Abstract
♦ Background: The impact of a low-glucose peritoneal dialysis (PD) regimen on biomarkers of peritoneal inflammation, fibrosis and membrane integrity remains to be investigated.
♦ Methods: In a randomized, prospective study, 80 incident PD patients received either a low-glucose regimen comprising Physioneal (P), Extraneal (E) and Nutrineal (N) (Baxter Healthcare Corporation, Deerfield, IL, USA) (PEN group), or Dianeal (control group) for 12 months, after which both groups continued with Dianeal dialysis for 6 months. Serum and dialysate levels of vascular endothelial growth factor (VEGF), decorin, hepatocyte growth factor (HGF), interleukin-6 (IL-6), macrophage migration inhibitory factor (MIF), hyaluronan (HA), adiponectin, soluble-intracellular adhesion molecule (s-ICAM), vascular cell adhesion molecule-1 (VCAM-1) and P-selectin, and dialysate cancer antigen 125 (CA125), were measured after 12 and 18 months. This paper focuses on results after 12 months, when patients in the PEN group changed to glucose-based PD fluid (PDF).
♦ Results: At the end of 12 months, effluent dialysate levels of CA125, decorin, HGF, IL-6, adiponectin and adhesion molecules were significantly higher in the PEN group compared to controls, but all decreased after patients switched to glucose-based PDF. Macrophage migration inhibitory factor level was lower in the PEN group but increased after changing to glucose-based PDF and was similar to controls at 18 months. Serum adiponectin level was higher in the PEN group at 12 months, but was similar in the 2 groups at 18 months. Body weight, residual renal function, ultrafiltration volume and total Kt/V did not differ between both groups. Dialysate-to-plasma creatinine ratio at 4 h was higher in the PEN group at 12 months and remained so after switching to glucose-based PDF.
♦ Conclusion: Changes in the biomarkers suggest that the PEN PD regimen may be associated with better preservation of peritoneal membrane integrity and reduced systemic vascular endothelial injury.
Keywords: Amino acid, biocompatibility, icodextrin, peritoneal dialysis, peritoneal inflammation, pH neutral
Preservation of the peritoneal membrane structure is central to the long-term success of peritoneal dialysis (PD). Constant exposure of the peritoneum to bio-incompatible glucose-based PD fluids (PDFs) results in deleterious changes to the peritoneal structure, which is associated with technique failure and unfavorable clinical outcome (1). Conventional PDFs contain unphysiologically high glucose concentrations to provide the osmotic drive, a low pH to prevent glucose caramelization during heat sterilization, and lactate to correct metabolic acidosis. During heat sterilization, glucose degradation products (GDPs) are generated and both glucose and GDPs result in the formation of advanced glycation end products (2). These components impair cellular functions in peritoneal resident and immune cells, resulting in the progressive replacement with extracellular matrix and fibrous tissue, and impairment of peritoneal function (3–5).
Alternative PDFs that utilize amino acids or icodextrin as the osmotic agent, or the partial substitution of lactate with bicarbonate, have been developed with the aim of preserving the dialytic function of the peritoneal membrane. The use of amino acid-based PDF is associated with modest nutritional benefit (6). Icodextrin-based PDF has proven efficacy in improving ultrafiltration, especially in high transporters, because of its sustained osmotic drive during a long dwell (7,8). Bicarbonate-lactate buffered PDFs have a physiological pH and less GDP formation compared to glucose-based lactate buffered PDFs. Previous studies have shown that bicarbonatelactate buffered PDFs can maintain acid-base balance, decrease intraperitoneal levels of inflammatory mediators, and possibly increase ultrafiltration compared with glucose-based lactate buffered PDFs (9,10). The local and systemic effects of conventional and alternative PDFs have recently been reviewed by García-Lopez et al. (11).
A PD regimen incorporating PDFs with different beneficial properties offers the theoretical advantage of reducing the exposure of the peritoneum to the bioincompatible nature of glucose-based PDF, but randomized prospective studies are few and of short duration. We recently reported our findings of a 12-month study that compared a novel low-glucose PD regimen comprising amino acid-based, icodextrin-based, and bicarbonate-lactate buffered PDFs (Physioneal [P], Extraneal [E] and Nutrineal [N] [PEN] group) with a conventional regimen using glucose-based PDF (control group), which showed that PEN treatment was associated with better preservation of daily urine volume and peritoneal membrane integrity (12). We have extended our observation period for a further 6 months, during which time patients in the PEN group were switched to conventional glucose-based PDF. Our objective was to confirm the causality of the observed changes in peritoneal membrane function and biomarkers during PEN treatment, and to extend our investigations to additional biomarkers pertinent to peritoneal inflammation, fibrosis and mesothelial cell mass.
The measurement of locally produced biomarkers in dialysate effluent represents a non-invasive means to assess peritoneal homeostasis, inflammation, and fibrosis. Established effluent biomarkers include cancer antigen 125 (CA125), vascular endothelial growth factor (VEGF), hyaluronan (HA), interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α (13), but interpretation of their levels in dialysate effluent is open to debate. For example, IL-6 is often considered a putative marker of peritoneal inflammation, but this is confounded by observations that IL-6 can down-regulate inflammatory molecules through the activation of IL-1 and TNF receptor antagonists, and dialysate IL-6 levels correlate positively with CA125, a marker of mesothelial cell mass and function (14,15). Given that IL-6 possesses anti-inflammatory properties, it is possible that IL-6 can assist in peritoneal membrane preservation. Measurement of VEGF and TNF-α in dialysis effluent may not truly reflect local production since trans-peritoneal transport of these biomarkers from the systemic circulation to dialysate has been reported (16,17). There is, therefore, a need to identify additional biomarkers that may provide a better reflection of peritoneal membrane changes.
Hepatocyte growth factor (HGF) and decorin are anti-fibrotic mediators that have been detected in spent PDF (18,19). Hepatocyte growth factor has been shown to ameliorate high glucose-induced epithelial-to-mesenchymal transition (EMT) and peritoneal fibrosis in animal models of PD and encapsulating peritoneal sclerosis (EPS) (20,21), whereas decorin plays an important role in extracellular matrix organization and inhibition of transforming growth factor (TGF)-β1 signaling (22,23). Macrophage migration inhibitory factor (MIF) is independently associated with inflammation in PD and hemodialysis patients, and its increased expression in the peritoneum is associated with peritoneal fibroblast activation during EPS (24,25). Atherosclerotic cardiovascular complications are a majority cause of morbidity and mortality in PD patients. Adiponectin is an adipose-derived hormone known to protect against cardiovascular disease. Plasma adiponectin levels are decreased in PD patients using conventional glucose-based PDF, whereas use of icodextrin-based PDF has been shown to increase adiponectin levels (26). Adhesion molecules present on mesothelial and endothelial cells play important roles in lymphocyte recruitment to sites of injury (27,28) and their synthesis can be increased by IL-6 during inflammatory processes. Given their putative roles in peritoneal membrane injury/preservation, in addition to the more established biomarkers, we have also measured serum and dialysate levels of decorin, HGF, MIF, adiponectin, soluble-intracellular adhesion molecule (s-ICAM), vascular cell adhesion molecule-1 (VCAM-1), and P-selectin to ascertain whether these molecules may be considered as additional biomarkers of peritoneal inflammation and fibrosis.
