Abstract
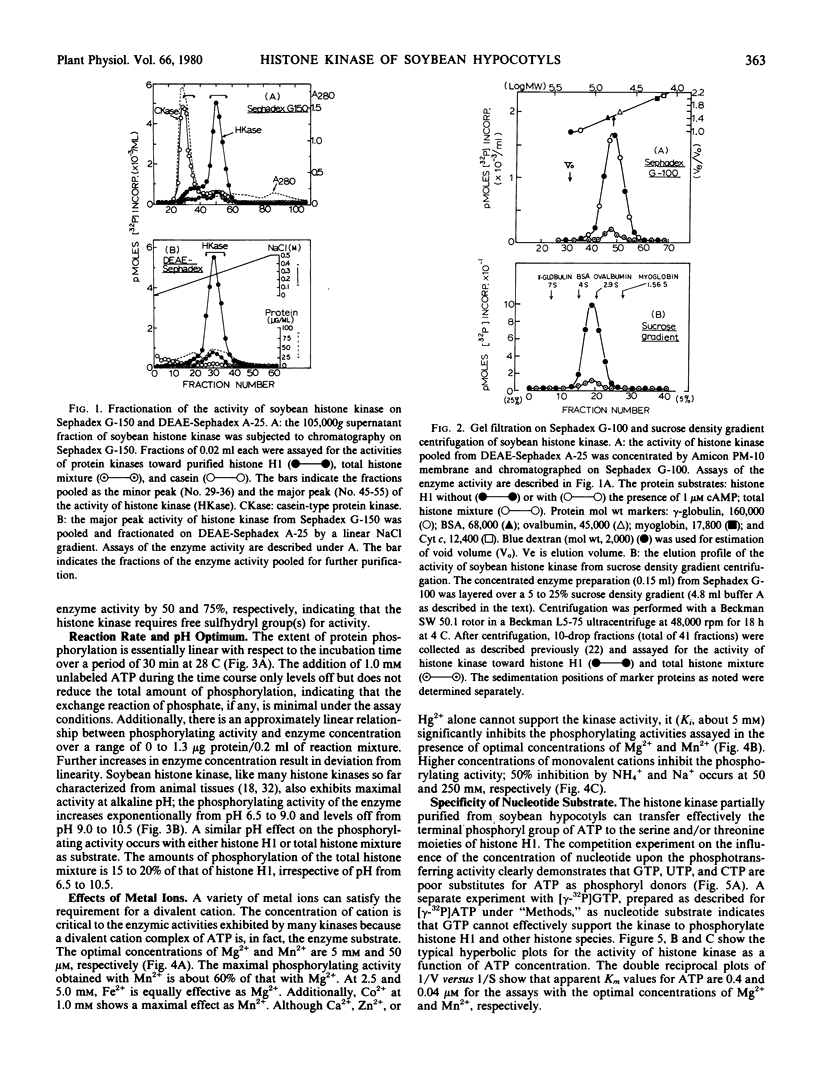
A histone-type protein kinase (EC 2.7.1.37) has been partially purified (320-fold) from the crude extracts of soybean hypocotyls by means of a combination of gel filtration and anion exchange procedures. The purified enzyme fraction is devoid of the activities of phosphoprotein phosphatase (EC 3.1.3.16), histone protease, and casein (or phosvitin)-type kinase. The soybean histone kinase uses ATP to phosphorylate specifically lysine-rich histone H1 from either pea seedlings or calf thymus.
