Abstract
Immunization with recombinant lipoprotein outer surface protein A vaccine is known to interfere with some serologic tests for Lyme disease. We tested sera from 152 vaccine recipients by using in-house and commercial Western blot assays and found that vaccination caused interference in up to 25% of recipients and can persist for over 6 years.
In addition to preventing Lyme disease, immunization with the recombinant lipoprotein outer surface protein A (rOspA) to prevent infection with Borrelia burgdorferi induced the production of antibodies which caused false-positive enzyme-linked immunosorbent assay (ELISA) results and interfered with the interpretation of Western blot (WB) tests (1, 4, 6). The potential difficulties to testing laboratories as a result of this interference appeared to be rendered a moot point when the vaccine manufacturer withdrew rOspA from the market in 2002, owing to financial considerations. However, the potential for long-term interference with diagnostic tests for Lyme disease in recipients of the vaccine has not to our knowledge been investigated. This paper reports on the findings obtained when vaccine recipients were tested for immunoglobulin G (IgG) antibodies to B. burgdorferi by using in-house-developed ELISA and WB test and commercial WB tests (Immunetics and Marblot). Test serum samples were obtained from 152 vaccine recipients who claimed to have had adverse reactions to the vaccine. The elapsed time from the last dose of vaccine to sample acquisition ranged from 5 months to 6 years and 7 months, with a median of 2.12 years, based on 134 individuals who provided that information. The in-house ELISA and WB were both produced by using low-passage B. burgdorferi strain B31, and the manufacture and use of these assays have been described previously (3, 5). Commercial WB tests were performed according to the manufacturer's recommended procedures for testing and interpretation of results. In addition to assessing WB test results by the Centers for Disease Control (CDC)/Dearborn criteria, all other bands, including reactivity to OspA, were recorded (2). Results of ELISA testing for IgG antibodies showed that 60% of the sera were nonreactive; however, the lack of reactivity did not correlate directly with the elapsed time since the last dose of vaccine. Testing for IgM antibodies, which was performed by using an in-house WB test, revealed reactivity in only 6% of the samples tested, none of which were considered positive.
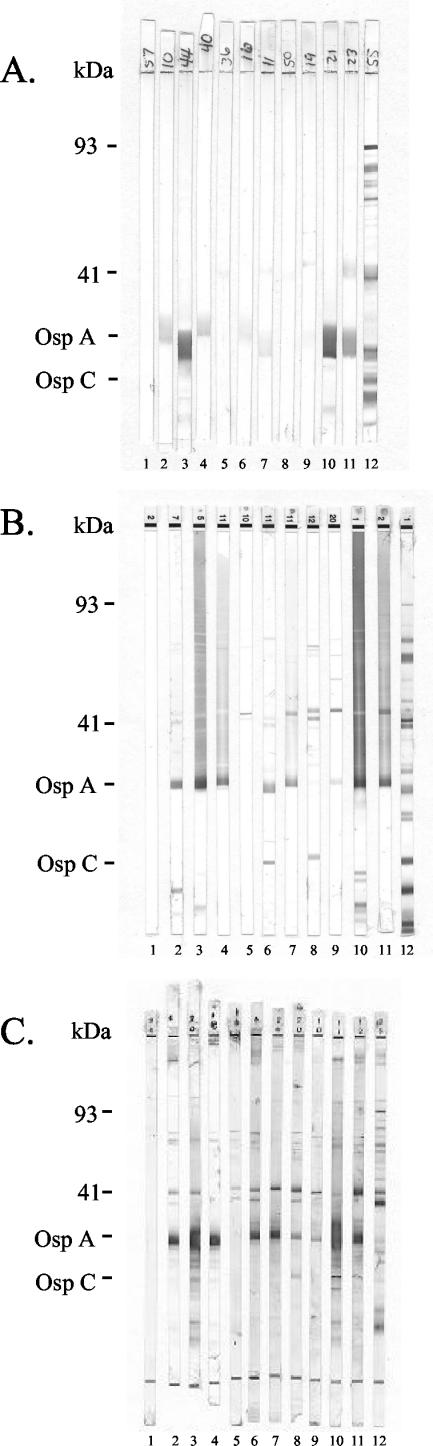
Results of WB testing for IgG antibodies to B. burgdorferi are summarized in Table 1. Analysis of results revealed that 62% of sera from individuals had some reactivity (at least one band) on the in-house WB test, with 86 and 99% of sera having some reaction on the Marblot and Immunetics WB tests, respectively. Reactivity to OspA was the most commonly detected band on each of the blots (49% for in-house, 62% for Marblot, and 91% for Immunetics), followed by bands corresponding to the 41-kDa flagellar antigen (14% for in-house, 30% for Marblot, and 81% for Immunetics). The percentage of sera showing at least one band, if one excludes the bands to OspA, is not out of line with what is expected when testing a population not infected with Lyme. Indeed, the