Abstract
We have used the polymerase chain reaction (PCR) to amplify up to 22 kb of the beta-globin gene cluster from human genomic DNA and up to 42 kb from phaga lambda DNA. We have also amplified 91 human genomic inserts of 9-23 kb directly from recombinant lambda plaques. To do this, we increased pH, added glycerol and dimethyl sulfoxide, decreased denaturation times, increased extension times, and used a secondary thermostable DNA polymerase that possesses a 3'-to 5'-exonuclease, or "proofreading," activity. Our "long PCR" protocols maintain the specificity required for targets in genomic DNA by using lower levels of polymerase and temperature and salt conditions for specific primer annealing. The ability to amplify DNA sequences of 10-40 kb will bring the speed and simplicity of PCR to genomic mapping and sequencing and facilitate studies in molecular genetics.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
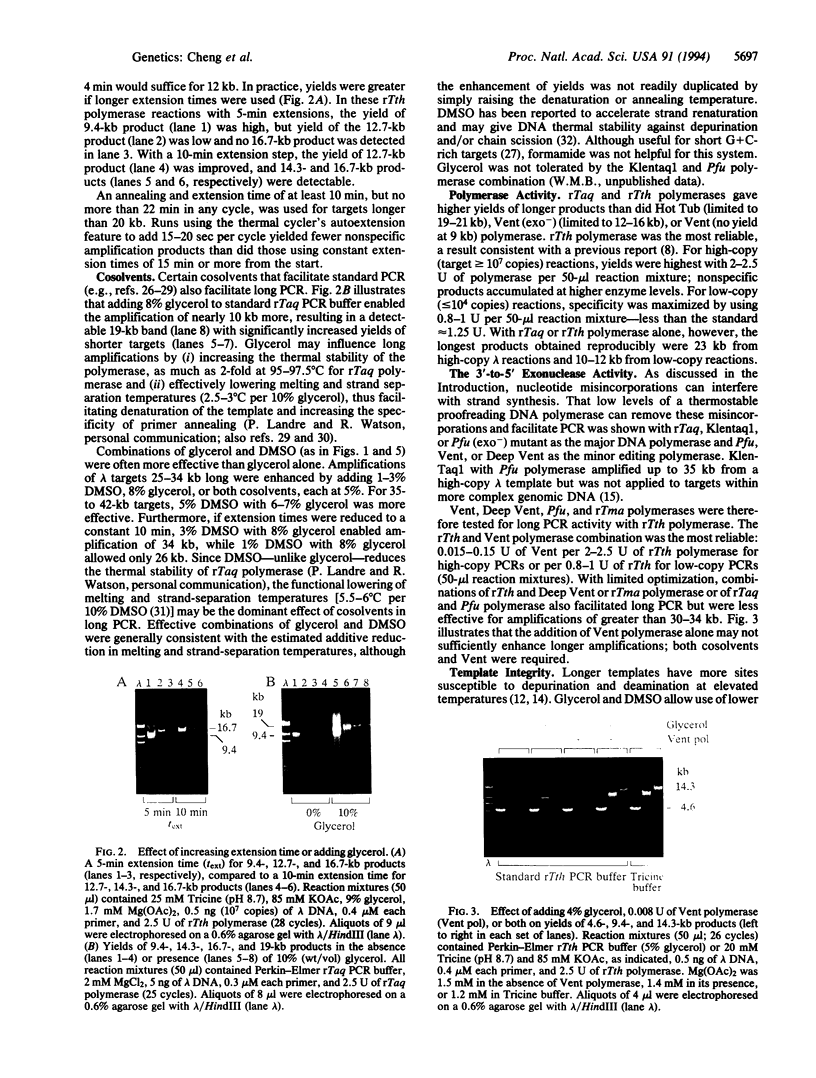
- Barnes W. M. PCR amplification of up to 35-kb DNA with high fidelity and high yield from lambda bacteriophage templates. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2216–2220. doi: 10.1073/pnas.91.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Frank M., Olson M. V. Electrophoretic separations of large DNA molecules by periodic inversion of the electric field. Science. 1986 Apr 4;232(4746):65–68. doi: 10.1126/science.3952500. [DOI] [PubMed] [Google Scholar]
- Chester N., Marshak D. R. Dimethyl sulfoxide-mediated primer Tm reduction: a method for analyzing the role of renaturation temperature in the polymerase chain reaction. Anal Biochem. 1993 Mar;209(2):284–290. doi: 10.1006/abio.1993.1121. [DOI] [PubMed] [Google Scholar]
- Chou Q., Russell M., Birch D. E., Raymond J., Bloch W. Prevention of pre-PCR mis-priming and primer dimerization improves low-copy-number amplifications. Nucleic Acids Res. 1992 Apr 11;20(7):1717–1723. doi: 10.1093/nar/20.7.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. H., Jagadeeswaran P., Wang R. R., Weissman S. M. Structural analysis of templates and RNA polymerase III transcripts of Alu family sequences interspersed among the human beta-like globin genes. Gene. 1981 Mar;13(2):185–196. doi: 10.1016/0378-1119(81)90007-x. [DOI] [PubMed] [Google Scholar]
- Erlich H. A., Arnheim N. Genetic analysis using the polymerase chain reaction. Annu Rev Genet. 1992;26:479–506. doi: 10.1146/annurev.ge.26.120192.002403. [DOI] [PubMed] [Google Scholar]
- Escara J. F., Hutton J. R. Thermal stability and renaturation of DNA in dimethyl sulfoxide solutions: acceleration of the renaturation rate. Biopolymers. 1980 Jul;19(7):1315–1327. doi: 10.1002/bip.1980.360190708. [DOI] [PubMed] [Google Scholar]
- Filichkin S. A., Gelvin S. B. Effect of dimethyl sulfoxide concentration on specificity of primer matching in PCR. Biotechniques. 1992 Jun;12(6):828–830. [PubMed] [Google Scholar]