Methods
Patients and Study Design
This multicenter, open-labeled, randomized, prospective study was approved by the Institutional Review Boards of all 8 participating hospitals and registered with the Hong Kong Clinical Trial Registry (trial number HKCTR-8). All participating patients gave written informed consent at the time of enrollment. Newly started continuous ambulatory PD patients were recruited from January 2006 to December 2008 and randomized to receive either a low-glucose regimen (PEN group) or conventional glucose-based PDF (control group). The PEN dialysis regimen included 1 exchange of amino acid-based PDF (Nutrineal; Baxter Healthcare Corporation, Deerfield, IL, USA), 1 – 2 exchanges of neutral pH, low GDP PDF (Physioneal; Baxter Healthcare Corporation, Deerfield, IL, USA), and 1 overnight exchange of icodextrin-based PDF (Extraneal; Baxter Healthcare Corporation, Deerfield, IL, USA) daily for 12 months. Patients in the control group received 3 – 4 exchanges of glucose-based PDF (Dianeal; Baxter Healthcare Corporation, Deerfield, IL, USA). After 12 months, patients in the PEN group changed to glucose-based PDF while controls continued with glucose-based PDF, and both groups were followed for another 6 months. Randomization, inclusion and exclusion criteria are described elsewhere (12). Although 150 patients consented to participate in this study, 126 completed the first 12 months of the study (12), of which 80 completed the 18 months’ study. Primary endpoints included biomarker levels for mesothelial cell mass, fibrosis and inflammation in dialysis effluent and/or serum, and residual renal function and daily urine volume.
Collection of PDF and Blood Samples
Overnight dialysate samples (50 mL) collected from patients at 12 and 18 months after the start of the study were centrifuged at 3,000 rpm for 10 min to remove cell debris, aliquoted into 5-mL samples and stored at -70°C until analysis. Clotted blood samples were collected at 12 and 18 months, centrifuged at 2,500 rpm for 10 min, serum aliquoted into 1-mL samples and stored at -70°C until analysis.
All laboratory procedures were conducted in the Department of Medicine at the University of Hong Kong, and analyzed in a blinded manner.
Laboratory Assays
Cancer antigen 125 level in PDF was measured using a commercial immunoassay according to the manufacturer’s instructions (Biocheck Inc., Genetimes Technology International Holding Limited, Hong Kong), and the range for detection was 5 – 400 U/mL. The levels of decorin, HGF, VEGF, HA, MIF, adiponectin, s-ICAM, VCAM-1 and P-selectin in spent PDF and serum samples were measured using commercial Duoset ELISAs according to the manufacturer’s instructions (R&D Systems, Genetimes Technology International Holding Limited, Hong Kong). The detection range was 30 – 2,000 pg/mL for decorin, VEGF, MIF, and s-ICAM, 125 – 8,000 pg/mL for HGF, 0.4 – 80 ng/mL for HA, 80 – 4,000 pg/mL for adiponectin, 15 – 1,000 pg/mL for VCAM-1, and 125 – 8,000 pg/mL for P-selectin. The levels of IL-6 and TNF-α in PDF and serum samples were determined using BD OptEIA ELISAs (BD Biosciences Pharmingen, Genetimes Technology International Holding Limited, Hong Kong), with detection ranges of 4.7 – 300 and 8 – 1,000 pg/mL respectively. In cases where biomarker levels were below the detection range, the ELISA was repeated with 2 further serial dilutions of the relevant standard. Recombinant proteins with known concentrations that fell within the lower part of the standard curve were then used to assess and confirm the reproducibility of the standard curves, and only values greater than mean optical density (OD) of respective blanks + 3 standard deviation (SD) were considered positive values. Icodextrin- and glucose-based PDF did not interfere with any of the assays (data not shown).
Clinical Study Parameters
At 12 and 18 months after the start of the study, the body mass index (BMI), Kt/V for urea, D/P creatinine ratio at 4 h, and the modified peritoneal equilibration test were measured (12). The ultrafiltration volume per day was determined by averaging the daily ultrafiltration volumes from the patients’ PD records over a 7-day period prior to Kt/V assessment. Urine volume was determined from a 24-h urine sample. Residual renal function was calculated as the mean of creatinine and urea clearance, normalized to the standard body surface area of 1.73 m2. Daily glucose load represents the glucose content patients were exposed to when using 2-L bags of either 1.36% or 2.27% glucose-based PDF.
Statistical Analysis
Statistical analysis was performed using Prism 6 for Windows (GraphPad Software, Inc., San Diego, California, USA). Analysis of covariance with Bonferroni’s multiple comparison post-test was used to compare differences between groups. Two-tailed p < 0.05 was considered statistically significant. Data are presented as median (horizontal line within the box), 25th and 75th percentiles (bottom and top of the box respectively), with the bars representing the lowest and highest values excluding outliers, the latter defined as values greater than 1.5 times the interquartile range above the third quartile or below the first quartile.
Results
Peritoneal dialysis fluid and serum samples from 80 patients who completed the 18-month study were included (PEN group, n = 43; control group, n = 37). Baseline characteristics were similar between the 2 groups (Table 1).
TABLE 1.
Baseline Demographics of PD Patients Randomized to Treatment with PEN or Glucose-Based PDF
CA125 Level in Spent PDF
After 12 months, dialysate CA125 levels were significantly higher in the PEN group compared to the control group (p < 0.001), but the level decreased after patients switched to glucose-based PDF (p < 0.001). Cancer antigen 125 level in controls was significantly lower than the PEN group throughout the course of the study (p < 0.001 and p < 0.05, PEN vs control at 12 months and 18 months, respectively) (Figure 1).
Figure 1 —
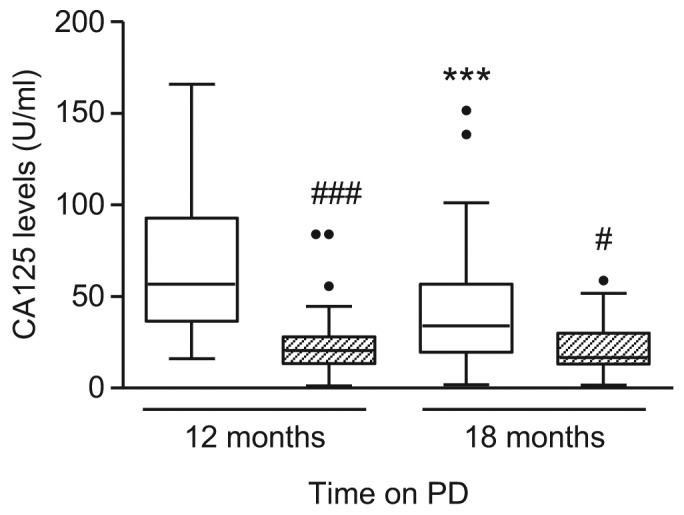
Effect of PEN and glucose-based PD on dialysate CA125 level. Box and whiskers plot showing dialysate CA125 level over time in PEN group (□) and control group ( ). Data are presented as median (horizontal line within box), 25th and 75th percentiles (bottom and top of the box respectively), and the lowest and highest values (bars), excluding outliers (•), the latter defined as values greater than 1.5 times the interquartile range above the third quartile or below the first quartile. Intra- and inter-group comparisons were analyzed with ANOVA. PD = peritoneal dialysis; CA = cancer antigen; PEN = Physioneal (P), Extraneal (E), Nutrineal (N); *** = p<0.001 (12 months vs 18 months in PEN Group); # = p<0.05; ### = p<0.001 (PEN vs control for the same time-point).