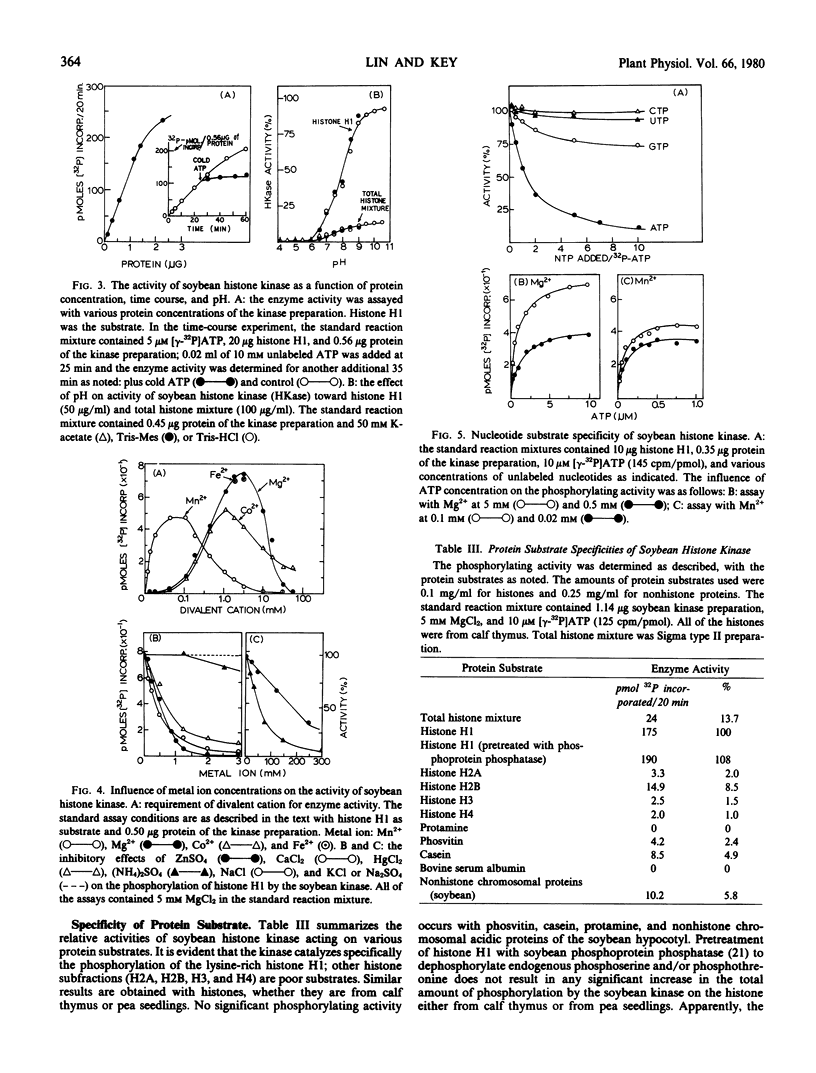
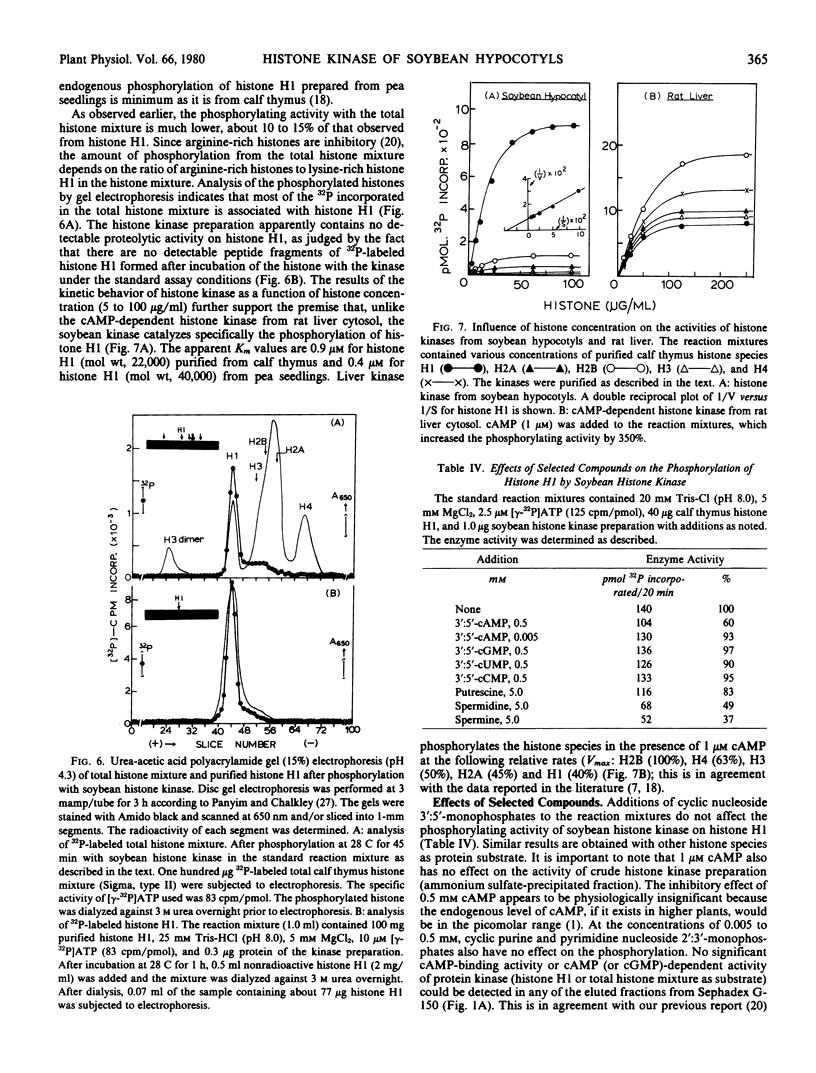
The histone kinase requires free sulfhydryl group(s) for activity, but not stability. The pH optimum is around 9 to 10. The apparent Km values for histone H1 of pea seedlings and calf thymus are 0.4 and 0.9 micromolar, respectively. The Km values for ATP are 40 nanomolar with the optimal concentration of Mn2+ (50 nanomolar) and 0.4 micromolar with that of Mg2+ (5 millimolar). The estimated molecular weight of the kinase is 52,000 by gel filtration or 48,600 by sedimentation constant (3.2 S). cAMP does not alter the sedimentation velocity of the kinase. The enzyme activity is unaffected by cyclic nucleoside monophosphates and plant growth substances. Like arginine-rich histones, a variety of divalent cations and polycations (polyamines) are inhibitory.
This cAMP-independent soybean histone kinase is not associated with the isolated ribosomes but shows highest specific activity in the nuclearchromatin fraction, suggesting that it may function in the regulation of histone H1 phosphorylation in the soybean hypocotyl.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Béliveau R., Bellemare G. Light-dependent phosphorylation of thylakoid membrane polypeptides. Biochem Biophys Res Commun. 1979 Jun 13;88(3):797–803. doi: 10.1016/0006-291x(79)91478-5. [DOI] [PubMed] [Google Scholar]
- Chapman K. S., Trewavas A., van Loon L. C. Regulation of the Phosphorylation of Chromatin-associated Proteins in Lemna and Hordeum. Plant Physiol. 1975 Feb;55(2):293–296. doi: 10.1104/pp.55.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Smith D. L., Bruegger B. B., Halpern R. M., Smith R. A. Occurrence and distribution of acid-labile histone phosphates in regenerating rat liver. Biochemistry. 1974 Aug 27;13(18):3785–3789. doi: 10.1021/bi00715a026. [DOI] [PubMed] [Google Scholar]
- Chen L. J., Walsh D. A. Multiple forms of hepatic adenosine 3':5'-monophosphate dependent protein kinase. Biochemistry. 1971 Sep 14;10(19):3614–3621. doi: 10.1021/bi00795a020. [DOI] [PubMed] [Google Scholar]
