Abstract
♦ Background: Residual renal function (RRF) is an important prognostic indicator in continuous ambulatory peritoneal dialysis (CAPD) patients. We determined the predictors of RRF loss in a cohort of incident CAPD patients.
♦ Methods: We reviewed the record of 645 incident CAPD patients. RRF loss is represented by the slope of decline of residual glomerular filtration rate (GFR) as well as the time to anuria.
♦ Results: The average rate of residual GFR decline was -0.083 ± 0.094 mL/min/month. The rate of residual GFR decline was faster with a higher proteinuria (r = -0.506, p < 0.0001) and baseline residual GFR (r = -0.560, p < 0.0001). Multivariate analysis showed that proteinuria, baseline residual GFR, and the use of diuretics were independent predictors of residual GFR decline. Cox proportional hazard model showed that proteinuria, glucose exposure, and the number of peritonitis episodes were independent predictors of progression to anuria, while a higher baseline GFR was protective. Each 1 g/day of proteinuria is associated with a 13.2% increase in the risk of progressing to anuria, each 10 g/day higher glucose exposure is associated with a 2.5% increase in risk, while each peritonitis episode confers a 3.8% increase in risk.
♦ Conclusions: Our study shows that factors predicting the loss of residual solute clearance and urine output are different. Proteinuria, baseline residual GFR, and the use of diuretics are independently related to the rate of RRF decline in CAPD patients, while proteinuria, glucose exposure, and the number of peritonitis episodes are independent predictors for the development of anuria. The role of anti-proteinuric therapy and measures to prevent peritonitis episodes in the preservation of RRF should be tested in future studies.
Keywords: Renal failure, survival, cardiovascular disease, peritonitis
Residual renal function (RRF) is well recognized as an important marker of outcomes in peritoneal dialysis (PD) (1). In dialysis patients, the presence of RRF is associated with easy fluid management, improved nutrition, reduced erythropoietin requirements, better potassium clearance, and improved quality of life (2). Published evidence suggests that a higher level of RRF is independently associated with a better survival in dialysis patients (1,3,4). For example, analysis of the CANUSA study has shown that every 0.5 mL/min higher RRF was associated with a 9% lower risk of death (5). More importantly, the survival benefit conveyed by RRF could not be explained by a better small solute clearance, and is possibly the result of a better preservation of endocrine function of the kidney and the better excretion of uremic toxins of middle molecular weight (6,7).
In recent years, the importance of identifying factors that protect and preserve RRF is increasingly being recognized among dialysis patients. For example, RRF is better preserved with PD than with hemodialysis (8,9), although a more recent study showed no difference in the rate of decline of RRF between PD and hemodialysis patients using biocompatible high flux membranes and ultra-pure water (10). Previous studies also showed that high baseline RRF, high peritoneal transporter, poorly controlled hypertension, persistent proteinuria, prior history of diabetes, congestive heart failure, and peripheral vascular disease are predictors of loss of RRF, while a higher serum calcium level, use of a calcium channel blocker, and use of an angiotensin-converting enzyme inhibitor are independently associated with decreased risk of losing RRF (9,11–15).
Unfortunately, most of the published reports have a small sample size or were conducted in the last century. To complicate the issue, there is increasing evidence of dissociation between the loss of residual solute clearance and urine volume (16,17). For example, in the balANZ study, the use of biocompatible PD solution delays the onset of anuria but has no effect on the decline of residual glomerular filtration rate (GFR) (17). In essence, our knowledge of the factors that preserve RRF in CAPD remains limited. We therefore performed a retrospective study to determine the predictors of RRF loss in a large cohort of unselected incident CAPD patients.
Patients and Methods
Patient Selection
We studied the clinical record of 645 incident CAPD patients between 1998 and 2009 in our center. We excluded patients who had a failed kidney allograft, planned to have elective living donor transplant or transfer to another renal center within 6 months. We also excluded 122 patients who were treated with machine-assisted PD, and another 118 patients who received more than two weeks of temporary hemodialysis before starting CAPD. The study is approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong, and all study procedures are in adherence to the Declaration of Helsinki.
Collection of Clinical Data
Clinical data were recorded by chart review of the whole team. Data collected included age, sex, underlying causes of renal disease, blood pressure, serum chemistry, peritonitis, exposure to aminoglycosides, treatment with angiotensin converting enzyme (ACE) inhibitor, angiotensin receptor blocker (ARB), calcium channel blocker (CCB), and diuretics, were recorded. Exposure to ACE inhibitor, ARB, CCB or diuretics was considered to be present when the patients received the corresponding drug for at least six months. The modified Charlson’s comorbidity index, which was validated in PD patients (18), was used to calculate a comorbidity score. Dietary protein intake was estimated by the normalized protein nitrogen appearance (NPNA). During the study period, peritonitis episodes were mostly treated according to the recommendations of the International Society for Peritoneal Dialysis (19–21), with empirical antibiotic treatment with cefazolin plus netilmicin or ceftazidime.
Assessment of RRF Loss
Residual GFR was measured around one month after CAPD and then every six months, or more frequently if clinically indicated. Residual GFR was calculated as the average of 24-hour urinary urea and creatinine clearances (22). The rate of RRF loss was represented by the rate of residual GFR decline and the time to anuria. The rate of residual GFR decline was computed by the least squares linear regression formula. Anuria was defined as absence of appreciable urine output as in our previous study (23).