). Data are presented as median (horizontal line within box), 25th and 75th percentiles (bottom and top of the box respectively), and the lowest and highest values (bars), excluding outliers (•), the latter defined as values greater than 1.5 times the interquartile range above the third quartile or below the first quartile. Intra- and inter-group comparisons were analyzed with ANOVA. PD = peritoneal dialysis; CA = cancer antigen; PEN = Physioneal (P), Extraneal (E), Nutrineal (N); *** = p<0.001 (12 months vs 18 months in PEN Group); # = p<0.05; ### = p<0.001 (PEN vs control for the same time-point).
Decorin, HGF, and VEGF Levels in Serum and PDF
Serum levels of decorin, HGF, and VEGF were comparable between the 2 groups throughout the course of the study (Table 2). Dialysate levels of decorin and HGF were higher in the PEN group after 12 months (p < 0.05), but, after PEN patients switched to glucose-based PDF, there was no difference between the 2 groups for both anti-fibrotic markers (Figure 2A and 2B). Dialysate level of VEGF was similar in both treatment groups throughout the course of the study (Figure 2C).
TABLE 2.
Level of Pro-/Anti-Inflammatory or Fibrotic Mediators in Serum
Figure 2 —
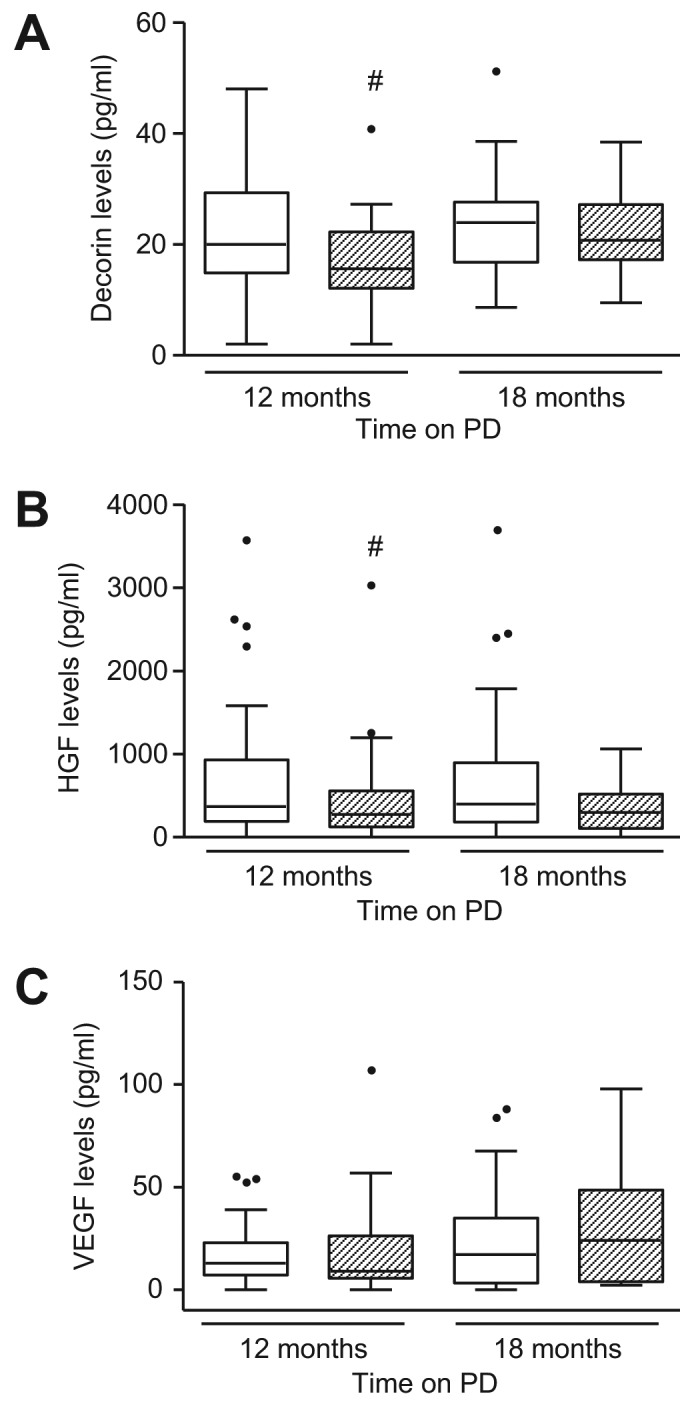
Effect of PEN and glucose-based PD on dialysate levels of decorin, HGF, and VEGF. Box and whiskers plot showing dialysate levels of (A) decorin, (B) HGF, and (C) VEGF over time in PEN group (□) and control group ( ). Data are presented as median (horizontal line within box), 25th and 75th percentiles (bottom and top of the box respectively), and the lowest and highest values (bars), excluding outliers (•), the latter defined as values greater than 1.5 times the interquartile range above the third quartile or below the first quartile. Intra- and inter-group comparisons were analyzed with ANOVA. PEN = Physioneal (P), Extraneal (E), Nutrineal (N); PD = peritoneal dialysis; HGF = hepatocyte growth factor; VEGF = vascular endothelial growth factor; # = p<0.05 (PEN vs control for the same time-point).
). Data are presented as median (horizontal line within box), 25th and 75th percentiles (bottom and top of the box respectively), and the lowest and highest values (bars), excluding outliers (•), the latter defined as values greater than 1.5 times the interquartile range above the third quartile or below the first quartile. Intra- and inter-group comparisons were analyzed with ANOVA. PEN = Physioneal (P), Extraneal (E), Nutrineal (N); PD = peritoneal dialysis; HGF = hepatocyte growth factor; VEGF = vascular endothelial growth factor; # = p<0.05 (PEN vs control for the same time-point).