- Dolby T. N., Ajiro K., Borun T. W., Gilmour R. S., Zweidler A., Cohen L., Miller P., Nieolini C. Physical properties of DNA and chromatin isolated from G1- and S-phase HeLa S-3 cells. Effects of histone H1 phosphorylation and stage-specific nonhistone chromosomal proteins on the molar ellipticity of native and reconstituted nucleoproteins during thermal denaturation. Biochemistry. 1979 Apr 3;18(7):1333–1344. doi: 10.1021/bi00574a033. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Kemp B. E., Krebs E. G. Phosphorylation of hydroxyproline in a synthetic peptide catalyzed by cyclic AMP-dependent protein kinase. J Biol Chem. 1979 Aug 10;254(15):6987–6990. [PubMed] [Google Scholar]
- Garrard W. T., Bonner J. Changes in chromatin proteins during liver regeneration. J Biol Chem. 1974 Sep 10;249(17):5570–5579. [PubMed] [Google Scholar]
- Jackson V., Shires A., Chalkley R., Granner D. K. Studies on highly metabolically active acetylation and phosphorylation of histones. J Biol Chem. 1975 Jul 10;250(13):4856–4863. [PubMed] [Google Scholar]
- Keates R. A. Cyclic nucleotide-independent protein kinase from pea shoots. Biochem Biophys Res Commun. 1973 Sep 18;54(2):655–661. doi: 10.1016/0006-291x(73)91473-3. [DOI] [PubMed] [Google Scholar]
- Keates R. A., Trewavas A. J. Protein kinase activity associated with isolated ribosomes from peas and lemna. Plant Physiol. 1974 Jul;54(1):95–99. doi: 10.1104/pp.54.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan T. A. Protein kinases and protein kinase substrates. Adv Cyclic Nucleotide Res. 1973;3:99–153. [PubMed] [Google Scholar]
- Lin P. P. Cyclic nucleotides in higher plants? Adv Cyclic Nucleotide Res. 1974;4(0):439–460. [PubMed] [Google Scholar]
- Lin P. P., Key J. L. Lysine-rich histone H1 kinase from soybean hypocotyl. Biochem Biophys Res Commun. 1976 Nov 22;73(2):396–403. doi: 10.1016/0006-291x(76)90721-x. [DOI] [PubMed] [Google Scholar]
- Lin P. P. Phosphoprotein Phosphatase of Soybean Hypocotyls: PURIFICATION, PROPERTIES, AND SUBSTRATE SPECIFICITIES . Plant Physiol. 1980 Sep;66(3):368–374. doi: 10.1104/pp.66.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. P., Wilson R. F., Bonner J. Isolation and properties of nonhistone chromosomal proteins from pea chromatin. Mol Cell Biochem. 1973 Jun 27;1(2):197–207. doi: 10.1007/BF01659330. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Murray M. G., Guilfoyle T. J., Key J. L. Isolation and Characterization of a Chromatin-associated Protein Kinase from Soybean. Plant Physiol. 1978 Jun;61(6):1023–1030. doi: 10.1104/pp.61.