Study of Peritoneal Transport
Standard peritoneal permeability test (PET) was performed around one month after CAPD by the method of Twardowski (24). Peritoneal transport characteristic was represented by dialysate-to-plasma ratios of creatinine at four hours (D/P4) and mass transfer area coefficients (MTAC) of creatinine as calculated by a formula previously described (25).
Dialysis Adequacy and Nutritional Indices
Dialysis adequacy and nutritional status were assessed at least yearly. The method of dialysis adequacy assessment has been described previously (26). Nutritional status was represented by serum albumin level, subjective global assessment (SGA) (27), and comprehensive malnutrition-inflammation score (MIS) (28), which has been validated in PD patients (29,30).
Statistical Analysis
Statistical analysis was performed by SPSS for Windows software version 15.0 (SPSS Inc., Chicago, IL). Results are expressed as mean ± SD unless otherwise specified. For peritoneal transport characteristics, baseline data are used for analysis. For other time-dependent parameters, including blood pressure, serum chemistry, dialysis adequacy and nutritional indices, average values (measured every six months) during the study period are used for analysis. To analyze the relation between era of starting dialysis and the rate of residual GFR decline and anuria, patients were stratified into three groups according to the year PD was started. Comparisons between parameters are performed by chi-square test, Student t-test, or Pearson’s correlation coefficient as appropriate.
A multiple linear regression model was constructed to determine independent predictors of the slope of residual GFR decline. In addition, we constructed a Cox proportional hazard model to determine independent predictors of time to anuria. In this part of analysis, diabetic status, presence of ischemic heart disease (IHD) and peripheral vascular disease (PVD), Charlson’s comorbidity score, HbA1c level, serum phosphate level, D/P4, dialysis effluent protein level, baseline proteinuria and residual glomerular filtration rate (GFR), the