- Glukhov A. I., Trofimova M. E., Gordeev S. A., Grebennikova T. V., Vinogradov S. V., Kiselev V. I., Kramarov V. M. Amplifikatsiia posledovatel'nostei DNK faga lambda metodom polimeraznoi tsepnoi reaktsii s ispol'zovaniem termostabil'noi DNK-polimerazy. Mol Biol (Mosk) 1991 Nov-Dec;25(6):1602–1610. [PubMed] [Google Scholar]
- Good N. E., Izawa S. Hydrogen ion buffers. Methods Enzymol. 1972;24:53–68. doi: 10.1016/0076-6879(72)24054-x. [DOI] [PubMed] [Google Scholar]
- Huang M. M., Arnheim N., Goodman M. F. Extension of base mispairs by Taq DNA polymerase: implications for single nucleotide discrimination in PCR. Nucleic Acids Res. 1992 Sep 11;20(17):4567–4573. doi: 10.1093/nar/20.17.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W., Wirsen C. O., Molyneaux S. J., Langworthy T. A. Comparative Physiological Studies on Hyperthermophilic Archaea Isolated from Deep-Sea Hot Vents with Emphasis on Pyrococcus Strain GB-D. Appl Environ Microbiol. 1992 Nov;58(11):3472–3481. doi: 10.1128/aem.58.11.3472-3481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Neumann R., Keyte J. Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res. 1988 Dec 9;16(23):10953–10971. doi: 10.1093/nar/16.23.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. H., Winistorfer S. C. A method for the amplification of unknown flanking DNA: targeted inverted repeat amplification. Biotechniques. 1993 Nov;15(5):894–904. [PubMed] [Google Scholar]
- Kainz P., Schmiedlechner A., Strack H. B. In vitro amplification of DNA fragments greater than 10 kb. Anal Biochem. 1992 Apr;202(1):46–49. doi: 10.1016/0003-2697(92)90203-j. [DOI] [PubMed] [Google Scholar]
- Kieleczawa J., Dunn J. J., Studier F. W. DNA sequencing by primer walking with strings of contiguous hexamers. Science. 1992 Dec 11;258(5089):1787–1791. doi: 10.1126/science.1465615. [DOI] [PubMed] [Google Scholar]
- Kong H., Kucera R. B., Jack W. E. Characterization of a DNA polymerase from the hyperthermophile archaea Thermococcus litoralis. Vent DNA polymerase, steady state kinetics, thermal stability, processivity, strand displacement, and exonuclease activities. J Biol Chem. 1993 Jan 25;268(3):1965–1975. [PubMed] [Google Scholar]
- Korge B. P., Gan S. Q., McBride O. W., Mischke D., Steinert P. M. Extensive size polymorphism of the human keratin 10 chain resides in the C-terminal V2 subdomain due to variable numbers and sizes of glycine loops. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):910–914. doi: 10.1073/pnas.89.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B. R., Kersulyte D., Brikun I., Berg C. M., Berg D. E. Direct and crossover PCR amplification to facilitate Tn5supF-based sequencing of lambda phage clones. Nucleic Acids Res. 1991 Nov 25;19(22):6177–6182. doi: 10.1093/nar/19.22.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawyer F. C., Stoffel S., Saiki R. K., Chang S. Y., Landre P. A., Abramson R. D., Gelfand D. H. High-level expression, purification, and enzymatic characterization of full-length Thermus aquaticus DNA polymerase and a truncated form deficient in 5' to 3' exonuclease activity. PCR Methods Appl. 1993 May;2(4):275–287. doi: 10.1101/gr.2.4.275. [DOI] [PubMed] [Google Scholar]
