Abstract
♦ Background: There is limited available evidence regarding the role of monitoring serum vancomycin concentrations during treatment of peritoneal dialysis (PD)-associated peritonitis.
♦ Methods: A total of 150 PD patients experiencing 256 episodes of either gram-positive or culture-negative peritonitis were included to investigate the relationship between measured serum vancomycin within the first week and clinical outcomes of cure, relapse, repeat or recurrence of peritonitis, catheter removal, temporary or permanent transfer to hemodialysis, hospitalization and death.
♦ Results: Vancomycin was used as an initial empiric antibiotic in 54 gram-positive or culture-negative peritonitis episodes among 34 patients. The median number of serum vancomycin level measurements in the first week was 3 (interquartile range; IQR 1 – 4). The mean day-2 vancomycin level, measured in 34 (63%) episodes, was 17.5 ± 5.2 mg/L. Hospitalized patients were more likely to have serum vancomycin levels measured on day 2 and ≥ 3 measurements in the first week. The peritonitis cure rates were similar between patients with < 3 and ≥ 3 measurements in the first week (77% vs 57%, p = 0.12) and if day-2 vancomycin levels were measured or not (68% vs 65%, p = 0.84). The average day-2 (18.0 ± 5.9 vs 16.6 ± 3.2, p = 0.5), first-week average (18.6 ± 5.1 vs 18.6 ± 4.3, p = 0.9) and nadir (14.5 ± 4.1 vs 13.6 ± 4.2, p = 0.5) vancomycin levels were comparable in patients who did or did not achieve peritonitis cure. Similar results were observed for all other clinical outcomes.
♦ Conclusion: The clinical outcomes of gram-positive and culture-negative peritonitis episodes are not associated with either the frequency or levels of serum vancomycin measurements in the first week of treatment when vancomycin is dosed according to International Society for Peritoneal Dialysis (ISPD) Guidelines.
Keywords: Kidney failure, chronic outcomes, peritoneal dialysis, peritonitis, therapeutic drug monitoring, vancomycin
Peritonitis is a frequent, major complication of peritoneal dialysis (PD) that often results in considerable morbidity, technique failure and mortality (1–3). The International Society for Peritoneal Dialysis (ISPD) Guidelines for PD-related Infections (4) recommend that initial empiric antibiotic therapy of peritonitis should include gram-positive cover, such as vancomycin. Vancomycin is a type of glycopeptide, which is recommended to be administered intermittently in a dose of 15 – 30 mg/kg of body weight in one exchange every 5 – 7 days daily via the intraperitoneal (IP) route for optimal efficacy (4). When antibiotics are administered intermittently via the IP route, ongoing effective eradication of peritoneal organisms during non-antibiotic PD exchanges requires back-diffusion of drug from the serum into the dialysate (5). Thus, monitoring serum antibiotic levels and adjusting dosages accordingly may be important for maximizing therapeutic efficacy and minimizing toxicity. The ISPD Guidelines recommend a dosing interval of 4 – 5 days to keep serum trough levels above 15 mg/L but also advise that it is best to obtain levels in view of the variability of losses due to residual renal function (4). However, the evidence underpinning these recommendations is sparse.
In a retrospective investigation of 31 episodes of gram-positive PD peritonitis, Mulhern et al. (6) observed that a cumulative 4-week trough serum vancomycin level < 12 mg/L or an initial 7-day trough serum level < 9 mg/L predicted significantly increased risks of peritonitis relapse. In contrast, Blunden et al. (7) reported that day-5 vancomycin levels did not predict cure or relapse of gram-positive or culture-negative peritonitis in 374 PD patients and that increasing vancomycin concentrations did not appear to improve cure rates. The limited available evidence has fuelled considerable uncertainty regarding the role of monitoring serum vancomycin concentrations in PD patients receiving this antimicrobial agent for PD-related peritonitis (8).
The aim of the present single-center observational cohort study was to investigate the relationship between measured serum vancomycin levels following initial empiric antibiotic therapy and subsequent clinical outcomes of confirmed gram-positive or culture-negative peritonitis.
