Abstract
Tendons like the flexor carpi ulnaris (FCU) that contain region-specific distributions of proteoglycans (PGs) as a result of the heterogeneous, multi-axial loads they are subjected to in vivo provide valuable models for understanding structure-function relationships in connective tissues. However, the contributions of specific PGs to FCU tendon mechanical properties are unknown. Therefore, the objective of this study was to determine how the location-dependent, viscoelastic mechanical properties of the FCU tendon are impacted individually by PG-associated glycosaminoglycans (GAGs) and by two small leucine-rich proteoglycans (SLRPs), biglycan and decorin. Full length FCU tendons from biglycan- and decorin-null mice were compared to wild type mice to evaluate the effects of specific SLRPs, while chondroitinase ABC digestion of isolated specimens removed from the tendon midsubstance was used to determine how chontroitin/dermatan sulfate (CS/DS) GAGs impact mechanics in mature FCU tendons. A novel combined genetic knockout/ digestion technique also was employed to compare SLRP-null and wild-type tendons in the absence of CS/DS GAGs that may impact properties in the mature state. In all genotypes, mechanical properties in the FCU tendon midsubstance were not affected by GAG digestion. Full-length tendons exhibited complex, multi-axial deformation under tension that may be associated with their in vivo loading environment. Mechanical properties were adversely affected by the absence of biglycan, and a decreased modulus localized in the center of the tendon was measured. These results help elucidate the role that local alterations in proteoglycan levels may play in processes that adversely impact tendon functionality including injury and pathology.
Keywords: flexor carpi ulnaris tendon, proteoglycan, biglycan, decorin, knockout
INTRODUCTION
The intricate structure and mechanical properties of tendon are endowed by interactions between its constituent molecules during development as well as in the mature state. Although the central solid component of tendon is a hierarchically organized, highly aligned network of collagen I, fibril characteristics are highly sensitive to interactions with the small leucine-rich proteoglycans (SLRPs) biglycan and decorin, suggesting a role for these molecules in mediating tendon development and potentially modulating fibril interactions in mature tissue [1–4]. However, recent studies have demonstrated that the impact of SLRPs on tendon structure and mechanics is tendon- and region-specific [2, 5]. For example, flexor digitorum longus (FDL) tendons from biglycan-null (Bgn−/−) mice exhibited inferior mechanical properties as compared to wild type and decorin-deficient (Dcn−/−) tendons while in the mouse patellar tendon, an increased static modulus was measured in Dcn−/− tendons and Bgn−/− tendons exhibited no mechanical differences from wild type [5, 6]. In rat tail tendon fascicles, the human medial collateral ligament (MCL) and the human patellar tendon, removal of SLRP-associated glycosaminoglycans (GAGs) by choindroitinase digestion did not induce mechanical changes under either shear or tension [7–10]. However, in the mouse Achilles tendon, removal of dermatan/chondroitin sulfate GAGs caused a significant reduction in modulus in the distal (i.e., calcaneal) third of the tendon midsubstance [11] while GAG depletion altered stress relaxation in bovine extensor tendon fascicles [12]. Finally, in the mouse supraspinatus tendon (SST), the absence of biglycan induced an increase in the transition stress between the toe and linear regions of the stress-strain curve only in the tendon midsubstance, and this mechanical alteration was concomitant with modified realignment of the collagen network under loading [13]. These findings demonstrate a tendon and region specificity to the role of SLRPs in tendon mechanics that are likely related to local functional demand. In support of this idea, the content of SLRPs and other proteoglycans (PGs) in the SST demonstrates regional variations dictated by in vivo mechanical requirements [14].
Like the SST, the mechanical environment and functional demands of the flexor carpi ulnaris (FCU) tendon are unique compared to its surrounding tissues. The FCU tendon is located on the medial side of the forearm and inserts distally into the pisiform bone on the palmar side of the wrist. However, in contrast to the digital flexor tendons, the FCU tendon does not pass underneath the flexor retinaculum. As a result, its insertion angle is not physically constrained and it experiences multiaxial loading during in vivo loading [15]. In particular, during wrist adduction, a joint motion partially controlled by the FCU muscle, the FCU tendon-pisiform enthesis is subject to high shear strains that have been linked to a fibrocartilage-like structure and composition in other tendons [16, 17]. Interestingly, the FCU insertion site is known to contain high levels of collagen II and aggrecan, a large proteoglycan typically associated with cartilaginous tissues that experience predominantly compression and shear [15]. Given its unique functional demands and heterogeneous loading environment, an understanding of the regionally-varying mechanical role(s) of PGs in the FCU tendon could clarify how these molecules contribute to mechanics in collagenous tissues, both as regulators of proper structural development and as potential mediators of force transmission between fibrils.