use of ACE inhibitor or ARB therapy, CCB, and diuretics, number of peritonitis episodes, and number of peritonitis episodes that required aminoglycoside therapy, were included as the independent variables. These parameters were selected for the construction of the Cox model because they had substantial association (p < 0.1) with the slope of residual GFR decline or time to anuria by univariate analysis, or they were previously reported as predictors of RRF decline. The effects of different medications were analyzed as separate independent variables. Backward stepwise analysis was used to identify independent predictors. Additional linear regression and Cox regression models were constructed for the subgroup analysis of diabetic patients only. Since there was a significant relation between proteinuria and the use of ACE inhibitor or ARB therapy, additional linear regression and Cox models were also constructed without including proteinuria in the model. A p value of less than 0.05 is considered statistically significant. All probabilities are two-tailed.
Results
We studied 645 incident CAPD patients. The demographic, clinical and biochemical information are summarized in Table 1 and Table 2, respectively. Patients who received ACE inhibitor or ARB therapy had less proteinuria than those who did not (0.98 ± 1.25 vs 1.44 ± 1.57 g/day, p = 0.001). The anti-proteinuric effect was significant in both diabetic patients (1.58 ± 1.62 vs 2.16 ± 1.83 g/day, p = 0.034) and non-diabetic ones (0.63 ± 0.81 vs 0.86 ± 1.01 g /day, p = 0.047).
TABLE 1.
Baseline Demographic and Clinical Data
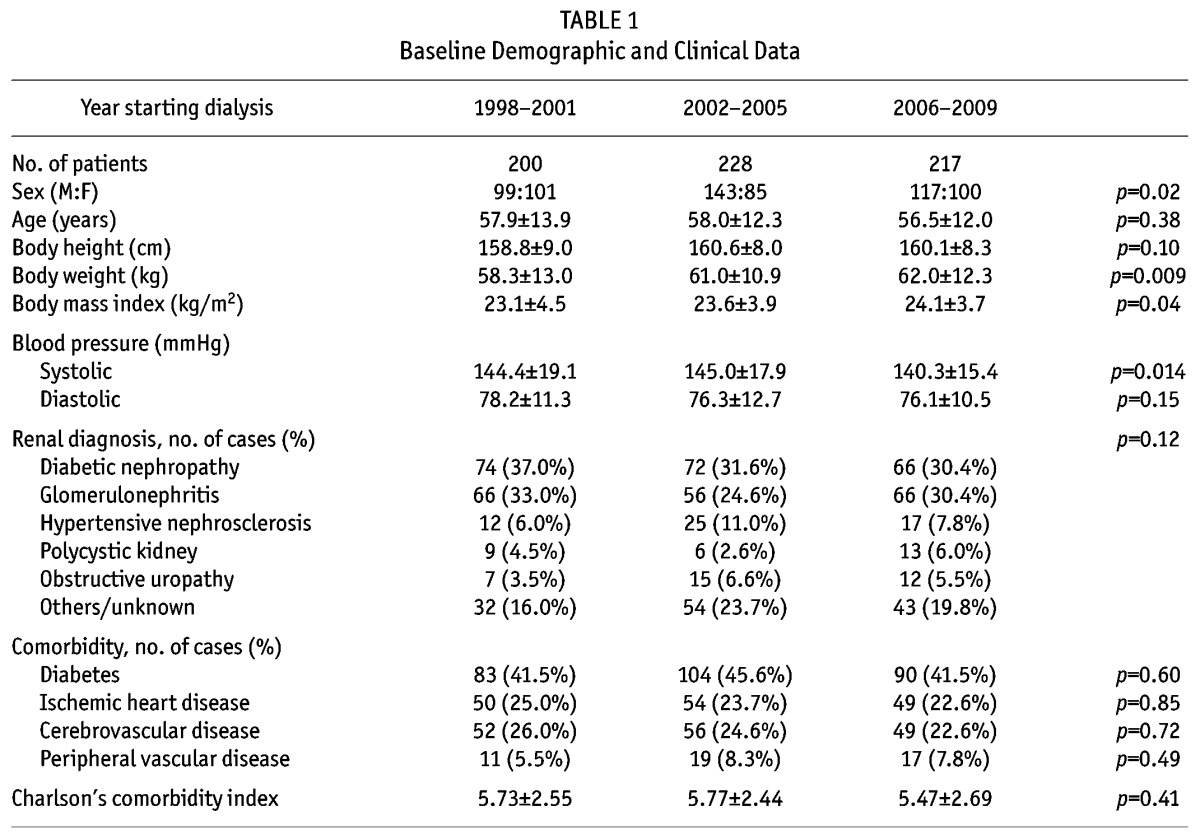
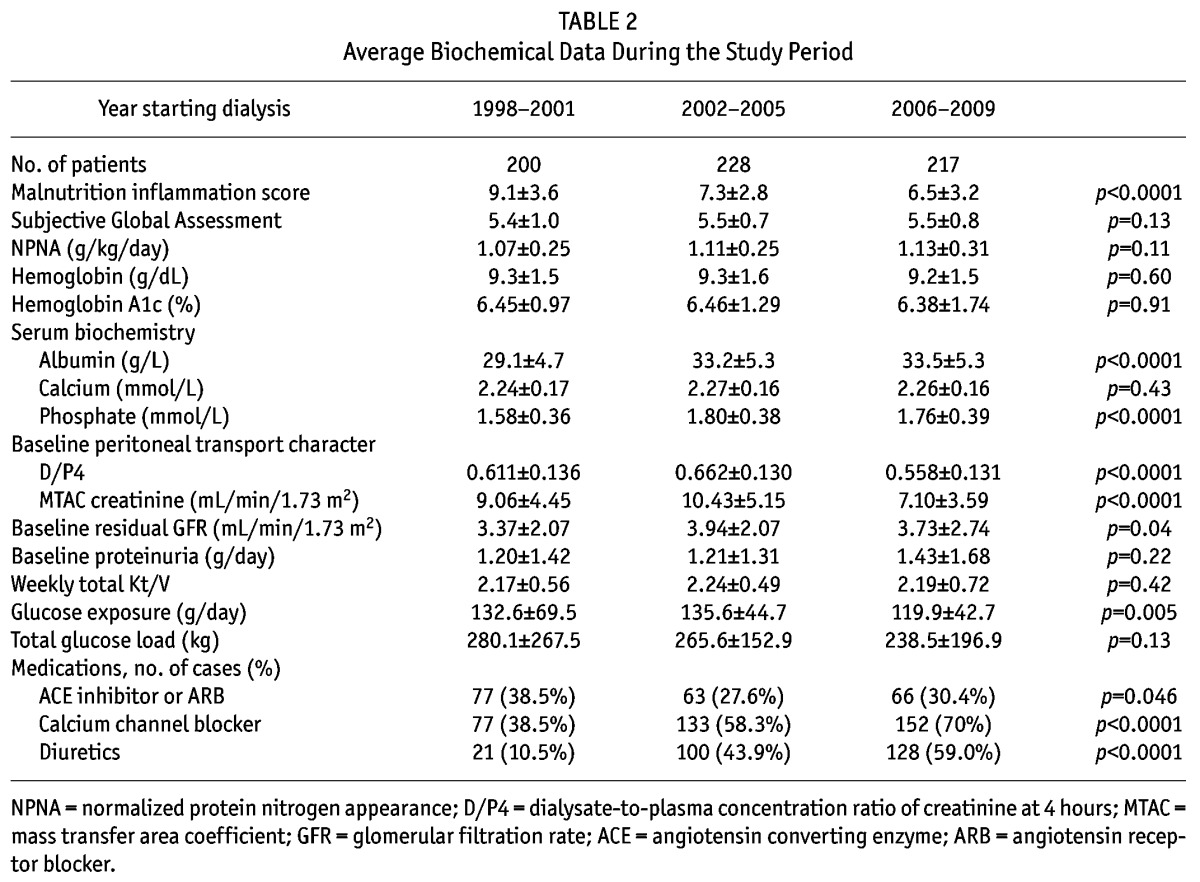
TABLE 2.
Average Biochemical Data During the Study Period
Slope of Residual Renal Function Decline
The patients were followed for an average of 66.3 ± 34.7 months. The average rate of residual GFR decline was -0.083 ± 0.094 mL/min/month. During the study period, there were a total of 1,502 peritonitis episodes, of which 454 episodes received aminoglycosides treatment. The peritonitis rate was one episode per 28.5 patient-months.