Methods
Patient Population
All patients receiving PD for end-stage kidney disease (ESKD) at Princess Alexandra Hospital who experienced either a gram-positive or culture-negative peritonitis episode between 1 January 2005 and 31 December 2011 were included. Clinical, demographic and treatment data were prospectively recorded on an electronic database and included age, gender, racial origin, cause of primary renal disease, coronary artery disease, peripheral vascular disease, cerebrovascular disease, diabetes mellitus, smoking status, body mass index (BMI), dates and microbiology of peritonitis episodes, PD modality and dialysis fluids used at the time of peritonitis, initial and subsequent antibiotic treatment regimens, and dates and levels of serum vancomycin measurements.
Peritonitis was defined as clinical features of peritonitis (abdominal pain or cloudy dialysate) and dialysate leukocytosis (white blood cell count > 100 /μL with > 50% neutrophils) (4). Empiric treatment of peritonitis was initiated as soon as possible and consisted of IP cephazolin or vancomycin in combination with gentamicin (0.6 mg/kg up to a maximum of 50 mg in one PD exchange each 24 hours). Vancomycin was selected for gram-positive cover in preference to cephazolin if the patients were clinically unwell, were allergic to cephalosporins or had a prior history of methicillin-resistant S. aureus (MRSA) colonization or infection. The agent was administered by the IP route in a dose of 30 mg/kg of body weight (maximum dose 2 g). The day of peritonitis presentation was defined as day 0. Unit protocols recommended trough serum vancomycin levels be performed on day 2 and thereafter every 2 – 3 days during the first week. Vancomycin levels were measured by particle-enhanced immuno-turbidimetry on a Beckman Coulter DxC-800 instrument. Further vancomycin was not administered unless the trough serum levels were below 15 mg/L. If the vancomycin level fell below 15 mg/L, a further dose of 30 mg/kg of body weight (maximum dose 2 g) was administered. Doses were not modified according to urine output, residual renal function or PD modality. PD catheters were removed if dialysate effluent remained cloudy after 5 days of appropriate antibiotic therapy (4).
The outcomes examined were peritonitis cure, relapse, recurrence or repeat PD peritonitis, hospitalization, catheter removal, temporary hemodialysis transfer (in which patients subsequently resumed PD), permanent hemodialysis transfer and patient death. A peritonitis episode was considered “cured” by antibiotics alone if the patient was symptom free, the PD effluent was clear and the episode was not complicated by relapse, catheter removal or death (9). Peritonitis relapse was defined as peritonitis occurring within 4 weeks of a prior episode in which