Abstract
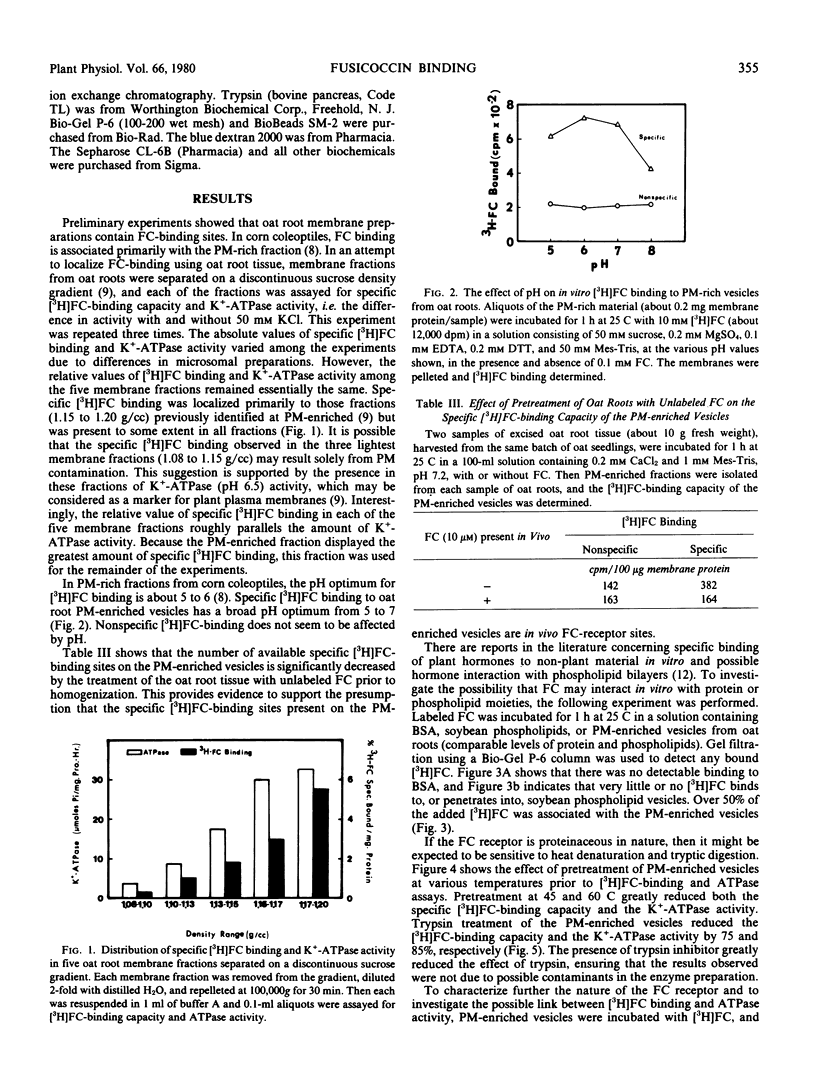
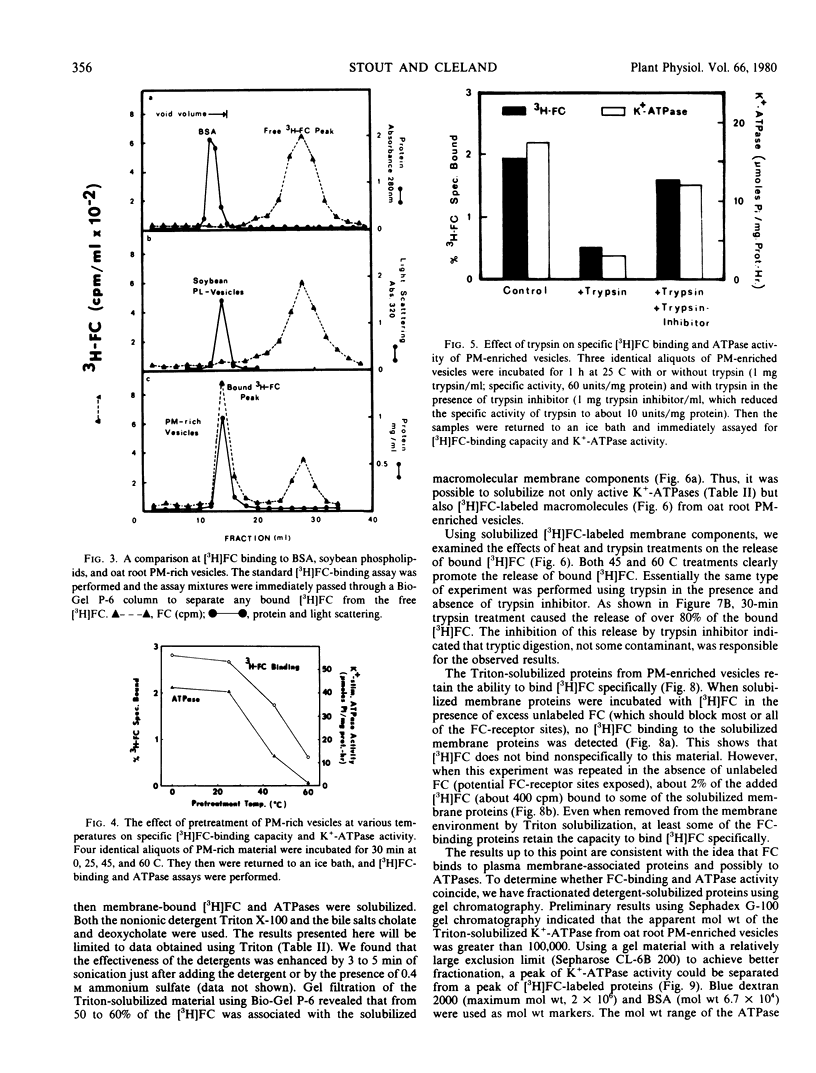
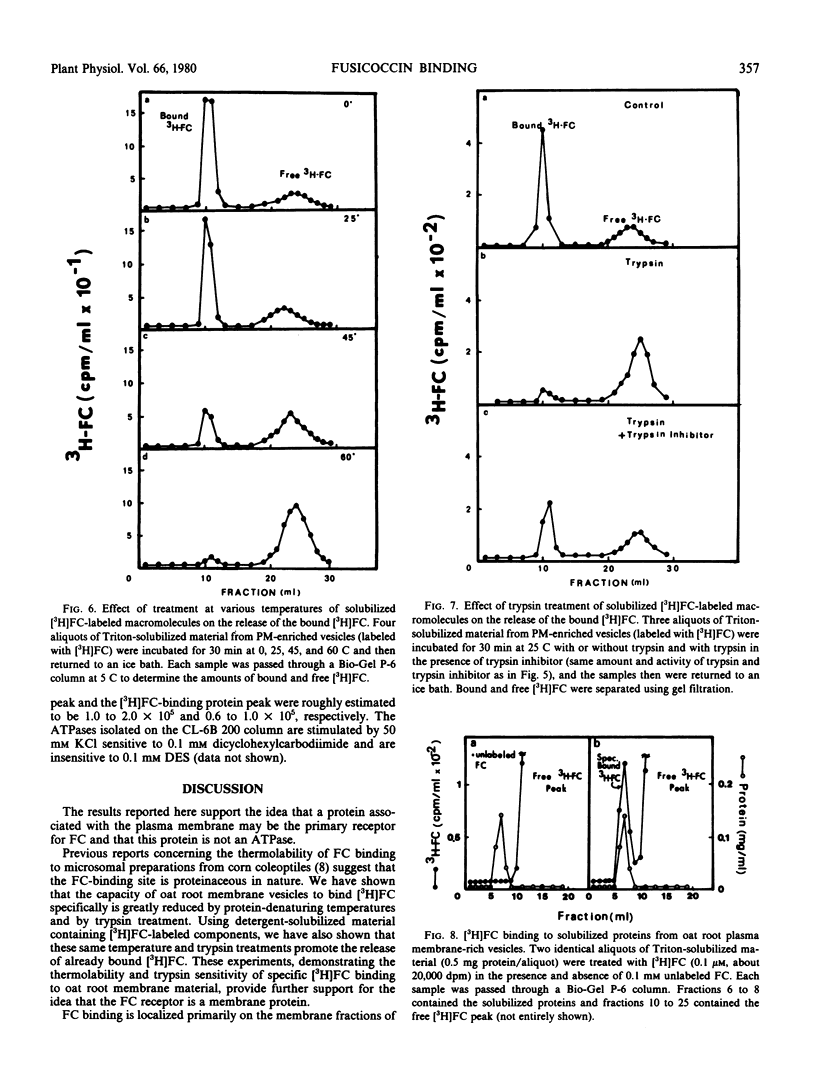
The possibility that fusicoccin (FC) binds to plasma membrane-associated ATPases of oat (cv. Victory) roots has been examined. Specific FC-binding in vitro is localized primarily on plasma membrane-enriched fractions. This FC-binding is greatly reduced by pretreatment of the membrane vesicles at temperatures above 45 C or with trypsin, and the same treatments cause the release of already bound FC. These results support the idea that the FC receptor is a protein located on the plasma membrane.