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Key J. L. 2,4-Dichlorophenoxyacetic Acid-enhanced Phosphorylation of Soybean Nuclear Proteins. Plant Physiol. 1978 Feb;61(2):190–198. doi: 10.1104/pp.61.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Pochron S. F., Baserga R. Histone H1 phosphorylation in cell cycle-specific temperature-sensitive mutants of mammalian cells. J Biol Chem. 1979 Jul 25;254(14):6352–6356. [PubMed] [Google Scholar]
- Ralph R. K., McCombs P. J., Tener G., Wojcik S. J. Evidence for modification of protein phosphorylation by cytokinins. Biochem J. 1972 Dec;130(4):901–911. doi: 10.1042/bj1300901a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D. D., Rubin P. M. Plant Pyruvate Dehydrogenase Complex: II. ATP-Dependent Inactivation and Phosphorylation. Plant Physiol. 1977 Jan;59(1):1–3. doi: 10.1104/pp.59.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: additional initiation factor required for formation of ternary complex (eIF-2.GTP.Met-tRNAf) and demonstration of inhibitory effect of heme-regulated protein kinase. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1079–1083. doi: 10.1073/pnas.76.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Schendel P. F., Wells R. D. The synthesis and purification of (gamma-32P)-adenosine triphosphate with high specific activity. J Biol Chem. 1973 Dec 10;248(23):8319–8321. [PubMed] [Google Scholar]
- Sierra J. M., de Haro C., Datta A., Ochoa S. Translational control by protein kinase in Artemia salina and wheat germ. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4356–4359. doi: 10.1073/pnas.74.10.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. L., Chen C. C., Bruegger B. B., Holtz S. L., Halpern R. M., Smith R. A. Characterization of protein kinases forming acid-labile histone phosphates in Walker-256 carcinosarcoma cell nuclei. Biochemistry. 1974 Aug 27;13(18):3780–3785. doi: 10.1021/bi00715a025. [DOI] [PubMed] [Google Scholar]
- Trewavas A. The Phosphorylation of Ribosomal Protein in Lemna minor. Plant Physiol. 1973 Apr;51(4):760–767. doi: 10.1104/pp.51.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon L. C., Trewavas A., Chapman K. S. Phosphorylation of Chromatin-associated Proteins in Lemna and Hordeum. Plant Physiol. 1975 Feb;55(2):288–292. doi: 10.1104/pp.55.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]


