J Pharm Biopharm. 2008;69:31–42. doi: 10.1016/j.ejpb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Amrutkar JR, Gattani SG. Chitosan-chondroitin sulfate based matrix tablets for colon specific delivery of indomethacin. AAPS PharmSciTech. 2009;10:670–677. doi: 10.1208/s12249-009-9253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiyaboonchai W, Limpeanchob N. Formulation and characterization of amphotericin B-chitosan-dextran sulfate nanoparticles. Int J Pharm. 2007;329:142–149. doi: 10.1016/j.ijpharm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Sarmento B, Ribeiro A, Veiga F, Ferreira D. Development and characterization of new insulin containing polysaccharide nanoparticles. Colloids Surf B Biointerfaces. 2006;53:193–202. doi: 10.1016/j.colsurfb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Dalpiaz A, Vighi E, Pavan B, Leo E. Fabrication via a nonaqueous nanoprecipitation method, characterization and in vitro biological behavior of N(6)-cyclopentyladenosine-loaded nanoparticles. J Pharm Sci. 2009;98:4272–4284. doi: 10.1002/jps.21710. [DOI] [PubMed] [Google Scholar]