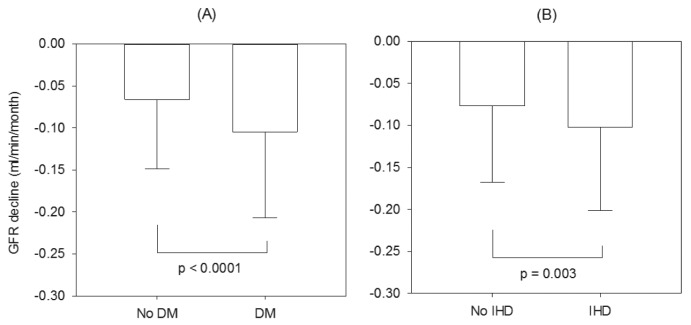
The rate of residual GFR decline was faster in patients with diabetes or IHD (Figure 1). Amongst the diabetic patients, the rate of residual GFR decline was also modestly but statistically faster amongst patients with a high HbA1c during the observation period (r = -0.163, p = 0.003). In contrast, the rate of residual renal function decline was similar between patients with and without peripheral artery disease or cardiovascular disease (details not shown). There was no significant relation between the year of starting PD and the rate of residual GFR decline (-0.076 ± 0.099, -0.091 ± 0.090 and -0.081 ± 0.091 mL/min/month for patients who started dialysis in 1998 – 2001, 2002 – 2005 and 2006 – 2009, respectively, p = 0.2).
Figure 1 —
Comparison of the rate of residual glomerular filtration rate (GFR) decline between (A) patients with and without diabetes (DM); and (B) patients with and without ischemic heart disease (IHD). Data are compared using unpaired Student t-test.
The rate of residual renal function decline had significant correlation with the proteinuria (r = -0.506, p < 0.0001) and baseline residual GFR (r = -0.560, p < 0.0001). The rate of residual GFR decline was also faster in patients with higher body mass index (r = -0.160, p < 0.0001), Charlson’s score (r = -0.174, p < 0.0001), D/P4 (r = -0.100, p = 0.014), dialysis effluent protein level (r = -0.129, p = 0.005), serum phosphate level (r = -0.168, p < 0.0001), and dietary protein intake (r = -0.293, p < 0.0001). In contrast, the rate of residual GFR decline did not correlate with the mean arterial blood pressure (r = -0.053, p = 0.2), glucose exposure (r = -0.020, p = 0.6), number of peritonitis episodes (r = 0.033, p = 0.4), or number of peritonitis episodes treated with aminoglycosides (r = 0.011, p = 0.8) during the study period. The rate of residual GFR decline did not correlate with any nutritional index or MIS score (details not shown). The rate of decline of residual GFR was slower in patients who received ACE inhibitor or ARB, but faster in those who received CCB or diuretic therapy (Figure 2).
Figure 2 —
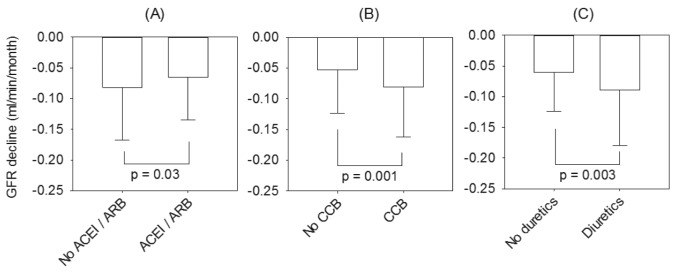
The effect of drug therapy on the rate of residual glomerular filtration rate (GFR) decline: (A) angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB); (B) calcium channel blockers (CCB); and (C) diuretics. Data are compared using unpaired Student t-test.
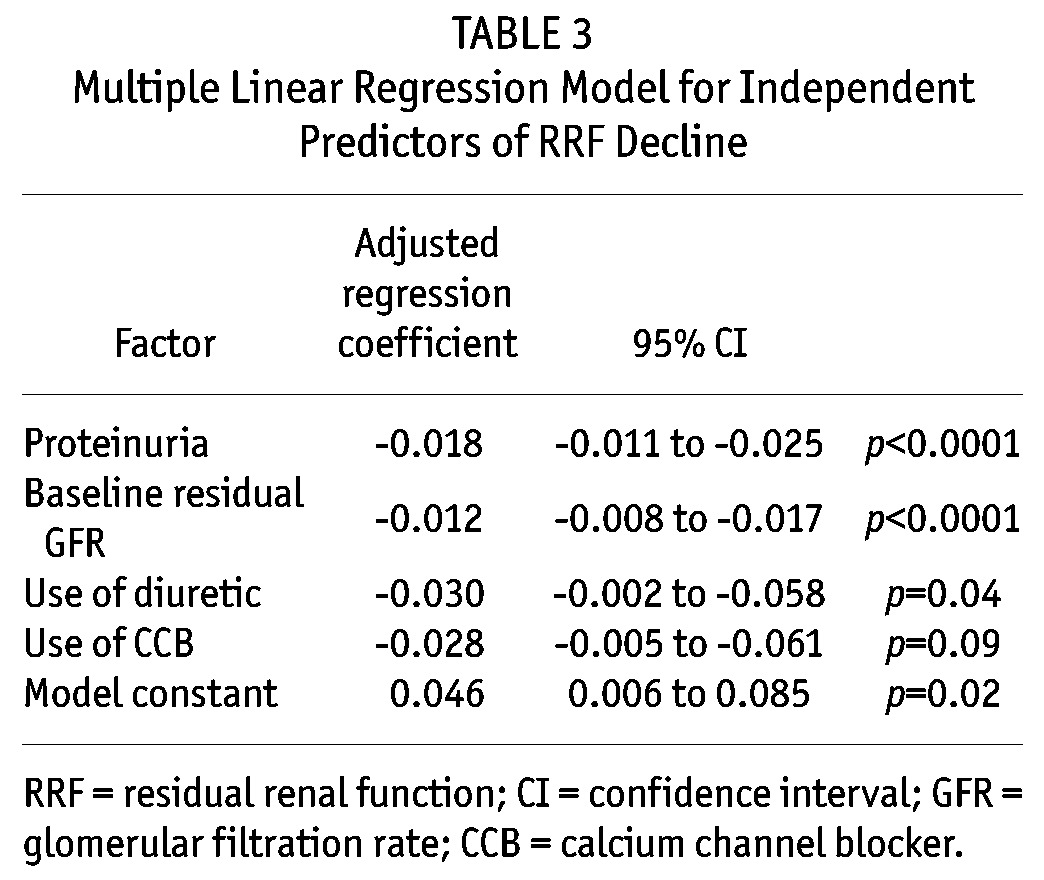