the dialysate culture yielded the same organism or was sterile (4,10,11). Peritonitis recurrence was defined as peritonitis occurring within 4 weeks of a prior episode in which the dialysate culture yielded a different organism to that of the original episode (4,10,11). Repeat peritonitis was defined as peritonitis occurring more than 4 weeks after a prior episode in which the dialysate culture yielded the same organism (10,12). Peritonitis-related death was defined as death occurring within 30 days of peritonitis onset (13).
Statistical Analysis
Results were expressed as frequencies and percentages for categorical variables, mean ± standard deviation for continuous normally distributed variables, and median (interquartile range; IQR) for continuous variables that were not normally distributed. Dichotomous and categorical data were compared using chi-square tests. Continuous normally distributed data were compared using two-tailed unpaired t-tests. Continuous non-normally distributed data were compared using Mann-Whitney tests. The independent predictors of day-2 serum vancomycin levels were assessed by generalized linear model analysis. The independent predictors of the clinical outcomes of peritonitis were determined by multivariable logistic regression using backward stepwise elimination based on the likelihood ratio until the most parsimonious model was identified. Day-2 vancomycin levels (or nadir vancomycin levels in the first week or average vancomycin level in the first week or frequency of serum vancomycin measurement in the first week), age, body mass index (BMI), diabetes mellitus, organism type (S. aureus, coagulase-negative staphylococci or other), PD modality at the time of peritontitis and anuria were included in the model as covariates. First-order interaction terms between the significant covariates were examined where appropriate. Data were analyzed using the software packages PASW Statistics for Windows release 21.0 (SPSS Inc., North Sydney, Australia). P values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
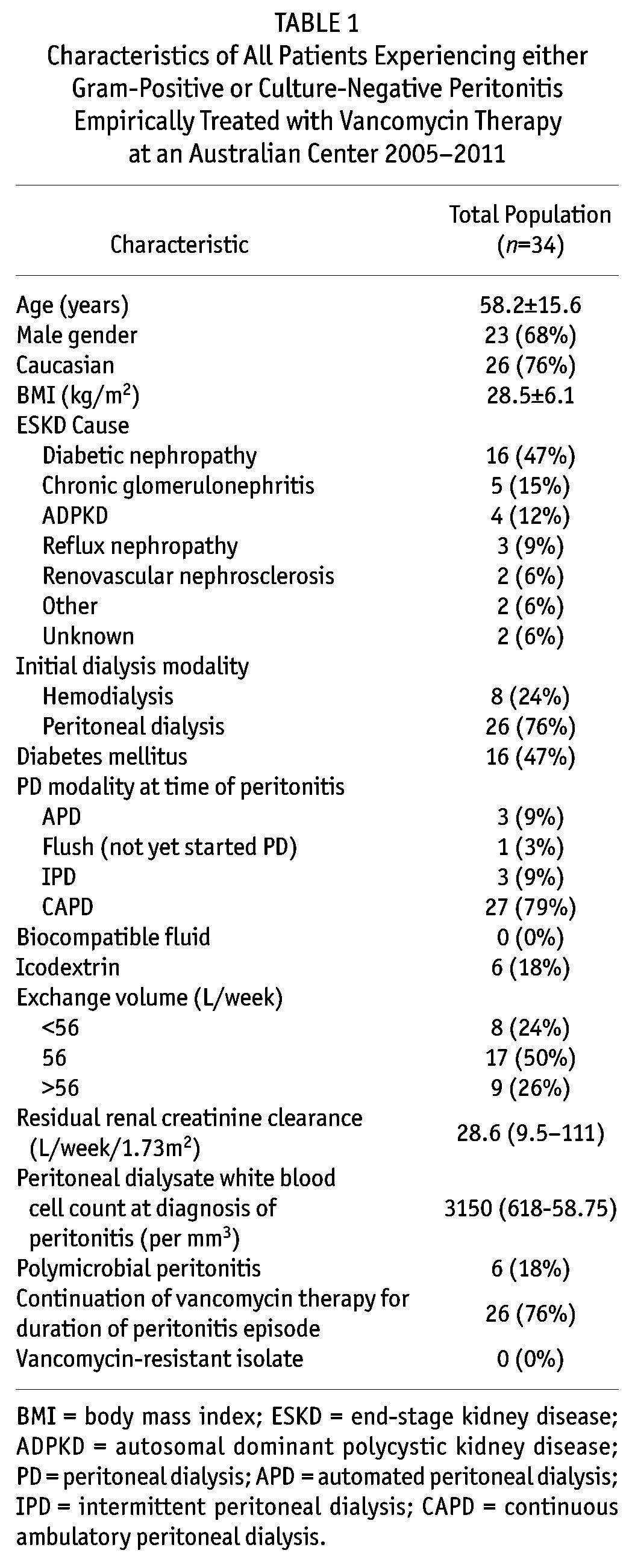
The mean overall peritonitis rate during the study period (2005 –) was 0.46 episodes per patient-year (25.9 patient-months per episode). Out of a total of 377 peritonitis episodes in 185 patients, 218 (58%) gram-positive peritonitis episodes were recorded in 115 patients and 71 (19%) culture-negative peritonitis episodes in 35 patients. This equated to mean gram-positive and culture-negative peritonitis rates of 0.27 and 0.09 episodes per patient-year, respectively. Fifty-four episodes (21%) of gram-positive or culture-negative episodes in 34 patients (23%) were initially treated with empiric vancomycin therapy. The baseline characteristics of these patients are shown in Table 1.
TABLE 1.