The objective of this study was to elucidate the effects of chondroitin/dermatan sulfate (CS/DS) GAGs and the SLRPs, biglycan and decorin on the location-dependent, viscoelastic mechanical properties of the FCU tendon using a combined GAG digestion and knockout model approach. We hypothesized that GAGs would have no effect on tensile mechanical properties in the tendon midsubstance, but that since SLRPs are important in the establishment of mechanical properties, the null tendons would exhibit inferior midsubstance properties both in the presence and absence of GAGs. We further hypothesized that SLRPs are important in developing regional differences in mechanics. Therefore, in full length, intact FCU tendon/pisiform complexes, the absence of SLRPs would lead to a region-dependent reduction of mechanical properties focused near the insertion.
METHODS
Study design
C57BL/6 wild-type (WT, n=52), biglycan-null (Bgn−/−, n=34) and decorin-null (Dcn−/−, n=25) mice were sacrificed at 150 days post-natal with IACUC approval. Each mouse was designated for either GAG quantification (WT: n=10 mice), Study 1 (WT: n=11 mice, Bgn−/−: n=11 mice; Dcn−/−: n=14 mice) or Study 2 (WT: n=31 mice, Bgn−/−: n=23; Dcn−/−: n=11).
Glycosaminoglycan Quantification
FCU tendon-pisiform complexes were dissected from the right or left forelimb and randomly assigned to phosphate buffered saline (PBS) incubation while the contralateral limb was assigned to chondroitinase ABC (ChABC) incubation. ChABC removes CS and DS GAGs, but does not hydrolyze keratin sulfate (KS). Pilot studies demonstrated that GAG removal was negligible in FCU-pisiform complexes incubated in ChABC (Figure 1, n=4 tendons/incubation condition), possibly signifying that the ChABC concentration was insufficient to remove all CS and DS GAGs near the insertion and in residual cartilage that may have remained on the pisiform. Therefore, the pisiform was sharply detached using a scalpel blade (n=6 tendons/incubation condition). Tendons were then incubated for 15 hours at 37°C in 200 μL PBS or 200 μL PBS with 2.5 U chondroitinase ABC (Sigma-Aldrich Corp., St. Louis, MO) supplemented with protease inhibitors (Roche, Basel Switzerland). The resulting concentration of ChABC was higher than in previously published work [18]. Following incubation, specimens were digested in 2.5 mg/mL proteinase K (Sigma-Aldrich, St. Louis, MO) in 0.05 M ammonium acetate for 18 hours at 65 °C, after which the enzyme proteinase K was inactivated by heating to 100 °C for 10 minutes. Quantification of glycosaminoglycan (GAG) content was then performed using the 1,9-dimethylmethylene blue (DMB) dye-binding assay [19].
Figure 1.
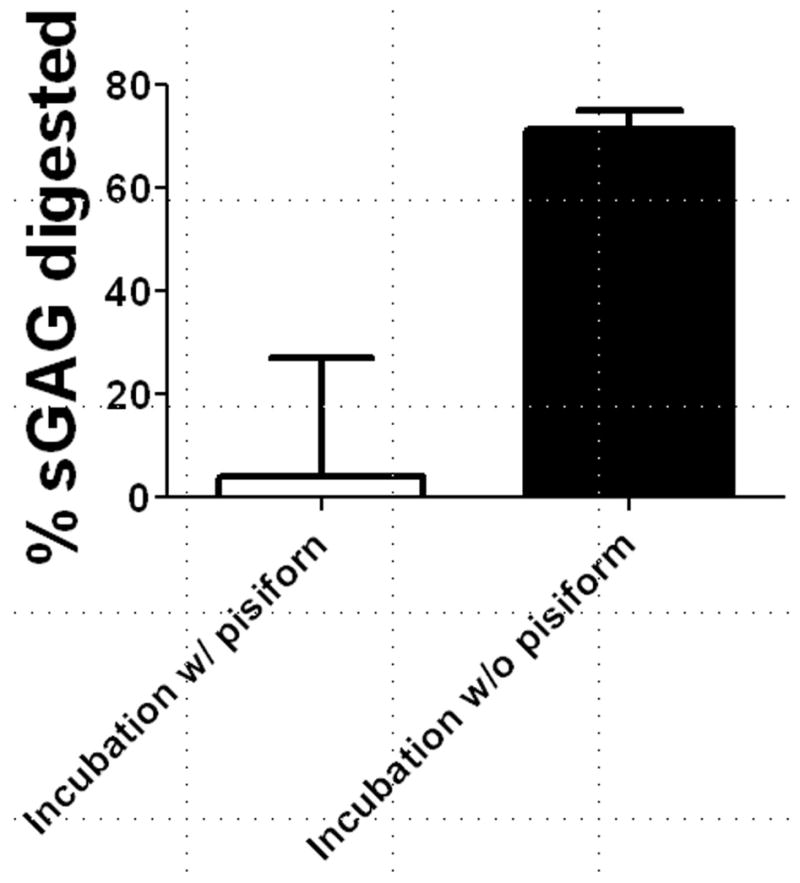
Percentage of CS/DS glycosaminoglycans removed after chondroitinase ABC digestion for FCU tendons with and without the pisiform. Removal of the pisiform allowed for more consistent and complete GAG digestion.