IL-6, MIF, HA, and TNF-α in Serum and PDF
PEN and control patients showed comparable serum levels of IL-6, MIF, and HA over time (Table 2). Dialysate IL-6 level was significantly higher in the PEN group after 12 months compared to controls, and the difference was sustained after patients switched to glucose-based PDF (p < 0.01 and p < 0.05 for 12 and 18 months, respectively) (Figure 3A). Dialysate MIF level was lower in PEN patients after 12 months (p < 0.05), but became similar in the 2 groups after PEN patients switched to glucose-based PDF (Figure 3B). Dialysate HA level marginally increased in both groups over time and there was no difference between groups throughout the study duration (Figure 3C). Serum and dialysate TNF-α levels were below the lower limit of the assay for both treatment groups throughout the course of the study (data not shown).
Figure 3 —
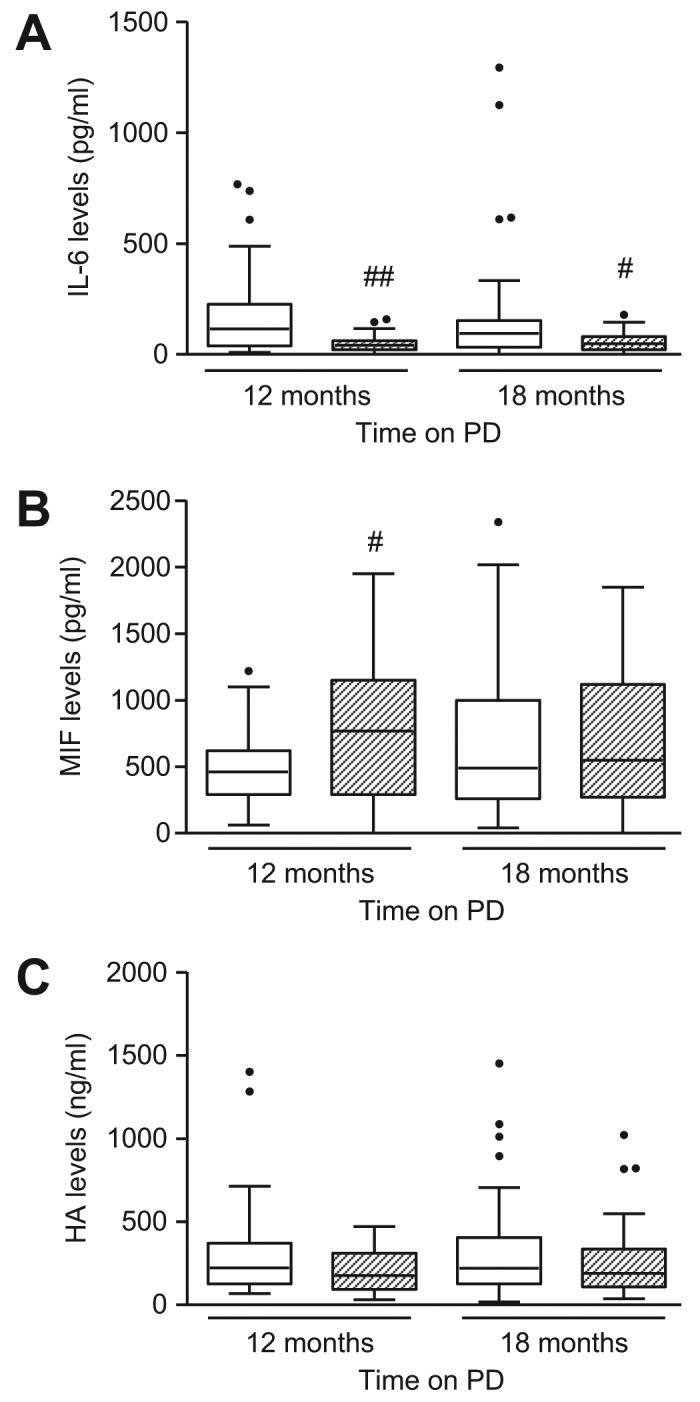
Effect of PEN and glucose-based PD on dialysate levels of IL-6, MIF, and hyaluronan. Box and whiskers plot showing dialysate levels of (A) IL-6, (B) MIF, and (C) HA over time in PEN group (□) and control group ( ). Data are presented as median (horizontal line within box), 25th and 75th percentiles (bottom and top of the box respectively), and the lowest and highest values (bars), excluding outliers (•), the latter defined as values greater than 1.5 times the interquartile range above the third quartile or below the first quartile. Intra- and inter-group comparisons were analyzed with ANOVA. PEN = Physioneal (P), Extraneal (E), Nutrineal (N); PD = peritoneal dialysis; IL-6 = interleukin-6; MIF = macrophage migration inhibitory factor; HA = hyaluronan; # = p<0.05, ## = p<0.01 (PEN vs control for the same time-point).
). Data are presented as median (horizontal line within box), 25th and 75th percentiles (bottom and top of the box respectively), and the lowest and highest values (bars), excluding outliers (•), the latter defined as values greater than 1.5 times the interquartile range above the third quartile or below the first quartile. Intra- and inter-group comparisons were analyzed with ANOVA. PEN = Physioneal (P), Extraneal (E), Nutrineal (N); PD = peritoneal dialysis; IL-6 = interleukin-6; MIF = macrophage migration inhibitory factor; HA = hyaluronan; # = p<0.05, ## = p<0.01 (PEN vs control for the same time-point).
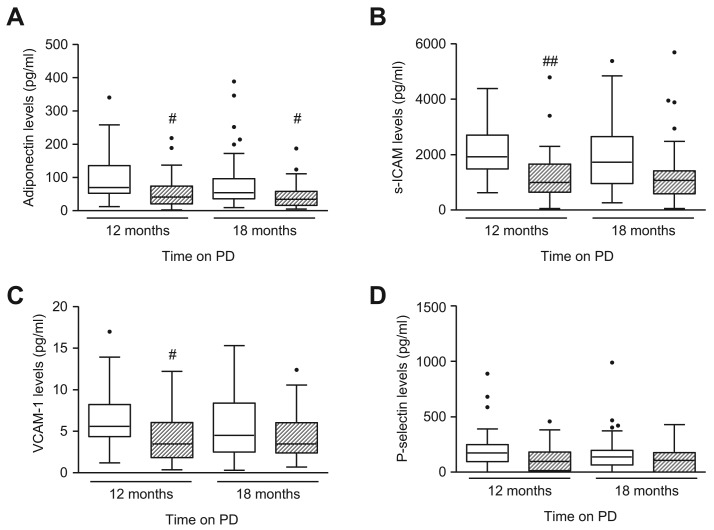
Adiponectin, s-ICAM, VCAM-1, and P-Selectin Levels in Serum and PDF
Serum adiponectin level was significantly higher in the PEN group after 12 months (p < 0.05), which decreased slightly after patients changed to glucose-based PDF, and the 2 groups showed similar levels at 18 months (Table 2). Similar changes were observed with serum levels of VCAM-1. Serum levels of s-ICAM and P-selectin did not change over time (Table 2). Dialysate adiponectin level was significantly higher in the PEN group compared to controls throughout the course of the study (p < 0.05 for both 12 and 18 months) (Figure 4A). Dialysate s-ICAM, and VCAM-1 levels were significantly higher in the PEN group after 12 months, but both came down after switching to glucose-based PDF (Figure 4B and 4C). The level of P-selectin was similar in both groups throughout the study duration (Figure 4D).