Characteristics of All Patients Experiencing either Gram-Positive or Culture-Negative Peritonitis Empirically Treated with Vancomycin Therapy at an Australian Center 2005-2011
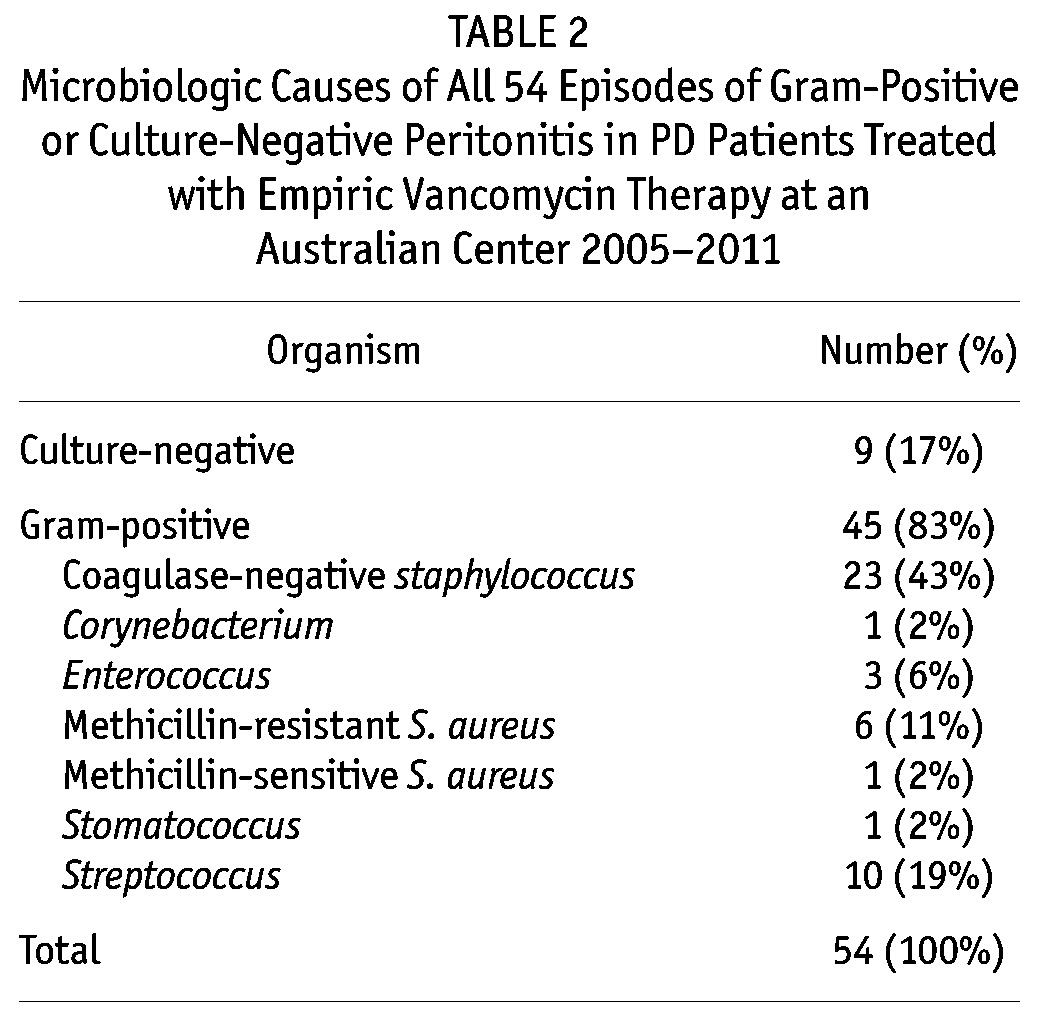
The microbiologic causes of gram-positive or culture-negative peritonitis episodes treated empirically with vancomycin therapy during the study period are shown in Table 2. Eighty-three per cent of cases involved gram-positive organisms (most commonly coagulase-negative staphylococci), whilst 17% were culture-negative. No vancomycin-resistant organisms were isolated from PD effluent.
TABLE 2.
Microbiologic Causes of All 54 Episodes of Gram-Positive or Culture-Negative Peritonitis in PD Patients Treated with Empiric Vancomycin Therapy at an Australian Center 2005-2011
Serum Vancomycin Levels and Monitoring Frequency in the First Week of Peritonitis Treatment
A median number of 3 (IQR 1 – 4) serum vancomycin measurements were performed in the first week of peritonitis treatment. Using multivariable logistic regression analysis, measuring serum vancomycin levels on 3 or more occasions in the first week was independently predicted by hospitalization for peritonitis (adjusted odds ratio (OR) 11.7, 95% confidence interval (CI) 1.11 – 124), but was not associated with age, gender, diabetes mellitus, automated PD (APD), weekly exchange volume, BMI or anuria.
Serum vancomycin levels were most commonly performed on day 2 in the first week in 34 (63%) of 54 gram-positive or culture-negative peritonitis episodes. Mean serum vancomycin levels on day 2 were 17.5 ± 5.2 mg/L (Figure 1). Levels fell below 15 mg/L in 25 (46%) cases. A day-2 vancomycin level < 15 mg/L was not independently predicted by age, diabetes mellitus, BMI, APD, weekly exchange volume or anuria.