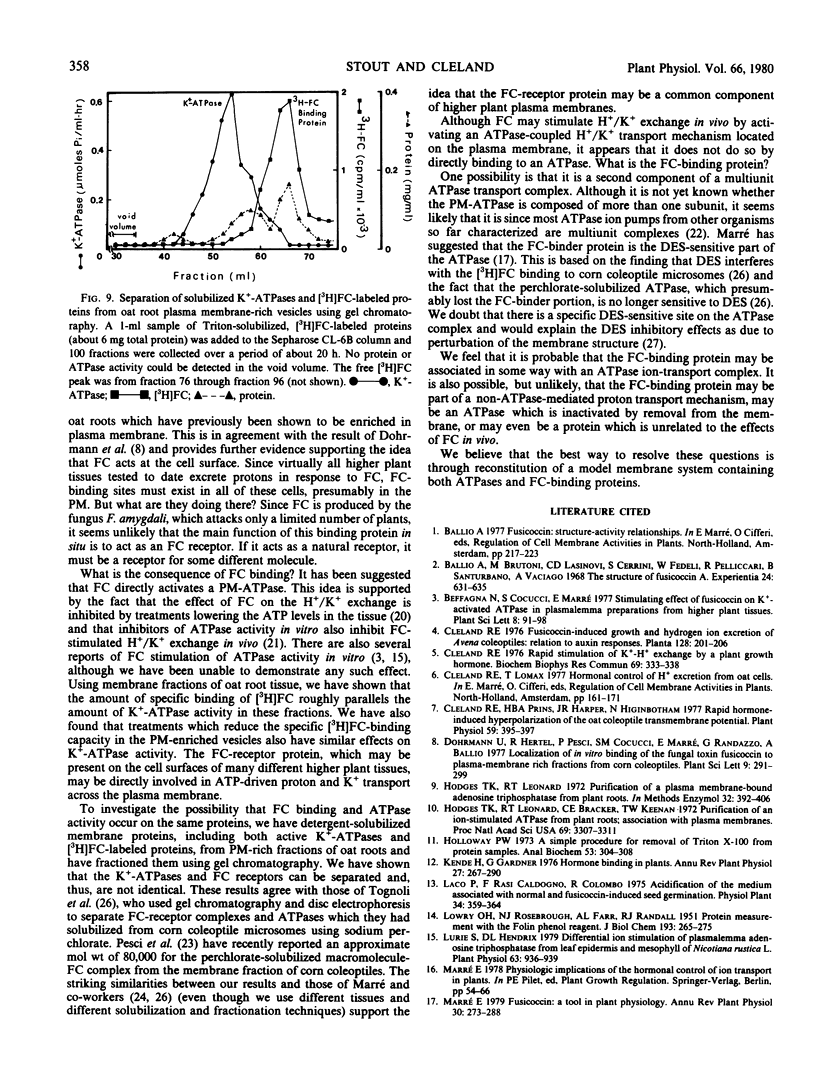
Both active ATPases and FC-binding proteins were solubilized using 1% Triton X-100. When this material was fractionated using gel chromatography, the ATPase activity could be separated from the FC-binding proteins. The identity of the FC-binding proteins is discussed with regard to the extensive evidence which supports the involvement of plasma membrane-ATPase H+/K+ pumps in FC-stimulated acidification and K+ uptake.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballio A., Brufani M., Casinovi C. G., Cerrini S., Fedeli W., Pellicciari R., Santurbano B., Vaciago A. The structure of fusicoccin A. Experientia. 1968 Jun 15;24(6):631–635. doi: 10.1007/BF02153818. [DOI] [PubMed] [Google Scholar]
- Cleland R. E., Prins H. B., Harper J. R., Higinbotham N. Rapid Hormone-induced Hyperpolarization of the Oat Coleoptile Transmembrane Potential. Plant Physiol. 1977 Mar;59(3):395–397. doi: 10.1104/pp.59.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E. Rapid stimulation of K -H exchange by a plant growth hormone. Biochem Biophys Res Commun. 1976 Mar 22;69(2):333–338. doi: 10.1016/0006-291x(76)90526-x. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lurie S. Differential Ion Stimulation of Plasmalemma Adenosine Triphosphatase from Leaf Epidermis and Mesophyll of Nicotiana rustica L. Plant Physiol. 1979 May;63(5):936–939. doi: 10.1104/pp.63.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal M. Experimental membranes and mechanisms of bioenergy transductions. Annu Rev Biophys Bioeng. 1976;5:119–175. doi: 10.1146/annurev.bb.05.060176.001003. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Collins T., Evers A., Dunham P. Membrane perturbation: studies employing a calcium-sensitive dye, arsenazo III, in liposomes. Proc Natl Acad Sci U S A. 1976 Feb;73(2):510–514. doi: 10.1073/pnas.73.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]