Figure 4 —
Effect of PEN and glucose-based PD on dialysate levels of adiponectin, s-ICAM, VCAM-1, and P-selectin. Box and whiskers plot showing dialysate levels of (A) adiponectin, (B) s-ICAM, (C) VCAM-1 and (D) P-selectin over time in PEN group (□) and control group ( ). Data are presented as median (horizontal line within box), 25th and 75th percentiles (bottom and top of the box respectively), and the lowest and highest values (bars), excluding outliers (•), the latter defined as values greater than 1.5 times the interquartile range above the third quartile or below the first quartile. Intra- and inter-group comparisons were analyzed with ANOVA. PEN = Physioneal (P), Extraneal (E), Nutrineal (N); PD = peritoneal dialysis; s-ICAM = soluble-intracellular adhesion molecule; VCAM-1 = vascular cell adhesion molecule-1; # = p<0.05; ## = p<0.01 (PEN vs control for the same time-point).
). Data are presented as median (horizontal line within box), 25th and 75th percentiles (bottom and top of the box respectively), and the lowest and highest values (bars), excluding outliers (•), the latter defined as values greater than 1.5 times the interquartile range above the third quartile or below the first quartile. Intra- and inter-group comparisons were analyzed with ANOVA. PEN = Physioneal (P), Extraneal (E), Nutrineal (N); PD = peritoneal dialysis; s-ICAM = soluble-intracellular adhesion molecule; VCAM-1 = vascular cell adhesion molecule-1; # = p<0.05; ## = p<0.01 (PEN vs control for the same time-point).
Body Mass, Residual Renal Function, Urine Output, Dialysis Adequacy, Peritoneal Membrane Characteristics, Glucose Exposure and Peritonitis
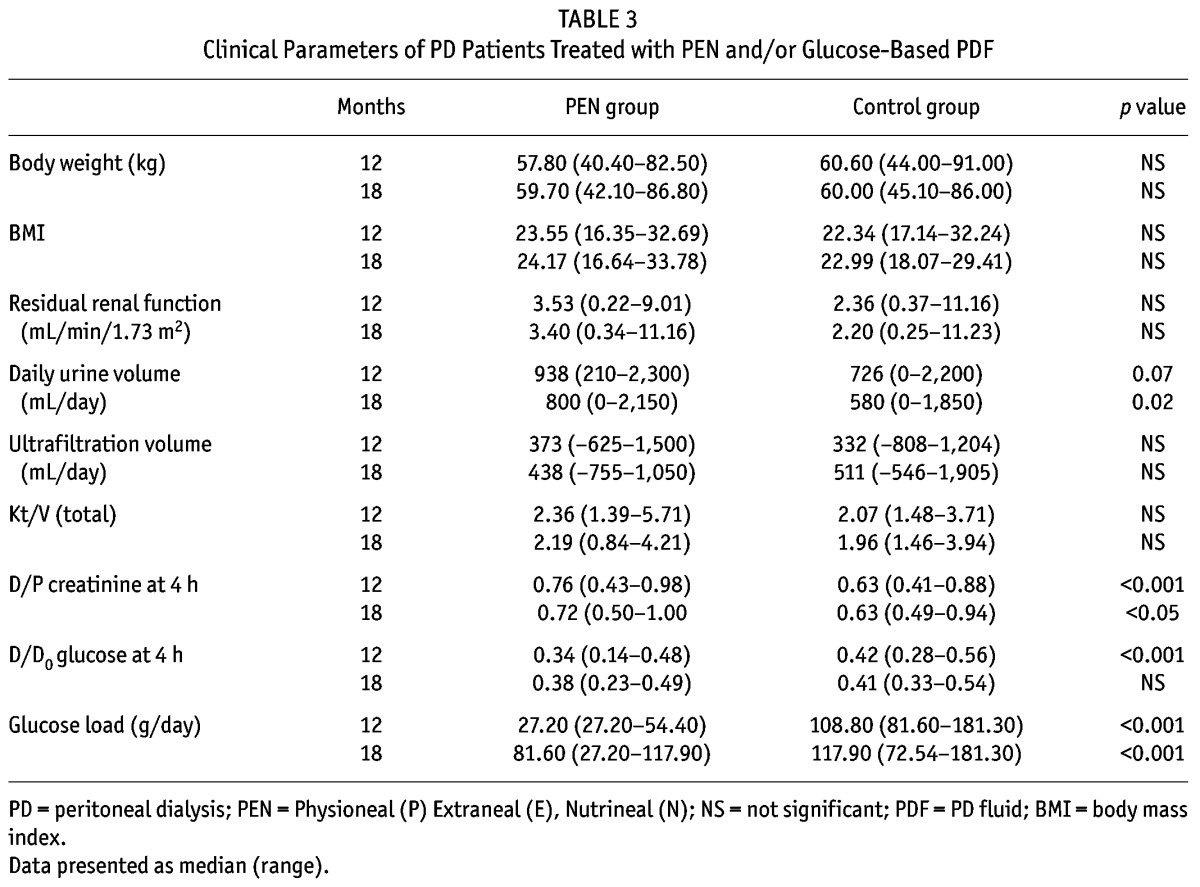
Mean body weight, BMI, residual renal function, daily ultrafiltration and total Kt/V were similar between the 2 groups throughout the study period (Table 3). Daily urine volume in PEN patients was numerically higher than that of the control group after 12 months but the difference did not reach statistical significance (p = 0.07). At 18 months, urine volume was statistically higher in patients who had received prior PD with the PEN regimen (p < 0.02) (Table 3). Dialysate/plasma creatinine ratio was significantly higher in the PEN group after 12 months (p < 0.001), and this difference was sustained after PEN patients switched to glucose-based PDF (p < 0.05). D/D0 glucose ratio was significantly lower in the PEN group after 12 months (p < 0.001), but the difference disappeared after PEN patients changed to glucose-based PDF. One patient in the PEN group developed peritonitis after changing to glucose-based PDF, whereas patients in the control group were peritonitis-free during the 12- to 18-month period.
TABLE 3.
Clinical Parameters of PD Patients Treated with PEN and/or Glucose-Based PDF
Discussion
Data from this study show that the use of a low-glucose PD regimen increased levels of anti-fibrotic markers and adiponectin, with a concomitant decrease in some of the inflammatory markers. Deteriorations in peritoneal permeability and daily urine output, as observed in controls, were prevented. Other investigators have also reported that the use of low GDP and neutral-pH PDF was associated with improvements in dialysate markers for peritoneal membrane integrity and inflammation (10,18,29).