Figure 1 —
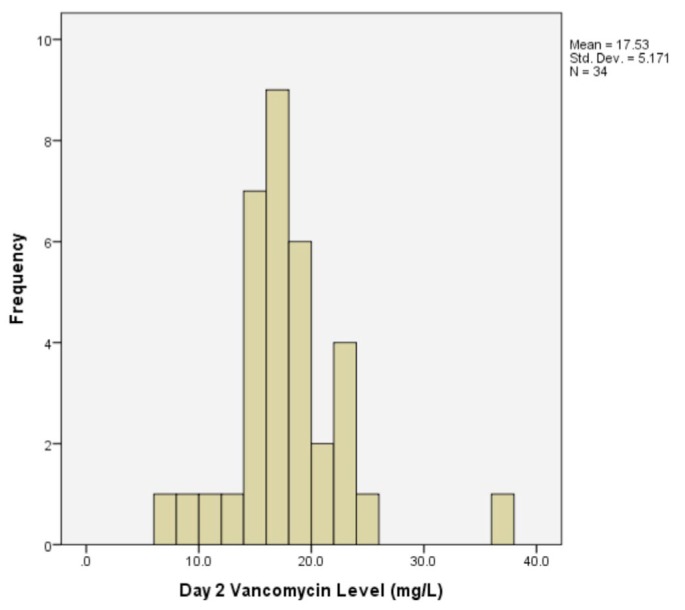
Histogram of serum vancomycin levels measured on day 2 in 34 gram-positive or culture-negative peritonitis episodes at an Australian Center 2005-2011.
The nadir serum vancomycin level during the first week was 14.2 ± 4.1 mg/L, whilst the average serum vancomycin level during the first week was 18.6 ± 4.8 mg/L.
Relationship Between Vancomycin Levels and Peritonitis Outcomes
The complications of gram-positive and culture-negative peritonitis episodes included relapse (13%), hospitalization (83%), catheter removal (13%) and death (2%). The overall cure rate was 67%. Compared with patients who had serum vancomycin levels performed on day 2, those who did not have vancomycin measurements had comparable outcomes and cure rates, except for lower rates of hospitalization (Table 3). Similarly, day-2 vancomycin levels were not significantly different between patients who did or did not experience resolution of peritonitis (Table 4), including the subgroup treated with APD (18.3 ± 1.2 vs 18.0 ± 2.6 mg/L, respectively, p = 0.85) and sub-groups with peritonitis due to coagulase-negative staphylococci (17.5 ± 7.2 vs 15.7 ± 5.0 mg/L, p = 0.69) and S. aureus (21.0 ± 5.7 vs 18.0 ± 2.6 mg/L, p = 0.46). Similar results were observed for nadir serum vancomycin levels in the first week and average serum vancomycin levels for each patient in the first week (Table 4). Using multivariable logistic regression analysis, day-2 serum vancomycin level was not associated with cure (adjusted per mg/L 1.13, 95% CI 0.89 – 1.45, p = 0.32). Similar results were found for nadir serum vancomycin level (OR per mg/L 1.10, 95% CI 0.88 – 1.37, p = 0.39) and average serum vancomycin level in the first week (OR per mg/L 1.06, 95% CI 0.89 – 1.325, p = 0.55).
TABLE 3.