percentage of sera with a band at 41 kDa is lower for the in-house WB test than we have previously reported (3). However, over 25% of the tested sera produced sufficient reactivity on the commercial WB tests to make the interpretation of test results difficult. In the case of Marblot assay, 25% of the WB tests showed significant graying in the high-molecular-mass region, with some tests also having multiple discrete bands (5%). According to the manufacturer, blot strips that exhibit extensive graying should be considered unreadable. An evaluation of Immunetics WB test strips revealed that over 25% of sera from vaccine recipients produced multiple discrete bands (6 or more), which made test interpretation difficult and required blinded reading by two or more technicians. Overlap between the populations yielding significant background on the two commercial tests was less than 50%. Despite the degree of WB test reactivity observed, only seven individuals were considered positive when evaluated by CDC/Dearborn criteria for interpretation (seven by Immunetics and one of those seven by Marblot). Overall evaluation of the three blot tests did not, in our opinion, indicate that any of the individuals tested had been infected with B. burgdorferi. This interpretation was based on experience from a previous study for which employee volunteers at our hospital were serially sampled and tested from baseline through 1 month after the third dose of vaccine. Results of that study showed reactivity on each of the WB tests similar to that reported here. None of the volunteers had a history of infection with B. burgdorferi. The in-house WB test, while showing less reactivity in general, tended to have a very broad band of reactivity around the 31- and often the 23-kDa regions, making determination of banding in those areas challenging. Each of the three WB tests used detected reactivity to 9 of the 10 CDC/Dearborn criteria bands with the population tested. Interestingly, the band that was not reacted with was different for each of the blots, with the in-house WB test showing no detection of bands at 58 kDa, the Marblot test showing no detection of bands at 28 kDa, and the Immunetics test showing no detection of bands at 45 kDa. The frequency of reactivity to criteria bands also differed between the assays, with the exception of the 41-kDa antigen (most frequent for each assay). The two most common criteria bands were the 28-kDa band and the 30- and 23-kDa (same frequency) bands for in-house WB assay, the 37- and 58-kDa bands for Marblot assay, and the 66- and 58-kDa bands for Immunetics assay. Figure 1 shows WB test strip results from selected samples of each of the three WB tests.
TABLE 1.
Summary of WB results for rOspA vaccine recipients (n = 152)
| WB assay type | Mean no. of bands (range)a
|
|
|---|---|---|
| CDC criteria | Total | |
| In house | 0.38 (0-4) | 1.3 (0-7) |
| Marblot | 0.058 (0-6) | 2.0 (0-9) |
| Immunetics | 1.84 (0-6) | 4.4 (0-15) |
Values presented represent the mean determined from counting bands designated in CDC/Dearborn criteria and from a total count of all bands present. In some instances, regions of broad bands (in particular, for Marblot WB strips) may have interfered with the determination of other discrete bands.
FIG. 1.