Cancer antigen 125 is a glycoprotein that is secreted specifically by mesothelial cells. It is a putative marker of mesothelial cell mass/turnover and peritoneal membrane integrity, although this has recently been questioned (15). After 12 months, patients treated with the PEN regimen demonstrated higher CA125 levels compared to controls. Our data demonstrate that the low-glucose PEN dialysis regimen may be more effective than conventional PD in preserving mesothelial cell mass, which is in keeping with independent studies that utilize different biocompatible regimens (9,10,29). Cancer antigen 125 levels decreased by approximately 40% when PEN patients were switched to glucose-based PDF but statistically remained significantly higher than controls, suggesting that the beneficial effect of PEN can be sustained after patients change to a less biocompatible regimen.
Mesothelial cells secrete decorin, a dermatan sulfate proteoglycan that possesses anti-fibrotic properties (19). Through its core protein, decorin has been shown to bind and neutralize the bioactivity of TGF-β1 (22). Margetts et al. demonstrated that adenovirus-mediated gene transfer of decorin into a rodent model of PD significantly reduced collagen synthesis in the mesenteric tissue (23). In this study, dialysate decorin levels were attributed, at least in part, to local production since they were significantly higher than circulating levels. Dialysate decorin levels in the PEN group were higher than that observed in controls after 12 months, but there was no difference between the treatment groups once PEN patients switched to glucose-based PDF. This would suggest that any beneficial effect of the PEN regimen on peritoneal fibrosis may have been negated once this regimen was stopped.
Hepatocyte growth factor is secreted by mesenchymal cells and targets neighboring epithelial cells that express HGF receptors. Increased HGF levels in mesothelial cells of rodent models of PD by genetic manipulation have been shown to reduce TGF-β1 secretion and peritoneal fibrosis respectively (30,31). High glucose concentration has been shown to reduce HGF synthesis in mesothelial cells, which is associated with a loss of cell viability, whilst the addition of exogenous HGF to mesothelial cells exposed to elevated glucose concentration can improve cell viability and mesothelial cell regeneration (32). In this study, the HGF level was 2.6-fold higher in the PEN group when compared to controls after 12 months. As observed with decorin, the level decreased once patients were switched to glucose-based PDF, thus highlighting the potential beneficial effect of the PEN regimen in mediating increased synthesis of anti-fibrotic mediators.
Dialysate VEGF level may reflect neoangiogenesis and inflammatory activation of mesothelial cells (29,33). Its level did not change in either treatment groups during the study duration, an observation also noted in previous biocompatibility studies (29).
Hyaluronan is a glycosaminoglycan that is synthesized by mesothelial cells, endothelial cells, and fibroblasts. The observation that dialysate HA levels were significantly higher than serum levels suggests local production. The role of HA in wound repair has been extensively studied in the skin, where HA has been shown to act as a promoter of early inflammation crucial for wound repair and skin regeneration. With regard to the peritoneum, previous studies have suggested that elevated HA levels may reflect peritoneal inflammation and tissue remodeling in PD patients (34,35). We have previously demonstrated that HA synthesis was increased in cultured mesothelial cells undergoing wound healing and remesothelialization (36). The data may indicate ongoing inflammation and tissue remodeling in both treatment groups.
Dialysate IL-6 level was 2.8-fold higher in the PEN group when compared to controls after 12 months, and this increase was sustained when patients were switched to glucose-based PDF. The biological function of IL-6 within the peritoneum is debatable. Some researchers have suggested that increased intraperitoneal IL-6 levels are associated with peritoneal inflammation and are a predictor of increasing peritoneal solute transport rate, whereas other researchers have suggested that preservation of mesothelial cell mass and peritoneal membrane integrity, indicated by increased CA125 levels, may contribute to increased IL-6 levels and improved host defense capability (37,38). The latter may explain the observed increase in locally produced intraperitoneal IL-6 levels in the PEN group compared to controls. Exposure of mesothelial cells to lactate buffered, glucose-based PDF results in the impairment of mesothelial cell functions and inhibition of IL-6 secretion (38), which could account for the reduced dialysate IL-6 levels in control patients.
Independent studies have demonstrated that there was no difference in dialysate TNF-α levels between patients treated with biocompatible PDF and conventional glucose-based PDF (18,29). We were unable to corroborate these findings since dialysate TNF-α levels were below the detectable range of the ELISA.
Macrophage migration inhibitory factor and adiponectin are regarded as representative markers of inflammatory and anti-inflammatory processes respectively. Macrophage migration inhibitory factor represents an early response pro-inflammatory cytokine that participates in leukocyte infiltration, T-cell activation and activation of cell signaling pathways. Gene expression of MIF in dialysis patients correlates with C-reactive protein levels (24), suggesting a role in peritoneal inflammation. Our observation that dialysate MIF level was significantly lower in patients maintained on the PEN regimen compared to controls indicates decreased peritoneal inflammation, a finding that was no longer observed once patients switched to glucose-based PDF. Lai et al. observed no significant difference in MIF levels in patients maintained on either a low GDP PD regimen or conventional glucose-based PD regimen (18), and this discrepancy may be due to differences in PD regimens or duration of the study period. Adiponectin is an adipose-derived cytokine that possesses anti-inflammatory and anti-atherogenic properties. It is found in abundance in the plasma of healthy individuals, whereas its levels are decreased in patients with increased cardiovascular risk, diabetes or obesity. Visceral obesity also induces secretion of pro-inflammatory mediators and macrophage infiltration in adipose tissue, which exacerbate the inflammatory condition. Recent studies have shown that adiponectin can preserve endothelial cell function, while decreased adiponectin level is accompanied by an increased release of atherogenic adhesion molecules, leading to increased leukocyte activation and leukocyte-endothelial cell interaction. Previous studies have demonstrated that conventional glucose-based PDF can increase intra-abdominal fat in PD patients and this may indirectly contribute to the inflammatory state of the peritoneum so often detected in chronic PD patients. We demonstrated that although serum adiponectin levels were similar between both groups at the start of the study, patients treated with the PEN regimen showed a progressive and sustained increase in circulating adiponectin levels over time, whereas adiponectin levels decreased in controls (12). Furthermore, dialysate adiponectin levels were significantly higher in the PEN group when compared to controls throughout the course of the study. It is unlikely that this difference was attributed to changes in body fat since body weight and BMI were comparable between both groups throughout the study. Whether icodextrin per se could account for the increase in adiponectin level observed in the PEN group remains to be investigated, but these results would suggest that the PEN regimen confers a more favorable environment to the systemic vasculature. Considering that cardiovascular complications are the leading cause of death in PD patients, the data have important clinical implications.
It is of interest to note a decrease in dialysate s-ICAM and VCAM-1 levels in controls compared to PEN-treated subjects. Whether the decrease in dialysate adhesion molecule levels signifies a reduction in destructive inflammation or reparative process is open to speculation. However, taking into account the established detrimental effects of exposing the peritoneal mesothelium to a high-glucose environment, the latter possibility would appear more likely. An alternative explanation is that exposure of the peritoneum to high glucose concentration could result in more pronounced vasculopathy and peritoneal fibrosis (1), thereby reducing the amount of viable tissue to synthesize adhesion molecules in controls. In this regard, previous studies have demonstrated that the use of amino acid-based or low-GDP PDF was associated with reduced peritoneal inflammation, neoangiogenesis and fibrotic markers, and better preservation of mesothelial cell ultrastructure and function (4,9,29,39).