Clinical Outcomes of 54 Gram-Positive or Culture-Negative Peritonitis Episodes in PD Patients During the Period 2005-2011*
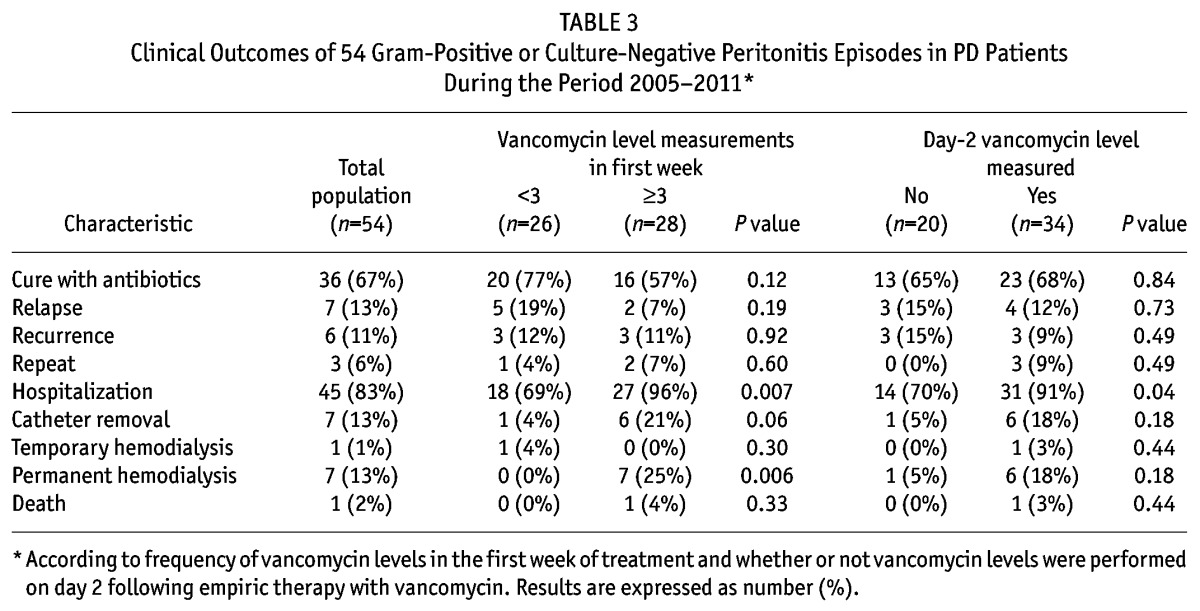
TABLE 4.
Comparison of Day-2, First-Week Nadir and First-Week Average Serum Vancomycin Levels*
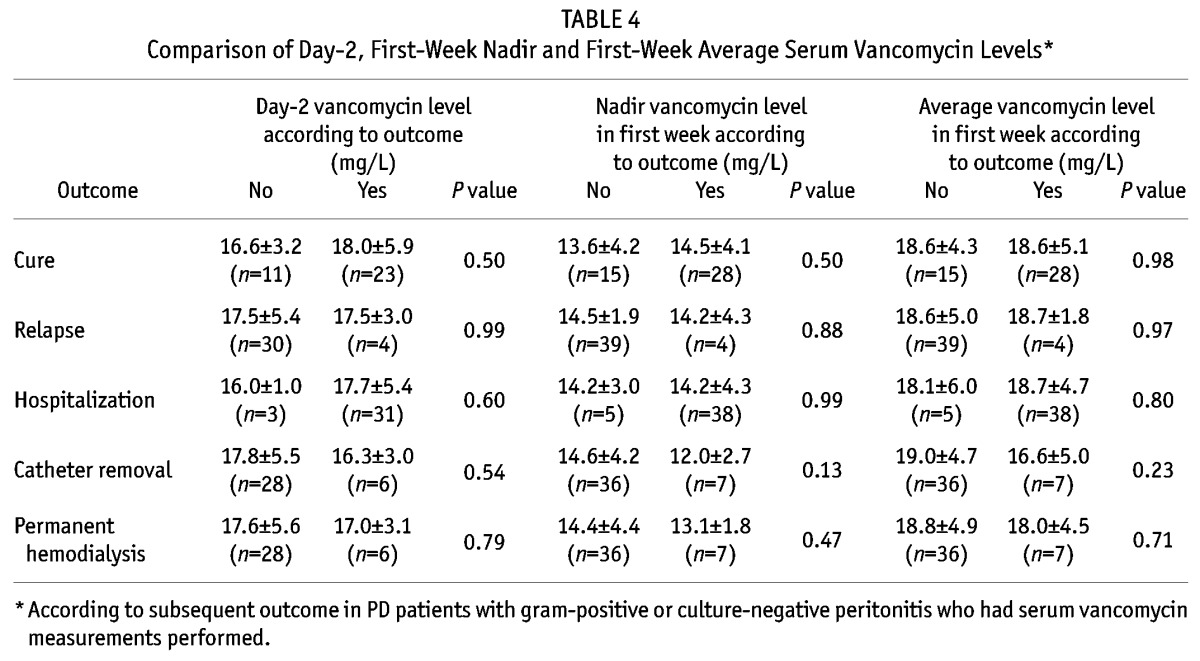
Compared with peritonitis episodes in which day-2 serum vancomycin levels were < 15 mg/L, those with levels ≥ 15 mg/L were associated with comparable rates of cure (60% vs 69%, respectively, p = 0.69). Similar results were observed for average serum vancomycin levels in the first week (71% vs 65%). Using multivariable logistic regression, cure rates were not independently predicted by day-2 serum vancomycin levels ≥ 15 mg/L (OR 1.90, 95% CI 0.42 – 8.65, p = 0.41) or average serum vancomycin levels ≥ 15 mg/L (OR 0.80, 95% CI 0.18 – 3.52, p = 0.77).