We went on to perform multiple linear regression to identify independent predictors of residual GFR decline. The final model is summarized in Table 3. In essence, proteinuria, baseline residual GFR, the use of diuretics and CCB are independent predictors of rapid residual GFR decline, although the result of CCB usage did not reach statistical significance. In this model, each 1 g/day of proteinuria is associated with an additional 0.22 mL/min decline in residual GFR in one year, and each 1 mL/min higher baseline GFR is associated with an additional 0.14 mL/min decline in residual GFR in one year. The result of the multiple linear regression model remains similar when only diabetic patients are analyzed; HbA1c does not appear as an independent predictor of the rate of residual GFR decline in diabetic patients (details not shown). Although we noted a significant anti-proteinuric effect of ACE inhibitor or ARB therapy, it is not independently associated with the rate of residual GFR decline even if proteinuria is not added to the linear regression model (details not shown).
TABLE 3.
Multiple Linear Regression Model for Independent Predictors of RRF Decline
Progression to Anuria
During this period, 378 patients (58.6%) progressed to anuria. Another 113 patients died before the development of anuria. During the same period, 42 patients were converted to long-term hemodialysis, 33 had kidney transplantation, 12 were transferred to other dialysis centers, and 1 had recovery of kidney function.
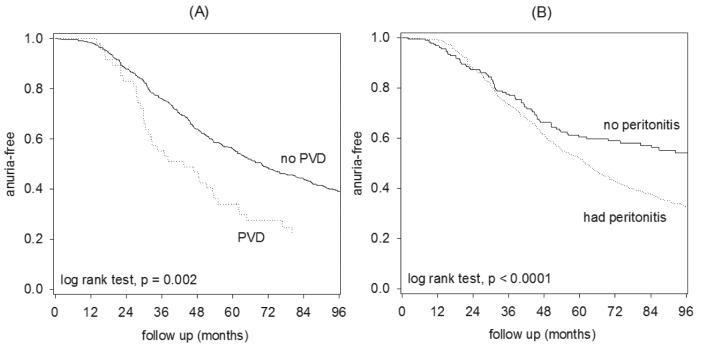
Univariate Cox analysis for the risk of progressing to anuria is summarized in Table 4. In short, patients with PVD (Figure 3), high body mass index, systolic blood pressure, serum phosphate level, proteinuria, MIS score, glucose exposure, baseline residual GFR, more peritonitis episodes, and a low SGA score were associated with the risk of developing anuria. In contrast, the risk of developing anuria was not related to diabetic status, presence of IHD, Charlson’s score, peritoneal transport status, dietary protein intake, or the use of aminoglycosides, ACE inhibitor or ARB, CCB, or diuretics (details not shown). There was no significant relation between the year of starting PD and the risk of progressing to anuria.
TABLE 4.
Cox Proportional Hazard Model for Independent Predictors of Anuria
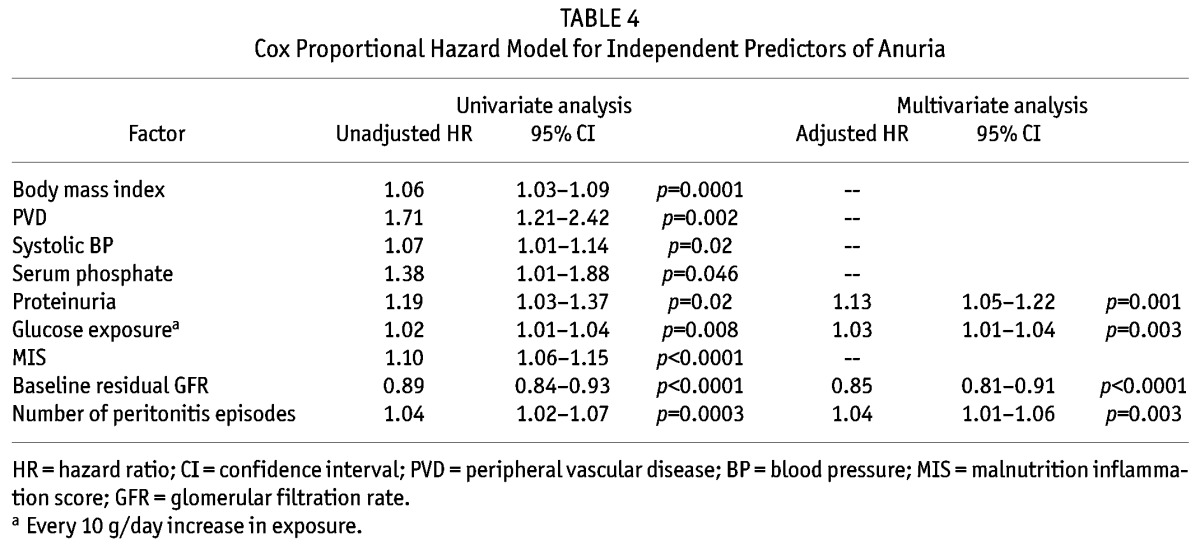
Figure 3 —
Kaplan Meier plot of the risk of developing anuria, comparing: (A) patients with and without peripheral vascular disease (PVD); and (B) patients with and without peritonitis episode.