There are a few limitations in our study. Expression of the biomarkers as their appearance rates may have been more appropriate since this would have eliminated the impact of dilution consequent to the ultrafiltration rate. This correction may only be applied when biomarkers increase linearly during a predefined dwell time; otherwise conveying the value of each biomarker as its concentration is more relevant (13). We only compared the concentration of biomarkers in overnight PD effluents, and the contribution of amino acid-based and neutral pH, low-GDP PDFs in modulating the synthesis of biomarkers was not determined. Without peritoneal biopsies, we were unable to directly correlate our results with pathophysiological changes within the peritoneum.
In conclusion, results from our study showed that the PEN PD regimen, which reduces the exposure of the peritoneum to high glucose and other unphysiological stimuli, can result in better preservation of mesothelial cell mass, a reduction in intraperitoneal inflammation, and possibly a delay or reduction of peritoneal fibrosis, which was associated with increased urine output and D/P creatinine ratios. Furthermore, there may be a beneficial effect on the systemic vasculature. Of the mediators of inflammation and fibrosis that were measured in this study, dialysate levels of decorin, IL-6, HA, s-ICAM and P-selectin were higher than their corresponding serum levels, suggesting that these locally produced effluent biomarkers may be used to assess peritoneal pathophysiology, although further studies are warranted to confirm this.
Disclosures
This study was supported by the Hong Kong Society of Nephrology Research Grant 2009, Baxter Healthcare Limited (Hong Kong), Research Grant Council General Research Fund (HKU 7830/09M), the Estate of the late Mr Chan Wing Hei, and Mr G. King. S. Yung is supported by the Endowment Fund established for the ‘Yu Chiu Kwong Professorship in Medicine’ awarded to T. M. Chan, and the Wai Hung Charitable Foundation Limited.
Acknowledgments
The Hong Kong PEN Study Group was coordinated by Dr. Sing Leung Lui at Tung Wah Hospital and Professor Tak Mao Chan at Queen Mary Hospital. Site investigators included Dr. Andrew Wong and Dr. Kin Yee Lo at Kwong Wah Hospital, Dr. Kin Shing Wong and Dr. Chik Cheung Chow at Pamela Youde Nethersole Eastern Hospital, Dr. Matthew K.L. Tong and Dr. Kwok Hong Chu at Princess Margaret Hospital, Dr. Tak Cheung Au and Dr. Chun Yu Yung at Tuen Mun Hospital, Dr. Chun Sang Li and Dr. Wai Leung Chak at Queen Elizabeth Hospital, Dr. Wai Kei Lo and Dr. Terence P.S. Yip at Tung Wah Hospital, Dr. Man Fai Lam at Queen Mary Hospital, and Dr. Yiu Wing Ho and Dr. Sunny Wong at United Christian Hospital. The investigators thank the nurses at the renal units and the patients for participating in this study.
REFERENCES
- 1. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 2002; 13:470–9. [DOI] [PubMed] [Google Scholar]
- 2. Schalkwijk CG, Posthuma N, ten Brink HJ, ter Wee PM, Teerlink T. Induction of 1,2-dicarbonyl compounds, intermediates in the formation of advanced glycation end-products, during heat-sterilization of glucose-based peritoneal dialysis fluids. Perit Dial Int 1999; 19:325–33. [PubMed] [Google Scholar]
- 3. Witowski J, Wisniewska J, Korybalska K, Bender TO, Breborowicz A, Gahl GM, et al. Prolonged exposure to glucose degradation products impairs viability and function of human peritoneal mesothelial cells. J Am Soc Nephrol 2001; 12:2434–41. [DOI] [PubMed] [Google Scholar]
- 4. Chan TM, Leung JK, Sun Y, Lai KN, Tsang RC, Yung S. Different effects of amino acid-based and glucose-based dialysate from peritoneal dialysis patients on mesothelial cell ultrastructure and function. Nephrol Dial Transplant 2003; 18:1086–94. [DOI] [PubMed] [Google Scholar]
- 5. Ha H, Cha MK, Choi HN, Lee HB. Effects of peritoneal dialysis solutions on the secretion of growth factors and extracellular matrix proteins by human peritoneal mesothelial cells. Perit Dial Int 2002; 22:171–7. [PubMed] [Google Scholar]
- 6. Li FK, Chan LY, Woo JC, Ho SK, Lo WK, Lai KN, et al. A 3-year, prospective, randomized, controlled study on amino acid dialysate in patients on CAPD. Am J Kidney Dis 2003; 42:173–83. [DOI] [PubMed] [Google Scholar]
- 7. Mistry CD, Gokal R, Peers E. A randomized multicenter clinical trial comparing isosmolar icodextrin with hyperosmolar glucose solutions in CAPD. MIDAS Study Group. Multicenter Investigation of Icodextrin in Ambulatory Peritoneal Dialysis. Kidney Int 1994; 46:496–503. [DOI] [PubMed] [Google Scholar]
- 8. Finkelstein F, Healy H, Abu-Alfa A, Ahmad S, Brown F, Gehr T, et al. Superiority of icodextrin compared with 4.25% dextrose for peritoneal ultrafiltration. J Am Soc Nephrol 2005; 16:546–54. [DOI] [PubMed] [Google Scholar]
- 9. Jones S, Holmes CJ, Krediet RT, Mackenzie R, Faict D, Tranaeus A, et al. Bicarbonate/lactate-based peritoneal dialysis solution increases cancer antigen 125 and decreases hyaluronic acid levels. Kidney Int 2001; 59:1529–38. [DOI] [PubMed] [Google Scholar]
- 10. Szeto CC, Chow KM, Lam CW, Leung CB, Kwan BC, Chung KY, et al. Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose-degradation products—a 1-year randomized control trial. Nephrol Dial Transplant 2007; 22:552–9. [DOI] [PubMed] [Google Scholar]
- 11. García-Lopez E, Lindholm B, Davies S. An update on peritoneal dialysis solutions. Nat Rev Nephrol 2012; 8:224–33. [DOI] [PubMed] [Google Scholar]
- 12. Lui SL, Yung S, Yim A, Wong KM, Tong KL, Wong KS, et al. A combination of biocompatible peritoneal dialysis solutions and residual renal function, peritoneal transport, and inflammation markers: a randomized clinical trial. Am J Kidney Dis 2012; 60:966–75. [DOI] [PubMed] [Google Scholar]
- 13. Lopes Barreto D, Krediet RT. Current status and practical use of effluent biomarkers in peritoneal dialysis patients. Am J Kidney Dis 2013; 62:823–33. [DOI] [PubMed] [Google Scholar]