Compared with patients who had serum vancomycin levels measured on at least 3 occasions in the first week, those who had less frequent vancomycin measurements had comparable outcomes and cure rates, except for lower rates of hospitalization (Table 3).
Discussion
In this single-center observational cohort study, the relationship between measured serum vancomycin levels on day 2 following initial empiric antibiotic therapy and subsequent clinical outcomes of confirmed gram-positive or culture-negative peritonitis was investigated. The study found that peritonitis cure rates were not associated with measurement of trough serum vancomycin on day 2, nadir serum vancomycin level in the first week, average serum vancomycin level in the first week or frequent (≥ 3) measurement of serum vancomycin levels in the first week. Other peritonitis outcomes (such as relapse, recurrence, repeat peritonitis, catheter removal, hospitalization, hemodialysis transfer and death) were also not associated with serum vancomycin levels, except that hospitalization was associated with more frequent measurement of serum vancomycin concentrations.
These observations are in keeping with those of a retrospective study of 407 episodes of gram-positive or culture-negative peritonitis at a single center in the United Kingdom, whereby day-5 vancomycin levels were not influenced by PD modality or the presence or absence of anuria, and were not associated with peritonitis relapse or cure (7). Moreover, increasing vancomycin concentrations did not appear to improve cure rates.
In contrast, Mulhern et al. (6) observed in 31 episodes of gram-positive peritonitis that a heightened risk of peritonitis relapse was associated with an initial 7-day trough serum vancomycin level < 9 mg/L or a cumulative 4-week trough < 12 mg/L. However, the initial empiric vancomycin dose administered in this study (15 mg/kg to a maximum of 1 g IP) was considerably lower than that employed either in our study (30 mg/kg up to a maximum of 2 g IP) or that of Blunden et al. (25 mg/kg rounded to the nearest 500 mg for anuric patients; increased by 25% if not anuric) (7). Consequently, average trough serum vancomycin levels in Mulhern’s study were considerably lower than those measured in the present study. Furthermore, whereas the studies of Blunden et al. (7) and Mulhern et al. (6) targeted trough serum vancomycin concentrations greater than 12 mg/L, the present study aimed to maintain trough serum vancomycin levels above 15 mg/L.
The ISPD Guidelines for the treatment of peritoneal dialysis-associated infections recommend that vancomycin should be administered in a dose of 15 – 30 mg/kg IP with further doses repeated every 5 – 7 days in continuous ambulatory PD or 3 – days in APD with the aim of maintaining trough serum vancomycin levels above 15 mg/L (4). The critical level of 15 mg/L was based on recent epidemiologic evidence of progressive increases in observed vancomycin minimal inhibitory concentration values for S. aureus with subsequent reports of treatment failures (14). Moreover, there is accumulating evidence suggesting that S. aureus exposure to trough serum vancomycin concentrations of < 10 mg/L can induce vancomycin resistance (15). Although the present study found no relationship between trough serum vancomycin levels and peritonitis cure or clinical outcomes, it is important to note that the majority of patients attained average trough serum vancomycin levels above 15 mg/L in the first week when ISPD-recommended vancomycin doses of 30 mg/kg were employed.
The present study has several limitations. First, the relatively small sample size meant that the possibility of a type 2 statistical error could not be excluded. Second, vancomycin levels were measured at variable time periods following the onset of peritonitis. Third, the non-randomized design of the study meant that there was a possibility that the results were confounded by indication bias. This scenario was suggested by the significant association between hospitalization and the frequency of serum vancomycin measurements, whereby sicker patients admitted to hospital would be expected to be monitored more closely.
In conclusion, the present study showed that the clinical outcomes of gram-positive and culture-negative peritonitis episodes are not associated with either the frequency or levels of serum vancomycin measurements in the first week of treatment when vancomycin is dosed according to ISPD Guidelines. Further studies are required. In particular, it would be valuable to study in a randomized controlled trial whether actively measuring to target vancomycin levels, and re-dosing as appropriate to maintain target levels, results in better outcomes than current best practice.