Two separate studies were performed to assess the role of SLRPs in FCU tendon mechanics. In Study 1, midsubstance sections of FCU tendons from WT and SLRP-null mice were digested in ChABC in order to separate the developmental effects of GAGs on tendon mechanical properties from their potential effects in mature tissue. In Study 2, mechanical testing was performed on whole FCU tendon-pisiform complexes from all three genotype groups to evaluate how the impact of SLRPs on tendon mechanics varies spatially.
Sample Preparation (Study 1)
FCU tendons were dissected, separated from the pisiform and incubated in PBS or ChABC as described in the previous section. Following digestion, tendon cross-sectional areas were measured using a laser-based device [24]. Although cross-sectional areas were not measured prior to incubation, previous studies have demonstrated that tendons swell after PBS incubation and (to a lesser extent) after ChABC incubation [18, 20]. However, since all genotype groups experienced the same two incubation conditions, swelling was not expected to impact the results of the study. After area measurement, 1 mm on each side of the 5 mm tendon was gripped between small sandpaper squares coated with cyanoacrylate. Tendons were then speckle-coated with Verhoeff’s stain to facilitate optical tracking. The sandpaper on both ends of the tendon was gripped using custom fixtures (yielding a 3 mm tendon gauge length) and tendons were loaded into a 37° C PBS bath connected to an Instron 5848 universal testing system (Instron, Norwood, MA).
Sample Preparation (Study 2)
FCU tendon-pisiform complexes were dissected as described in the previous section, but the pisiform was not separated and the pisiform enthesis remained intact. In each mouse, only one randomly-chosen tendon (right or light) was used. Tendon cross-sectional areas were measured using a laser-based device [24]. Using cyanoacrylate, the proximal side of the tendon was then glued between two small squares of sandpaper at a distance of 5 mm from the pisiform insertion. After speckle coating (see previous section), tendon-pisiform complexes were gripped with custom fixtures and loaded into a 37° C PBS bath connected to an Instron 5848 universal testing system (Instron, Norwood, MA).
Mechanical Testing and Viscoelastic Data Analysis (Studies 1 and 2)
After speckle-coating each tendon with Verhoeff’s stain to facilitate optical strain measurement, specimens were tested according to the following protocol: 1) preload to 0.005 N, 2) preconditioning, 3) stress relaxation to 4% strain, 4) sinusoidal frequency sweep, 5) return to gauge length, and 6) ramp to failure at 0.1% strain/second. Preconditioning consisted of 10 cycles of triangle wave strain between 0% and 4% at a frequency of 0.25 Hz. The frequency sweep consisted of 10 cycles of 0.125% amplitude sinusoidal strain at frequencies of 0.01, 0.1, 1, 5, and 10 Hz. The dynamic modulus |E*| (i.e., the stress amplitude divided by the strain amplitude) and the tangent of the phase angle δ between the stress and strain (tanδ, a measure of viscoelasticity) were computed for each frequency.
Optical Tracking and Quasi-Static Data Analysis (Study 2)
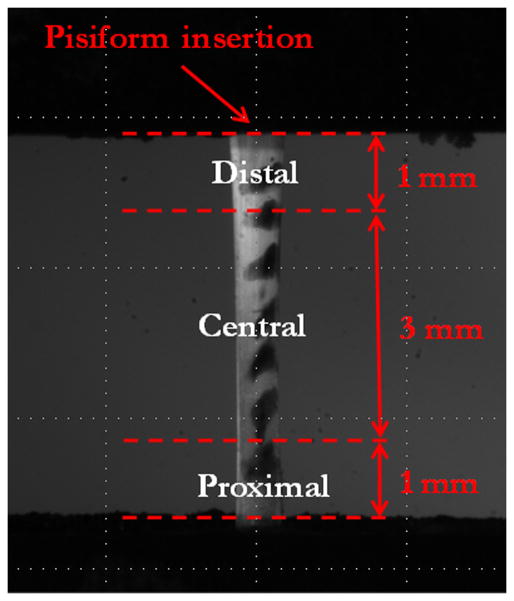
Step 6 was tracked optically and analyzed using Vic2D (Correlated Solutions, Columbia, SC) to determine the vertical strains within the distal (0–1 mm from the pisiform insertion), central (1–4 mm from the pisiform insertion) and proximal (4–5 mm from the pisiform insertion) regions (Figure 2) and the angle of the principal axis θp in the central region was calculated in the linear portion of the stress-strain curve [21]. In each region, the quasi-static parameters toe modulus Etoe, linear modulus Elin, and transition strain γtrans between the toe and linear regions of the stress-strain curve were determined assuming uniform tensile stress throughout the tendon. γtrans was computed based on a bilinear fit as described previously [22]. The maximum stress σmax prior to failure was also calculated for the entire tendon. Note that the principal angle was not computed in Study 1 or in the proximal and distal regions since gripping of the tendon was expected to prevent shear deformations associated with a non-zero θp in locations bounded by the fixtures.
Figure 2.