- 14. Oh KH, Jung JY, Yoon MO, Song A, Lee H, Ro H, et al. Intraperitoneal interleukin-6 system is a potent determinant of the baseline peritoneal solute transport in incident peritoneal dialysis patients. Nephrol Dial Transplant 2010; 25:1639–46. [DOI] [PubMed] [Google Scholar]
- 15. Visser CE, Brouwer-Steenbergen JJ, Betjes MG, Koomen GC, Beelen RH, Krediet RT. Cancer antigen 125: a bulk marker for the mesothelial mass in stable peritoneal dialysis patients. Nephrol Dial Transplant 1995; 10:64–9. [PubMed] [Google Scholar]
- 16. Zweers MM, de Waart DR, Smit W, Struijk DG, Krediet RT. Growth factors VEGF and TGF-beta1 in peritoneal dialysis. J Lab Clin Med 1999; 134:124–32. [DOI] [PubMed] [Google Scholar]
- 17. Zemel D, Imholz AL, de Waart DR, Dinkla C, Struijk DG, Krediet RT. Appearance of tumor necrosis factor-alpha and soluble TNF-receptors I and II in peritoneal effluent of CAPD. Kidney Int 1994; 46:1422–30. [DOI] [PubMed] [Google Scholar]
- 18. Lai KN, Lam MF, Leung JC, Chan LY, Lam CW, Chan IH, et al. A study of the clinical and biochemical profile of peritoneal dialysis fluid low in glucose degradation products. Perit Dial Int 2012; 32:280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yung S, Thomas GJ, Stylianou E, Williams JD, Coles GA, Davies M. Source of peritoneal proteoglycans. Human peritoneal mesothelial cells synthesize and secrete mainly small dermatan sulfate proteoglycans. Am J Pathol 1995; 146:520–9. [PMC free article] [PubMed] [Google Scholar]
- 20. Yu MA, Shin KS, Kim JH, Kim YI, Chung SS, Park SH, et al. HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J Am Soc Nephrol 2009; 20:567–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsuoka T, Maeda Y, Matsuo K, Naiki Y, Tamai Y, Sakaguchi M, et al. Hepatocyte growth factor prevents peritoneal fibrosis in an animal model of encapsulating peritoneal sclerosis. J Nephrol 2008; 21:64–73. [PubMed] [Google Scholar]
- 22. Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, et al. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 1992; 360:361–4. [DOI] [PubMed] [Google Scholar]
- 23. Margetts PJ, Gyorffy S, Kolb M, Yu L, Hoff CM, Holmes CJ, et al. Antiangiogenic and antifibrotic gene therapy in a chronic infusion model of peritoneal dialysis in rats. J Am Soc Nephrol 2002; 13:721–8. [DOI] [PubMed] [Google Scholar]
- 24. Zaza G, Pontrelli P, Pertosa G, Granata S, Rossini M, Porreca S, et al. Dialysis-related systemic microinflammation is associated with specific genomic patterns. Nephrol Dial Transplant 2008; 23:1673–81. [DOI] [PubMed] [Google Scholar]
- 25. Honda K, Nitta K, Horita S, Tsukada M, Itabashi M, Nihei H, et al. Histologic criteria for diagnosing encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patients. Adv Perit Dial 2003; 19:169–75. [PubMed] [Google Scholar]
- 26. Furuya R, Odamaki M, Kumagai H, Hishida A. Beneficial effects of icodextrin on plasma level of adipocytokines in peritoneal dialysis patients. Nephrol Dial Transplant 2006; 21:494–8. [DOI] [PubMed] [Google Scholar]
- 27. Suassuna JH, Das Neves FC, Hartley RB, Ogg CS, Cameron JS. Immunohistochemical studies of the peritoneal membrane and infiltrating cells in normal subjects and in patients on CAPD. Kidney Int 1994; 46:443–54. [DOI] [PubMed] [Google Scholar]
- 28. Li FK, Davenport A, Robson RL, Loetscher P, Rothlein R, Williams JD, et al. Leukocyte migration across human peritoneal mesothelial cells is dependent on directed chemokine secretion and ICAM-1 expression. Kidney Int 1998; 54:2170–83. [DOI] [PubMed] [Google Scholar]
- 29. Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, et al. The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (Balance) on the peritoneal membrane. Kidney Int 2004; 66:408–18. [DOI] [PubMed] [Google Scholar]
- 30. Maeda Y, Matsuoka T, Matsuo K, Naiki Y, Sakaguchi M, Hasegawa H. Gene therapy with HGF cDNA in patients on peritoneal dialysis. J Clin Pathol 2008; 61:781–2. [DOI] [PubMed] [Google Scholar]
- 31. Matsuo K, Maeda Y, Naiki Y, Matsuoka T, Tamai Y, Yonekawa S, et al. Possible effects of hepatocyte growth factor for the prevention of peritoneal fibrosis. Nephron Exp Nephrol 2005; 99:e87–94. [DOI] [PubMed] [Google Scholar]
- 32. Naiki Y, Matsuo K, Matsuoka T, Maeda Y. Possible role of hepatocyte growth factor in regeneration of human peritoneal mesothelial cells. Int J Artif Organs 2005; 28:141–9. [DOI] [PubMed] [Google Scholar]
- 33. Mandl-Weber S, Cohen CD, Haslinger B, Kretzler M, Sitter T. Vascular endothelial growth factor production and regulation in human peritoneal mesothelial cells. Kidney Int 2002; 61:570–8. [DOI] [PubMed] [Google Scholar]
- 34. Yung S, Coles GA, Davies M. IL-1 beta, a major stimulator of hyaluronan synthesis in vitro of human peritoneal mesothelial cells: relevance to peritonitis in CAPD. Kidney Int 1996; 50:1337–43. [DOI] [PubMed] [Google Scholar]
- 35. Yamagata K, Tomida C, Koyama A. Intraperitoneal hyaluronan production in stable continuous ambulatory peritoneal dialysis patients. Perit Dial Int 1999; 19:131–7. [PubMed] [Google Scholar]
- 36. Yung S, Thomas GJ, Davies M. Induction of hyaluronan metabolism after mechanical injury of human peritoneal mesothelial cells in vitro. Kidney Int 2000; 58:1953–62. [DOI] [PubMed] [Google Scholar]
- 37. Martikainen TA, Teppo AM, Gronhagen-Riska C, Ekstrand AV. Glucose-free dialysis solutions: inductors of inflammation or preservers of peritoneal membrane? Perit Dial Int 2005; 25:453–60. [PubMed] [Google Scholar]
- 38. Witowski J, Topley N, Jorres A, Liberek T, Coles GA, Williams JD. Effect of lactate-buffered peritoneal dialysis fluids on human peritoneal mesothelial cell interleukin-6 and prostaglandin synthesis. Kidney Int 1995; 47:282–93. [DOI] [PubMed] [Google Scholar]
- 39. Mortier S, Faict D, Schalkwijk CG, Lameire NH, De Vriese AS. Long-term exposure to new peritoneal dialysis solutions: effects on the peritoneal membrane. Kidney Int 2004; 66:1257–65. [DOI] [PubMed] [Google Scholar]