A Cox proportional hazard model was also constructed to determine the independent predictors of time to anuria. The final model is summarized in Table 4. In this model, higher proteinuria, glucose exposure, and number of peritonitis episode were independent predictors of progression to anuria, while a higher baseline residual GFR was protective. In this model, each 1 g/day of proteinuria is associated with a 22.3% increase in the risk of progressing to anuria, each 1 mL/min higher baseline GFR is associated with an 11.3% reduction in the risk, while each episode of peritonitis is associated with a 7.9% increase in the risk. The result of the Cox model remains similar when only diabetic patients are analyzed; HbA1c does not appear as an independent predictor of progressing to anuria in diabetic patients (details not shown). Similarly, ACE inhibitor or ARB therapy is not independently associated with the risk of progressing to anuria even if proteinuria is not added to the Cox model (details not shown).
Discussion
In this observational study, we found that proteinuria, baseline residual GFR, and the use of diuretics are independently related to the rate of decline of residual renal function in Chinese CAPD patients, while proteinuria, baseline residual GFR, glucose exposure, and the number of peritonitis episodes are independent predictors for the development of anuria.
Our results are by and large in line with previous reports. The overall rate of residual renal function decline, in terms of the slope of GFR, is similar to 0.05 to 0.11 mL/min/month as reported in previous reports of other PD patients (12,14). We found that proteinuria accounts for over 25% of the variability of residual GFR decline, and is an independent predictor of progressing to anuria. Similarly, Jansen et al. (11) found that a higher urinary protein loss is independently associated with the rate of GFR decline in the first 12 months of dialysis.
We found that a higher baseline residual GFR is independently associated with a high rate of residual renal function decline (as determined by the slope of GFR decline with time) but a lower risk of developing anuria. We believe the lower risk of developing anuria in patients with a high baseline residual GFR could readily be explained by the phenomenon of lead time bias. In fact, our observation is similar to previous reports. For example, when the rate of GFR decline is determined by slope calculation, both Liao et al. (14) and Liu et al. (31) found that a higher baseline GFR is independently associated with faster decline of residual GFR. On the other hand, when time to anuria is used as the end point, Johnson et al. (12) reported that a higher baseline GFR is independently associated with a lower risk.
Similar to previous studies (9,14,15,31), we found that patients with diabetes and PVD had more rapid deterioration of residual GFR, although the effect became insignificant after multivariate analysis to adjust for confounding factors. Contrary to the general expectation, we found that the number of total peritonitis episodes, rather than the degree of exposure to aminoglycosides, is related to the risk of progressing to anuria. Similarly, Liao et al. (14) found that the episodes of peritonitis independently predicted the decline of residual GFR, while Badve et al. (32) reported that the use of aminoglycosides for PD-associated peritonitis does not affect residual renal function. Although blood pressure and diabetic control are well documented to affect the rate of renal function decline in pre-dialysis patients, we found that systolic blood pressure had only a weak association with the risk of anuria, and HbA1c level had only a modest correlation with the slope of GFR decline. Moreover, the effect of both of them disappeared with multivariate analysis to control for confounding factors. Our observation is consistent with previous reports by Jansen et al. (11) and Sung et al. (33).
In this study, we found that the slope of GFR decline was slower in patients receiving ACE inhibitor or ARB, and faster in those treated with CCB or diuretics. Our finding is consistent with the result of previous observational studies (9,14) as well as randomized control trials (34,35). The protective effect of renin-angiotensin system blockade, however, disappeared after multivariate analysis to adjust for confounding factors. We believe our results do not disprove the efficacy of such a treatment because a retrospective study like ours is likely biased by the treatment indication. Moreover, there is a strong internal correlation between proteinuria and ACE inhibitor or ARB treatment that could not be reliably adjusted for during multivariate analysis.
In this study, we found that diuretic treatment and high glucose exposure (from hypertonic PD solution) are associated with more rapid decline of residual renal function, but causality could not be established in an observational study. On one hand, excessive volume depletion and glucose toxicity may accelerate the loss of residual renal function. However, it is also possible that diuretics and hypertonic exchange are more likely needed for volume control in patients with rapid loss of residual renal function. In our opinion, diuretic treatment is essentially a non-modifiable risk factor in this situation and, since the detrimental effect of chronic volume overload is well documented in PD patients (36), the use of diuretic treatment should not be discouraged. Since the number of patients treated with glucose-free solutions (e.g., icodextrin) was small, we were unable to analyze the effect of glucose-sparing strategies on the rate of residual GFR decline.