Regions of the full-length FCU tendon. The tendon was speckle-coated with Verhoeff’s stain to allow for optical tracking of strain within each region during the ramp-to-failure.
Statistics
In Study 1, a two-way ANOVA was performed to compare quasi-static parameters across incubation and genotype and to compare viscoelastic parameters across incubation and genotype at each frequency. In Study 2, a one way ANOVA was performed to compare quasi-static parameters across genotypes at each location and viscoelastic parameters across genotypes at each frequency. Bonferroni post-hoc analysis was used to correct for multiple comparisons and significance was set at p ≤0.05.
RESULTS
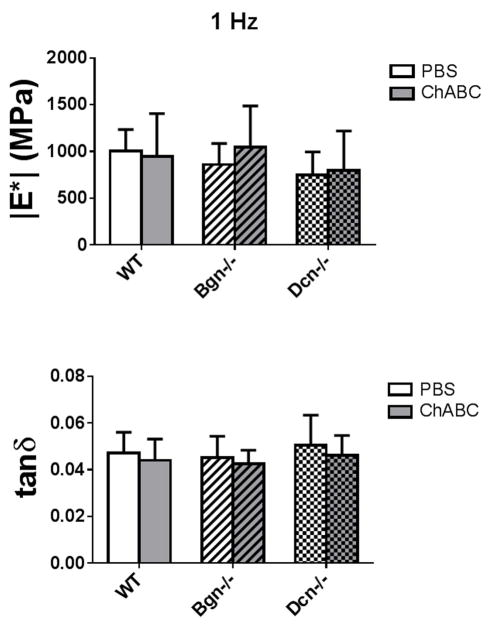
To examine the role of CS/DS GAGs, FCU tendons were incubated with and without ChABC. GAG digestion was variable and generally ineffective in FCU tendon-pisiform complexes. In contrast, 72% of sulfated GAGs were removed in tendons incubated in ChABC after detachment of the pisiform (Figure 1). However, for all genotypes, no differences in viscoelastic properties (|E*| and tanδ) were found between incubation conditions for FCU tendon midsubstance specimens tested in Study 1 (Figure 3). Similarly, for all incubation conditions, no differences in viscoelastic properties were found among genotypes.
Figure 3.
(a) Dynamic modulus (|E*|) and (b) tangent of the phase angle between stress and strain (tanδ) for FCU tendon midsubstance specimens stretched at 1 Hz in Study 1. No differences in |E*| or tanδ were found between genotypes or between incubation conditions.
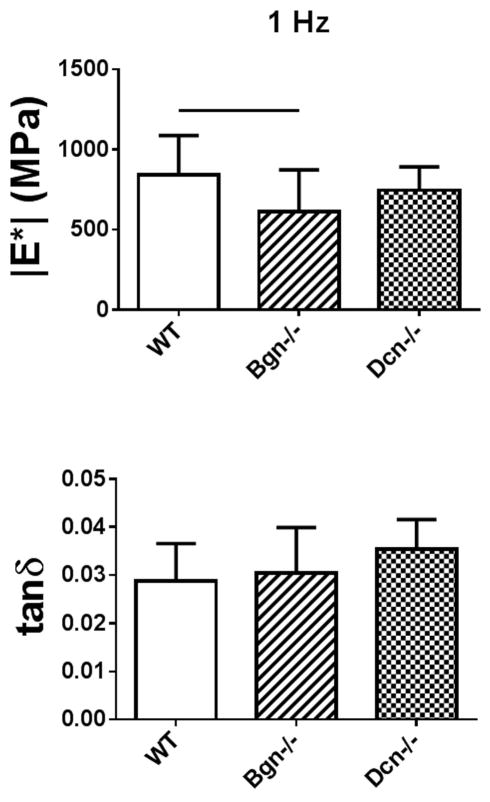
In Study 2, full-length Bgn−/− FCU tendons were less resistant to deformation (lower |E*|) than wild type (Figure 4). However, tanδ, a measure of energy dissipation relative to energy storage, was similar in all genotypes. Failure properties were altered by genotype, as the maximum stress σmax was lower in Bgn−/− as compared to both Dcn−/− and wild type (Figure 5).
Figure 4.
(a) Dynamic modulus (|E*|) and (b) tangent of the phase angle between stress and strain (tanδ) for full-length FCU tendons stretched at 1 Hz in Study 2. |E*| was reduced in Bgn−/− tendons relative to wild type, but no differences in tanδ were found between genotypes.
Figure 5.