Disclosures
Professor David Johnson is a consultant for Baxter Healthcare Pty Ltd and has previously received research funds from this company. He has also received speakers’ honoraria and research grants from Fresenius Medical Care and has previously been a consultant to Gambro. He is a current recipient of a Queensland Government Health Research Fellowship. The remaining authors have no competing financial interests to declare.
Acknowledgments
The invaluable assistance provided by Ms Lauren Jaffrey, Data Manager, is gratefully acknowledged. Wen Tang was supported by International Society of Peritoneal Dialysis Scholarship, grants from National Natural Science Foundation of China (Project 30900681), Beijing Municipal Science & Technology Commission (D09050704310905) and Fund of Peking University Third Hospital (76496-02). David Johnson is a current recipient of a Queensland Government Health Research Fellowship.
REFERENCES
- 1. Ghali JR, Bannister K, Brown FG, Rosman JB, Wiggins KJ, Johnson DW, et al. Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit Dial Int 2011; 31:651–62. [DOI] [PubMed] [Google Scholar]
- 2. Johnson DW, Dent H, Hawley CM, McDonald SP, Rosman JB, Brown F, et al. Associations of dialysis modality and infectious mortality in incident dialysis patients in Australia and New Zealand. Am J Kid Dis 2009; 53:290–7. [DOI] [PubMed] [Google Scholar]
- 3. Jose M, Johnson DW, Mudge DW, Tranaeus A, Voss D, Walker R, et al. Peritoneal dialysis practice in Australia and New Zealand: A call to action. Nephrology 2011; 16:19–29. [DOI] [PubMed] [Google Scholar]
- 4. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423. [DOI] [PubMed] [Google Scholar]
- 5. Booranalertpaisarn V, Eiam-Ong S, Wittayalertpanya S, Kanjanabutr T, Na Ayudhya DP. Pharmacokinetics of ceftazidime in CAPD-related peritonitis. Perit Dial Int 2003; 23:574–9. [PubMed] [Google Scholar]
- 6. Mulhern JG, Braden GL, O’Shea MH, Madden RL, Lipkowitz GS, Germain MJ. Trough serum vancomycin levels predict the relapse of gram-positive peritonitis in peritoneal dialysis patients. Am J Kidney Dis 1995; 25:611–5. [DOI] [PubMed] [Google Scholar]
- 7. Blunden M, Zeitlin D, Ashman N, Fan SL. Single UK Center experience on the treatment of PD peritonitis—antibiotic levels and outcomes. Nephrol Dial Transplant 2007; 22:1714–9. [DOI] [PubMed] [Google Scholar]
- 8. Johnson DW. Do antibiotic levels need to be followed in treating peritoneal dialysis-associated peritonitis? Semin Dial 2011; 24:445–6. [DOI] [PubMed] [Google Scholar]
- 9. Cho Y, Badve SV, Hawley CM, McDonald SP, Brown FG, Boudville N, et al. Effects of climatic region on peritonitis risk, microbiology, treatment and outcomes: a multicenter registry study. Perit Dial Int 2013. Jan-Feb; 33(1):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piraino B, Bernardini J, Brown E, Figueiredo A, Johnson DW, Lye WC, et al. ISPD – position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int 2011; 31:614–30. [DOI] [PubMed] [Google Scholar]
- 11. Burke M, Hawley CM, Badve SV, McDonald SP, Brown FG, Rosman JB, et al. Relapsing and recurrent peritoneal dialysis-associated peritonitis: A multi-center registry study. Am J Kidney Dis 2011; 58:429–36. [DOI] [PubMed] [Google Scholar]
- 12. Thirugnanasambathan T, Hawley CM, Badve SV, McDonald SP, Brown FG, Boudville N, et al. Repeated peritoneal dialysis-associated peritonitis: a multicenter registry study. Am J Kidney Dis 2012; 59:84–91. [DOI] [PubMed] [Google Scholar]
- 13. Boudville N, Kemp A, Clayton P, Lim W, Badve SV, Hawley CM, et al. Recent peritonitis associates with mortality among patients treated with peritoneal dialysis. J Am Soc Nephrol 2012; 23:1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vandecasteele SJ, De Vriese AS. Recent changes in vancomycin use in renal failure. Kidney Int 2010; 77:760–4. [DOI] [PubMed] [Google Scholar]
- 15. Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr., Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66:82–98. [DOI] [PubMed] [Google Scholar]