An important observation that we obtained from this study is that the predicting factors for the slope of residual GFR decline are somewhat different from those for the risk of developing anuria. It seems likely that in dialysis patients, residual renal function is lost in two pathways. On one hand, there is a gradual deterioration due to the underlying kidney disease and progressive renal scarring. On the other, a considerable proportion of patients develop anuria suddenly because of acute events (for example, sudden shut down of renal circulation, acute infection, or exposure to nephrotoxic agents). Our results indicate that clinical research on residual renal function should use both GFR slope and development of anuria as clinical end points.
There are a number of limitations to our study. First, because of the retrospective nature of our study and the practical logistics we used to identify the cases, we omitted patients who had very rapid loss of residual renal function and developed anuria within three months after the initiation of dialysis. For the same reason, we do not have data on all hospital admission or non-peritonitis infections. In our center, aminoglycosides are seldom used in non-peritonitis infections, and this part of our data should be accurate. Unfortunately, we do not have data on the exposure to other nephrotoxic drugs such as nonsteroidal anti-inflammatory agents or iodinated contrast, nor do we have data on the use of temporary hemodialysis support during the course of CAPD. In addition, we do not have data on the exact duration of each peritonitis episode; it is possible that the total duration of peritoneal inflammation, rather than the actual number of peritonitis episodes, is the determining factor of residual renal function loss. As mentioned above, any acute infection or cardiac events may precipitate acute irreversible loss of residual renal function, but this hypothesis could not be tested in our present study.
In addition, we only studied CAPD patients from a single center. Although this approach excludes several important confounding factors and makes our study population homogeneous, the validity of our results in other patient populations (for example, patients from other countries or those on hemodialysis or machine-assisted PD) is uncertain. Since hemodialysis and machine-assisted PD have both been reported to cause a more rapid decline of residual renal function as compared to CAPD (9,16), we could not exclude the possibility that the effect of dialysis modality on residual renal function decline may overwhelm other clinical factors that we identify.
Taken together, our results indicate that baseline residual GFR, proteinuria, diuretic treatment, glucose exposure, and peritonitis are major factors affecting the rate of RRF decline. We consider proteinuria and peritonitis are possibly reversible factors, and the role of anti-proteinuric therapy and measures to prevent peritonitis episodes in the preservation of RRF should be tested in future studies.
Disclosures
The authors declare no conflict of interest.
Acknowledgments
This study was supported in part by the Chinese University of Hong Kong (CUHK) research account 6901031. The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Vilar E, Farrington K. Emerging importance of residual renal function in end-stage renal failure. Semin Dial 2011; 24:487–94. [DOI] [PubMed] [Google Scholar]
- 2. Raimann JG, Kitzler TM, Levin NW. Factors affecting loss of residual renal function(s) in dialysis. Contrib Nephrol 2012; 178:150–6. [DOI] [PubMed] [Google Scholar]
- 3. Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int 2006; 69:1726–32. [DOI] [PubMed] [Google Scholar]
- 4. Liao CT, Chen YM, Shiao CC, Hu FC, Huang JW, Kao TW, et al. Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol Dial Transplant 2009; 24:2909–14. [DOI] [PubMed] [Google Scholar]
- 5. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Adequacy of dialysis and nutrition in continuous peritoneal dialysis associated with clinical outcomes. J Am Soc Nephrol 1996; 7:198–207. [DOI] [PubMed] [Google Scholar]
- 6. Szeto CC, Lai KN, Wong TY, Law MC, Leung CB, Yu AW, et al. Independent effects of residual renal function and dialysis adequacy on nutritional status and patient outcome in continuous ambulatory peritoneal dialysis (CAPD). Am J Kidney Dis 1999; 34:1056–64. [DOI] [PubMed] [Google Scholar]
- 7. Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis 1999; 33:523–34. [DOI] [PubMed] [Google Scholar]
- 8. Horinek A, Misra M. Does residual renal function decline more rapidly in hemodialysis than in peritoneal dialysis? How good is the evidence? Adv Perit Dial 2004; 20:137–40. [PubMed] [Google Scholar]
- 9. Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 2000; 11:556–64. [DOI] [PubMed] [Google Scholar]
- 10. McKane W, Chandna SM, Tattersall JE, Greenwood RN, Farrington K. Identical decline of residual renal function in high-flux biocompatible hemodialysis and CAPD. Kidney Int 2002; 61:256–65. [DOI] [PubMed] [Google Scholar]
- 11. Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 2002; 62:1046–53. [DOI] [PubMed] [Google Scholar]