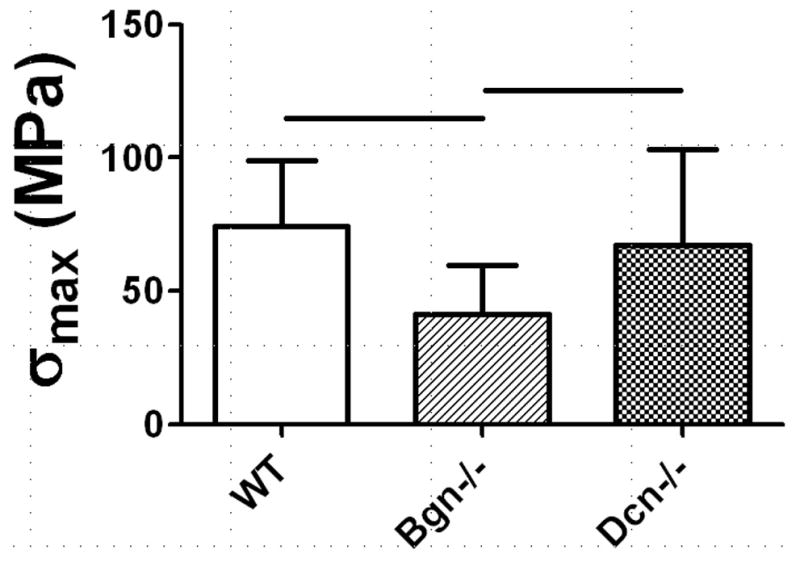
Maximum stress (σmax) for full-length FCU tendons stretched to failure at 0.1% strain/second in Study 2. σmax was reduced in Bgn−/− tendons relative to wild type and Bgn−/−.
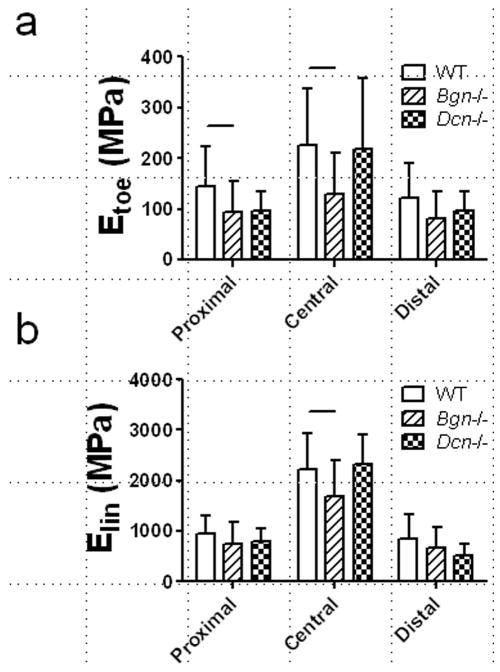
Genotype-related mechanical alterations in the full-length FCU tendon were regionally dependent (Figure 6). In particular, the toe-modulus Etoe was lower in Bgn−/− tendons than in wild type only in the proximal region and central regions (i.e., away from the tendon insertion). The linear modulus Elin was reduced in Bgn−/− tendons as compared to wild type only in the central region. Finally, while the principal angle in the central region was unaltered by genotype, the principal angle for full-length FCU tendons was generally high (~35°), indicative of substantial shear strain (Figure 7).
Figure 6.
(a) Optically-measured toe modulus (Etoe) and linear modulus (Elin) in each region of full-length FCU tendons stretched to failure at 0.1% strain/second in Study 2. Etoe was reduced in Bgn−/− tendons relative to wild type in the proximal and central regions, while Elinear was reduced in Bgn−/− tendons relative to wild type in just the central region.
Figure 7.
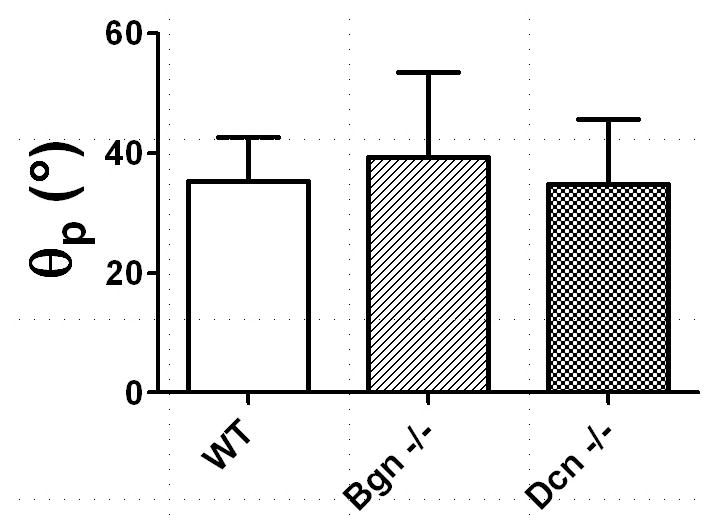
Angle of the principal axis (θp) with respect to vertical in the central region of full-length FCU tendons. No differences in θp were found among groups, but the nonzero values of θp suggest substantial induced shear strain in the tendon midsubstance.