WB strips from the in-house (A), Marblot (B), and Immunetics (C) assays used to test for IgG antibodies on control and vaccine recipient samples are shown. The control strips (negative, lane 1; positive, lane 12) were unique for each blot assay. Strips 2 through 11 show results for the same 10 vaccine recipients' samples tested with each of the three WB assays. These samples were selected to demonstrate typical reactivity observed in this study. The number of doses and elapsed time (in years and months) since the last vaccine dose for each recipient (ordered left to right [strips 2 to 11]) were as follows: lane 2, 2 doses, 1 year and 3 months; lane 3, 3 doses, 1 year and 6 months; lane 4, 3 doses, 5 years and 9 months; lane 5, 1 dose, 2 years and 6 months; lane 6, 2 doses, 2 years and 6 months; lane 7, 2 doses, 6 years and 7 months; lane 8, 1 dose, 1 year and 6 months; lane 9, 2 doses, 2 years and 8 months; lane 10, 3 doses, 3 years and 7 months, lane 11, 3 doses, 3 years and 7 months.
This study was initiated as a result of observations made when reviewing WB test results obtained when sera from vaccine recipients claiming adverse reactions to the vaccine were tested in an effort to rule out possible infection with B. burgdorferi. Results confirmed earlier reports that immunization with rOspA could result in an immune response that would interfere with subsequent serologic testing for infection with B. burgdorferi (1, 4, 6). Furthermore, our findings demonstrate that the degree of interference encountered varies greatly depending on the manufacturer of the WB test used and that the interference can persist for years. Indeed, of the three individuals whose sera were obtained over 5 years after their last dose of vaccine, two had positive ELISA results and all three had reactivity detected with all three WB tests (between 1 and 11 bands).
Results from this study indicate that reference laboratories should require notification of vaccine status when testing for Lyme disease to aid in the interpretation of results. Furthermore, as a high percentage of vaccine recipients show reactivity to two or more bands used as part of the CDC/Dearborn criteria, laboratories should consider formulating an alternative algorithm for use in interpreting WB test results from vaccine recipients.
In addition to documenting long-term interference with WB test interpretation as a result of immunization with rOspA, our findings showed extreme interassay variation with regard to the amount of reactivity detected for a given sample. This finding was somewhat surprising, as all three assays were manufactured by using the B31 strain of B. burgdorferi, none of the patients tested were believed to have been infected with B. burgdorferi, and the elapsed time since the last dose of vaccine exceeded 6 years in one case (median, 2.12 years). Thus, this test population should have appreciable levels of antibodies to only OspA and any other B. burgdorferi proteins that share antigenic determinants with OspA, in addition to the expected amount of cross-reactivity detected in patients not infected with Lyme disease (3). These results suggest that interpretation of WB test results of vaccine recipients can remain a problem for several years and that results from different WB tests for such individuals should be interpreted with caution.
REFERENCES
- 1.Aguero-Rosenfeld, M. E., J. Roberge, C. A. Carbonaro, J. Nowakowski, R. B. Nadelman, and G. P. Wormser. 1999. Effect of OspA vaccination of Lyme disease serologic testing. J. Clin. Microbiol. 37:3718-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1995. Recommendation for test performance and interpretation from Second National Conference on Serologic Diagnosis of Lyme Disease. Morb. Mortal. Wkly. Rep. 44:590-591. [PubMed] [Google Scholar]
- 3.Fawcett, P. T., K. M. Gibney, C. D. Rose, S. B. Dubbs, and R. A. Doughty. 1992. Frequency and specificity of antibodies that cross-react with Borrelia Burgdorferi antigens. J. Rheumatol. 19:582-587. [PubMed] [Google Scholar]
- 4.Fawcett, P. T., C. D. Rose, S. Budd, and K. M. Gibney. 2001. Effect of immunization with recombinant OspA on serologic test for Lyme borreliosis. Clin. Diagn. Lab. Immunol. 8:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fawcett, P. T., C. D. Rose, and K. M. Gibney. 1995. Comparative evaluation of adsorption with E. coli on ELISA tests from Lyme borreliosis. J. Rheumatol. 22:684-688. [PubMed] [Google Scholar]
- 6.Molloy, P. J. V. P. Berardi, D. H. Persing, and L. H. Sigal. 2000. Detection of multiple reactive protein species by immunoblotting after outer recombinant surface protein A Lyme disease vaccination. Clin. Infect. Dis. 31:42-47. [DOI] [PubMed] [Google Scholar]