- 12. Johnson DW, Mudge DW, Sturtevant JM, Hawley CM, Campbell SB, Isbel NM, et al. Predictors of decline of residual renal function in new peritoneal dialysis patients. Perit Dial Int 2003; 23:276–83. [PubMed] [Google Scholar]
- 13. Caravaca F, Dominguez C, Arrobas M. Predictors of loss of residual renal function in peritoneal dialysis patients. Perit Dial Int 2002; 22:414–7. [PubMed] [Google Scholar]
- 14. Liao CT, Shiao CC, Huang JW, Hung KY, Chuang HF, Chen YM, et al. Predictors of faster decline of residual renal function in Taiwanese peritoneal dialysis patients. Perit Dial Int 2008; 28(Suppl 3):S191–5. [PubMed] [Google Scholar]
- 15. Tian SL, Tian XK, Han QF, Axelsson J, Wang T. Presence of peripheral arterial disease predicts loss of residual renal function in incident CAPD patients. Perit Dial Int 2012; 32:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michels WM, Verduijn M, Grootendorst DC, le Cessie S, Boeschoten EW, Dekker FW, et al. Decline in residual renal function in automated compared with continuous ambulatory peritoneal dialysis. Clin J Am Soc Nephrol 2011; 6:537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol 2012; 23:1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beddhu S, Zeidel ML, Saul M, Seddon P, Samore MH, Stoddard GJ, et al. The effects of comorbid conditions on the outcomes of patients undergoing peritoneal dialysis. Am J Med 2002; 112:696–701. [DOI] [PubMed] [Google Scholar]
- 19. Keane WF, Alexander SR, Bailie GR, Boeschoten E, Gokal R, Golper TA, et al. Peritoneal dialysis-related peritonitis treatment recommendations:1996 update. Perit Dial Int 1996; 16:557–73. [PubMed] [Google Scholar]
- 20. Keane WF, Bailie GR, Boeschoten E, Gokal R, Golper TA, Holmes CJ, et al. Adult peritoneal dialysis-related peritonitis treatment recommendations: 2000 update. Perit Dial Int 2000; 20:396–411. [PubMed] [Google Scholar]
- 21. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. ISPD Ad Hoc Advisory Committee: Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31. [PubMed] [Google Scholar]
- 22. Van Olden RW, Krediet RT, Struijk DG, Arisz L. Measurement of residual renal function in patients treated with continuous peritoneal dialysis. J Am Soc Nephrol 1996; 7:745–8. [DOI] [PubMed] [Google Scholar]
- 23. Szeto CC, Wong TY, Chow KM, Leung CB, Law MC, Wang AY, et al. Impact of dialysis adequacy on the mortality and morbidity of anuric Chinese patients receiving continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 2001; 12:355–60. [DOI] [PubMed] [Google Scholar]
- 24. Twardowski ZJ. PET – a simpler approach for determining prescriptions for adequate dialysis therapy. Adv Perit Dial 1990; 6:186–91. [PubMed] [Google Scholar]
- 25. Krediet RT, Boeschoten EW, Zuyderhoudt FMJ, Strackee J, Arisz L. Simple assessment of the efficacy of peritoneal transport in continuous ambulatory peritoneal dialysis patients. Blood Purification 1986; 4:194–203. [DOI] [PubMed] [Google Scholar]
- 26. Szeto CC, Wong TY, Leung CB, Wang AY, Law MC, Lui SF, et al. Importance of dialysis adequacy in mortality and morbidity of Chinese CAPD patients. Kidney Int 2000; 58:400–7. [DOI] [PubMed] [Google Scholar]
- 27. Enia G, Sicus C, Alati G, Zoccali C. Subjective global assessment of nutrition in dialysis patients. Nephrol Dial Transplant 1993; 8:1094–8. [PubMed] [Google Scholar]
- 28. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 2001; 38:1251–63. [DOI] [PubMed] [Google Scholar]
- 29. Chan JY, Che KI, Lam KM, Chow KM, Chung KY, Li PK, et al. Comprehensive malnutrition inflammation score as a marker of nutritional status in Chinese peritoneal dialysis patients. Nephrology (Carlton) 2007; 12:130–4. [DOI] [PubMed] [Google Scholar]
- 30. Ho LC, Wang HH, Chiang CK, Hung KY, Wu KD. Malnutrition-inflammation score independently determined cardiovascular and infection risk in peritoneal dialysis patients. Blood Purif 2010; 30:16–24. [DOI] [PubMed] [Google Scholar]
- 31. Liu JH, Wang SM, Chen CC, Hsieh CL, Lin SY, Chou CY, et al. Relation of ankle-brachial index to the rate of decline of residual renal function in peritoneal dialysis patients. Nephrology (Carlton) 2011; 16:187–93. [DOI] [PubMed] [Google Scholar]
- 32. Badve SV, Hawley CM, McDonald SP, Brown FG, Boudville NC, Wiggins KJ, et al. Use of aminoglycosides for peritoneal dialysis-associated peritonitis does not affect residual renal function. Nephrol Dial Transplant 2012; 27:381–7. [DOI] [PubMed] [Google Scholar]
- 33. Sung SA, Hwang YH, Kim S, Kim SG, Oh J, Chung W, et al. Loss of residual renal function was not associated with glycemic control in patients on peritoneal dialysis. Perit Dial Int 2011; 31:154–9. [DOI] [PubMed] [Google Scholar]
- 34. Li PK, Chow KM, Wong TY, Leung CB, Szeto CC. Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann Intern Med 2003; 139:105–12. [DOI] [PubMed] [Google Scholar]
- 35. Suzuki H, Kanno Y, Sugahara S, Okada H, Nakamoto H. Effects of an angiotensin II receptor blocker, valsartan, on residual renal function in patients on CAPD. Am J Kidney Dis 2004; 43:1056–64. [DOI] [PubMed] [Google Scholar]
- 36. Brunkhorst R. Hypervolemia, arterial hypertension and cardiovascular disease: a largely neglected problem in peritoneal dialysis. Clin Nephrol 2008; 69:233–8. [DOI] [PubMed] [Google Scholar]