DISCUSSION
In this study, the effects of CS/DS GAGs and the SLRPs biglycan and decorin on the mechanical properties of the murine FCU tendon were investigated for the first time. A novel approach combining CS/DS GAG digestion and knockout mouse models was used to isolate the impact of biglycan and decorin on tendon development from their possible role in force transmission in mature tissue. Although investigations using knockout models have helped to elucidate the developmental impact of SLRP deficiency, results from these studies are confounded by the effect that GAG-mediated fibril interactions may have on tendon mechanics after development is complete. However, by removing GAGs from the tendons of wild type and biglycan- and decorin-null mice, a direct comparison of the mechanical properties of the underlying collagen network in mouse tendons formed in the presence and absence of specific SLRPs was performed for the first time. It was hypothesized that the collagen network in the biglycan- and decorin-null mice would exhibit inferior mechanical properties as a result of improper fibril formation during development [2]. However, no differences were found among genotypes in FCU midsubstance specimens incubated in either PBS or ChABC, indicating that the tensile mechanics of the FCU tendon are unaffected by biglycan or decorin deficiency during development. Furthermore, ChABC digestion did not alter mechanical properties in tendons of all genotypes, a finding consistent with previous investigations of rat tail fascicles, the human MCL and the human patellar tendon [7–10].
In Study 2, as hypothesized, the mechanical properties of full-length Bgn−/− FCU tendons were found to be reduced in comparison with wild type and Dcn−/− tendons. The measured moduli (650 MPa<|E*|<850 MPa) and the impact of biglycan were consistent with a previous study investigating the mouse FDL tendon (400 MPa<E<1100 MPa) [5], which is likely to experience similar stresses and strains in vivo. However, the mechanical alterations in the FCU were surprisingly restricted primarily to the central portion, where high levels of biglycan were not expected [14]. This finding can be explained by the large principal angles measured in the tendon midsubstance that signify substantial vertical shear strain within the image plane (Figure 7). Due to shear deformation in the midsubstance, the measured “tensile” force used to calculate the modulus within each region of the tendon is not indicative of the tensile force in the central portion of the tendon, where a significant shear force is present. In particular, the measured force is given by the sum of the tensile and shear forces. Thus, an increase in the measured modulus could reflect either an increase in the tensile modulus or an increase in the shear modulus. Since Study 1 demonstrated that the tensile modulus in the FCU tendon midsubstance is not impacted by genotype and the analysis of the principal angle (Figure 7) indicates that the induced shear strain is not impacted by genotype, the decrease in the measured tensile modulus in the central region of Bgn−/− tendons for a given tensile strain (i.e., in the toe or linear region) is likely due to a decreased shear modulus G. This reduction of G in the midsubstance of Bgn−/− tendons could result from either decreased force transmission between collagen fibrils or altered fibril properties as a result of abnormal development.
The large principle angle of the FCU tendon (indicating that tensile deformation induces substantial shear strain) may be related to its in vivo function. Unlike the finger flexor tendons, the FCU tendon does not pass underneath the flexor retinaculum. Therefore, its motion is not constrained and it experiences significant shear deformation during wrist adduction. Interestingly, the maximum range of motion of a typical human hand in adduction is 40–60° from vertical, while the measured principle angle was 35° from horizontal (i.e., 55° from the vertical). Thus, although the range of motion for the mouse wrist could differ from the human wrist, the structure of the FCU tendon may be tuned to reflect the deformations that it experiences in vivo. This finding is clinically relevant, as the FCU tendon is often transferred to the radial wrist extensors to improve flexion and extension in patients with muscle spasticity and other neuromuscular disorders [23]. When performing these procedures, hand surgeons should consider the unique and specialized properties of the FCU tendon, which may limit its functionality after transfer.
The absence of biglycan-related effects at the tendon insertion may be related to the presence of aggrecan and type II collagen. Near the human FCU tendon-pisiform enthesis, high levels of these cartilage-associated molecules were found [15]. Unlike the SLRPs decorin and biglycan which possess 1 and 2 CS/DS GAG chains (respectively), aggrecan is a massive proteoglycan with over 100 CS/DS/KS GAG chains attached to each core protein. Thus, it is possible that the effects of biglycan near the bone may be minimal compared to those of the much larger molecule aggrecan. Additionally, biglycan interacts differently with type II collagen than with type I collagen [24], and the structural and mechanical implications of the absence of biglycan may differ in the FCU tendon-pisiform insertion due to its higher type II/type I collagen ratio.
The primary limitation in this study is that PG content and fibril properties/characteristics were not measured in the tested tendons due to their small size (thickness ~100 μm). Furthermore, determination of GAG content before and after ChABC incubation was performed only on WT tendons. However, these assays were not necessary to demonstrate that, as hypothesized, FCU tendon mechanics are impacted by SLRPs in a region-specific manner. In particular, our finding that biglycan impacts the mechanical properties of the FCU tendon midsubstance is based on comparisons using a well-established strain of Bgn−/− mice known to express negligible levels of biglycan in all tissue types [25]. Moreover, this finding strongly supports the assumption that biglycan expression in the FCU tendon is non-negligible and--taken together with the known ubiquity of decorin in tendon--suggests that the measured absence of mechanical changes following chondroitinase ABC digestion in the tendon midsubstance implies that CS/DS GAGs do not impact the mechanical properties of the FCU tendon collagen network, but that this finding cannot be interpreted more broadly. In addition, the large proteoglycan content of the human FCU tendon is known [15]. Therefore, a potential mechanism for the findings of the current study (i.e., increased aggrecan content near the insertion) was proposed based on previous work. Nevertheless, while a region-specific assessment of fibril diameter in the FCU tendon would be challenging due to its small size, such a future investigation could shed light on the mechanism for decreased shear modulus in the midsubstance of full-length, Bgn−/− FCU tendons.