- Lawyer F. C., Stoffel S., Saiki R. K., Myambo K., Drummond R., Gelfand D. H. Isolation, characterization, and expression in Escherichia coli of the DNA polymerase gene from Thermus aquaticus. J Biol Chem. 1989 Apr 15;264(11):6427–6437. [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Lundberg K. S., Shoemaker D. D., Adams M. W., Short J. M., Sorge J. A., Mathur E. J. High-fidelity amplification using a thermostable DNA polymerase isolated from Pyrococcus furiosus. Gene. 1991 Dec 1;108(1):1–6. doi: 10.1016/0378-1119(91)90480-y. [DOI] [PubMed] [Google Scholar]
- Maga E. A., Richardson T. Amplification of a 9.0-kb fragment using PCR. Biotechniques. 1991 Aug;11(2):185–186. [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Myers T. W., Gelfand D. H. Reverse transcription and DNA amplification by a Thermus thermophilus DNA polymerase. Biochemistry. 1991 Aug 6;30(31):7661–7666. doi: 10.1021/bi00245a001. [DOI] [PubMed] [Google Scholar]
- Nelson D. L. Applications of polymerase chain reaction methods in genome mapping. Curr Opin Genet Dev. 1991 Jun;1(1):62–68. doi: 10.1016/0959-437x(91)80043-l. [DOI] [PubMed] [Google Scholar]
- Ochman H., Gerber A. S., Hartl D. L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988 Nov;120(3):621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohler L. D., Rose E. A. Optimization of long-distance PCR using a transposon-based model system. PCR Methods Appl. 1992 Aug;2(1):51–59. doi: 10.1101/gr.2.1.51. [DOI] [PubMed] [Google Scholar]
- Pomp D., Medrano J. F. Organic solvents as facilitators of polymerase chain reaction. Biotechniques. 1991 Jan;10(1):58–59. [PubMed] [Google Scholar]
- Ponce M. R., Micol J. L. PCR amplification of long DNA fragments. Nucleic Acids Res. 1992 Feb 11;20(3):623–623. doi: 10.1093/nar/20.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose E. A. Applications of the polymerase chain reaction to genome analysis. FASEB J. 1991 Jan;5(1):46–54. doi: 10.1096/fasebj.5.1.1991584. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G. DNA probes: applications of the principles of nucleic acid hybridization. Crit Rev Biochem Mol Biol. 1991;26(3-4):227–259. doi: 10.3109/10409239109114069. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wong D. M., Weinstock P. H., Wetmur J. G. Branch capture reactions: displacers derived from asymmetric PCR. Nucleic Acids Res. 1991 May 11;19(9):2251–2259. doi: 10.1093/nar/19.9.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D. Y., Ugozzoli L., Pal B. K., Qian J., Wallace R. B. The effect of temperature and oligonucleotide primer length on the specificity and efficiency of amplification by the polymerase chain reaction. DNA Cell Biol. 1991 Apr;10(3):233–238. doi: 10.1089/dna.1991.10.233. [DOI] [PubMed] [Google Scholar]