A second limitation of this investigation is the variable number of specimens per group in Study 2 (11<n<31). At the time Study 2 was conducted, a disparate number of mice per genotype were available for use. However, the number of mice in each group is sufficiently large (n>10) to avoid any type II error based on power analysis. Moreover, randomly removing 20 specimens from the WT group and 12 specimens from the Bgn−/− group did not affect the reported results.
An attempt was made to separate the developmental effects of biglycan deficiency from its influence on mature tendon properties using the combined ChABC digestion/knock out mouse model approach, but effective removal of GAGs was not possible in full length FCU tendons with the pisiform insertion intact. Furthermore, 28% of GAGs remained after ChABC digestion of midsubstance specimens and GAG removal was less complete than in rat tail tendon fascicles digested using a similar protocol (~10% residual GAGs) [18], most likely reflecting the content of KS GAGs from aggrecan, lumican and fibromodulin molecules that are not hydrolyzed by ChABC. Nevertheless, the percentage of remaining GAGs in the current investigation was higher than studies of murine Achilles tendons (~50% residual GAGs) that were able to detect differences in mechanical properties after digestion [11]. Thus, while the focus of the current investigation is on CS/DS GAGs, if GAGs of any form were to play a role in FCU tendon mechanics, 72% removal of these molecules should be sufficient to induce measureable changes. A final limitation was that grip effects could influence mechanical data in the proximal and distal regions of the tendon. However, these effects should not impact the inter-group comparisons performed in this study.
This study demonstrates that that proteoglycans in tendon may play an important role in regulating multi-axial deformations. This finding is of particular interest in tendons like the FCU and supraspinatus tendon, which experience complex loading environments in vivo. The current investigation and future studies assessing the region-dependent mechanical properties of these tendons under simple shear and compression will be instructive for understanding the implications of altered PG content as the result of exercise, injury, aging or pathological changes [26–28].
CONCLUSIONS
The effects of CS/DS GAGs and the SLRPs biglycan and decorin on the location-dependent, viscoelastic mechanical properties of the FCU tendon were investigated using a novel combined GAG digestion and knockout mouse model. For all genotypes, ChABC digestion did not alter midsubstance tendon mechanics, suggesting that CS/DS GAGs by themselves have little effect on the mechanical properties of the FCU. However, mechanical properties of Bgn−/− FCU tendons were generally reduced in comparison with wild type and Dcn−/− tendons and these alterations were primarily restricted primarily to the midsubstance. Therefore, biglycan (but not decorin) impacts mechanical properties in the FCU tendon. The absence of biglycan-related effects at the tendon insertion may be related to the presence of aggrecan and type II collagen and suggests a unique role for PGs in tissue like the FCU that experience heterogeneous and multi-axial loads. These data provide guidelines for understanding how injury- or disease-related changes in PG content may impact tendon function.
Table 1. Summary of Results from Study 1.
Summary of data from Study 1. Cross-sectional area and viscoelastic mechanical parameters were not significantly different between genotypes or incubation conditions.
| Genotype | Incubation | A (mm2) | |E*| (MPa) | tanδ | σ max (MPa) |
|---|---|---|---|---|---|
| WT | PBS | 0.025 ± 0.0047 | (10 ± 2.3) × 102 | 0.047 ± 0.0089 | (8.2 ± 3.2) × 101 |
| ChABC | 0.023 ± 0.0041 | (9.5 ± 4.6) × 102 | 0.044 ± 0.0091 | (9.0 ± 3.4) × 101 | |
| Bgn−/− | PBS | 0.028 ± 0.0047 | (8.6 ± 2.3) × 102 | 0.045 ± 0.0092 | (10 ± 4.0) × 101 |
| ChABC | 0.027 ± 0.0070 | (10 ± 4.2) × 102 | 0.042 ± 0.0059 | (11 ± 6.1) × 101 | |
| Dcn−/− | PBC | 0.030 ± 0.0054 | (7.5 ± 2.5) × 102 | 0.051 ± 0.013 | (7.3 ± 3.8) × 101 |
| ChABC | 0.032 ± 0.010 | (8.0 ± 4.2) × 102 | 0.046 ± 0.0083 | (7.9 ± 3.2) × 101 |
Acknowledgments
The authors thank Dr. Joseph Sarver for helpful discussions.
Footnotes
DECLARATION OF INTEREST
The authors report no conflict of interest and are alone responsible for the content and writing of this paper. This study was supported by NIH/NIAMS.
References
- 1.Chen S, Birk DE. The regulatory roles of small leucine-rich proteoglycans in extracellular assembly. FEBS J. 2013 doi: 10.1111/febs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang G, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98(6):1436–49. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 3.Redaelli A, et al. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons--a computational study from molecular to microstructural level. J Biomech. 2003;36(10):1555–69. doi: 10.1016/s0021-9290(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 4.Cribb AM, Scott JE. Tendon response to tensile stress: an ultrastructural investigation of collagen:proteoglycan interactions in stressed tendon. J Anat. 1995;187(Pt 2):423–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson PS, et al. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J Biomech Eng. 2005;127(1):181–5. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- 6.Dourte LM, et al. Influence of Decorin on the Mechanical, Compositional, and Structural Properties of the Mouse Patellar Tendon. Journal of Biomechanical Engineering-Transactions of the Asme. 2012;134(3) doi: 10.1115/1.4006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lujan TJ, et al. Contribution of glycosaminoglycans to viscoelastic tensile behavior of human ligament. J Appl Physiol. 2009;106(2):423–31. doi: 10.1152/japplphysiol.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lujan TJ, et al. Effect of dermatan sulfate glycosaminoglycans on the quasi-static material properties of the human medial collateral ligament. J Orthop Res. 2007;25(7):894–903. doi: 10.1002/jor.20351. [DOI] [PubMed] [Google Scholar]
- 9.Fessel G, Snedeker JG. Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biology. 2009;28(8):503–510. doi: 10.1016/j.matbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Svensson RB, et al. Tensile force transmission in human patellar tendon fascicles is not mediated by glycosaminoglycans. Connect Tissue Res. 2011;52(5):415–21. doi: 10.3109/03008207.2010.551569. [DOI] [PubMed] [Google Scholar]
- 11.Rigozzi S, Muller R, Snedeker JG. Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. J Biomech. 2009;42(10):1547–52. doi: 10.1016/j.jbiomech.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Legerlotz K, Riley GP, Screen HR. GAG depletion increases the stress-relaxation response of tendon fascicles, but does not influence recovery. Acta Biomater. 2013;9(6):6860–6. doi: 10.1016/j.actbio.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connizzo BK, et al. Effect of Age and Proteoglycan Deficiency on Collagen Fiber Re-Alignment and Mechanical Properties in Mouse Supraspinatus Tendon. Journal of Biomechanical Engineering. 2012 doi: 10.1115/1.4023234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matuszewski PE, et al. Regional Variation in Human Supraspinatus Tendon Proteoglycans: Decorin, Biglycan, and Aggrecan. Connect Tissue Res. 2012 doi: 10.3109/03008207.2012.654866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamczyk C, et al. An immunohistochemical study of the extracellular matrix of entheses associated with the human pisiform bone. J Anat. 2008;212(5):645–53. doi: 10.1111/j.1469-7580.2008.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat. 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- 17.Milz S, et al. An immunohistochemical study of the triangular fibrocartilage complex of the wrist: regional variations in cartilage phenotype. Journal of Anatomy. 2007;211(1):1–7. doi: 10.1111/j.1469-7580.2007.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Screen HR, et al. The influence of noncollagenous matrix components on the micromechanical environment of tendon fascicles. Ann Biomed Eng. 2005;33(8):1090–9. doi: 10.1007/s10439-005-5777-9. [DOI] [PubMed] [Google Scholar]
- 19.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–8. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 20.Screen HR, et al. The influence of swelling and matrix degradation on the microstructural integrity of tendon. Acta Biomater. 2006;2(5):505–13. doi: 10.1016/j.actbio.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Andarawis-Puri N, Ricchetti ET, Soslowsky LJ. Rotator cuff tendon strain correlates with tear propagation. J Biomech. 2009;42(2):158–63. doi: 10.1016/j.jbiomech.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lake SP, et al. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. J Orthop Res. 2009;27(12):1596–602. doi: 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thometz JG, Tachdjian M. Long-term follow-up of the flexor carpi ulnaris transfer in spastic hemiplegic children. J Pediatr Orthop. 1988;8(4):407–12. doi: 10.1097/01241398-198807000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Vynios DH, et al. The interactions of cartilage proteoglycans with collagens are determined by their structures. Biochimie. 2001;83(9):899–906. doi: 10.1016/s0300-9084(01)01332-3. [DOI] [PubMed] [Google Scholar]
- 25.Young MF, et al. Biglycan knockout mice: new models for musculoskeletal diseases. Glycoconj J. 2002;19(4–5):257–62. doi: 10.1023/A:1025336114352. [DOI] [PubMed] [Google Scholar]
- 26.Dunkman AA, et al. Decorin Expression Is Important for Age-Related Changes in Tendon Structure and Mechanical Properties. Matrix Biol. 2012 doi: 10.1016/j.matbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinemeier KM, et al. Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise. Scand J Med Sci Sports. 2011 doi: 10.1111/j.1600-0838.2011.01414.x. [DOI] [PubMed] [Google Scholar]
- 28.Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact. 2005;5(1):22–34. [PubMed] [Google Scholar]