Abstract
Introduction
Previous studies suggest that indicators of central adiposity such as waist-to-hip ratio (WHR) and waist circumference may be altered by HIV infection, antiretroviral (ARV) treatment or both.
Methods
Waist and hip circumference and body mass index (BMI) were measured among participants of the Women’s Interagency HIV Study (WIHS) semiannually from 1999 to 2004. Generalized linear models evaluated longitudinal patterns of these measures and associations with demographic and clinical characteristics.
Results
WHR was significantly larger while BMI, waist and hip circumference were significantly smaller at almost all eleven semiannual visits among 942 HIV-infected compared to 266 HIV-uninfected women. Among HIV-uninfected women, mean waist and hip circumference and BMI increased over the 5 year study period (waist: +4.1 cm or 4.4%, hip: +3.76 cm or 3.5% and BMI +2.43 kg/m2 or 8.2%), while WHR remained stable. Among the HIV-infected women, waist and hip circumference, BMI and WHR did not significantly change.
Independent predictors of smaller BMI among HIV-infected women included White race, HCV seropositivity, current smoking, higher viral load and lower CD4. Independent predictors of larger WHR among HIV-infected women included age, White and Other non-African-American race, higher CD4 and PI use. Use of a HAART regimen was not an independent predictor of either BMI or WHR..
Conclusions
HIV-infected women had higher WHR compared to HIV-uninfected women, despite lower BMI, waist and hip measurements. BMI, waist and hip circumference increased over 5 years among the HIV-uninfected women, but remained stable in the HIV-infected women. Among HIV-infected women, PI use was associated with larger WHR, although HAART use itself was not appreciably associated with either BMI or WHR.
Keywords: anthropometrics, HIV, women, waist-to-hip ratio
Introduction
The impact of highly active antiretroviral therapy (HAART) on HIV morbidity and mortality remains highly favorable a decade since its introduction in resource-rich1,2 and more recently in resource-limited settings.3 A variety of metabolic side-effects associated with HAART, including body fat changes, have been described 4,5 and continue to be a concern. Most publications about the impact of HIV and HAART on body fat changes have focused on men and initially suggested a mixed process of fat accumulation and fat loss.6 More recent studies suggest that fat loss predominates.7–9 However, some have suggested that in the US, an epidemic of obesity among HIV-infected persons has become more common than wasting,10 paralleling the rise in obesity in the general US population. 11
A recent study of patterns of body circumference measurements over 4 years among HIV-infected and –uninfected men found that waist and hip circumference increased in all men, regardless of HIV status or HAART use. Among HIV-infected men, WHR increased most in the HAART-treated group due to more rapid increases in waist than hip circumference.12
Both waist circumference and waist-to-hip ratio (WHR) are important markers of central adiposity and predictors of coronary heart disease in the general population, especially among women.13,14 As women comprise a growing proportion of HIV-infected individuals worldwide,15 it becomes increasingly important to understand the impact of HIV and HAART use on body fat distribution and cardiovascular risk among women. The objectives of this study therefore are to describe the longitudinal patterns of body mass index (BMI), WHR and waist and hip circumferences over time in a cohort of HIV-infected and –uninfected women, and to relate these patterns to demographic and clinical characteristics.
Methods
Study Population
The Women’s Interagency HIV Study (WIHS) is a prospective, multicenter cohort study established in 1994 to investigate health changes in women with and at risk for HIV infection. Of the original 2,625 participants enrolled at six sites (Bronx, Brooklyn, Chicago, Los Angeles, San Francisco and Washington, DC) between October 1994 and November 1995, 2056 were HIV-infected and 569 were HIV-uninfected. Participants were recruited from community outreach and hospital-based programs and are evaluated every six months by standardized interviews and physical examination, as described elsewhere.16,17 Phlebotomy for the determination of CD4 cell count, plasma HIV-1 RNA, HIV antibody for those previously testing HIV seronegative, and other laboratory parameters is performed. HCV antibody was determined at enrollment. Information about the use of all medications, including antiretroviral agents, is recorded at each study visit. Medications are prescribed by each participant’s primary care provider and not the study. The institutional review board for each participating site approved the WIHS study protocol.
Anthropometric measurements
WIHS participants underwent anthropometric measurements, standardized according to the Third National Health and Examination Survey (NHANES III) procedures18 at eleven semiannual study visits (WIHS study visits 10 – 20, referred to here as V10-V20) during the 5 year study period (April/October 1999 – April/October 2004). The measurements used in this analysis, performed in duplicate, include height, weight, and waist and hip circumference. Participants wore lightweight clothing and removed shoes and heavy objects prior to being weighed. Each examiner completed a standardized training and certification program prior to the collection of anthropometric data and was recertified every two years. One exercise physiologist conducted training and certification of all examiners at each site. The upper and lower 1% of the anthropometric data points were reviewed by at least two investigators (JJ, SG, and/or KA) and those observations likely to be a result of error were set to missing. This did not reduce the number of participants in the study group.
Exclusion criteria
Of the 2625 women in the original WIHS cohort, 452 died and 351 withdrew from the study at or before visit 9, leaving 1822 participants available for anthropometric measurements at visit 10. Of these, 15 who had seroconverted to HIV were excluded and 434 women were excluded because of pregnancy or use of prednisone (topical and inhaled corticosteroids use excepted), megesterol, oxandrolone, DHEA or nandrolone during WIHS visits V8-V20, leaving 1373 potential participants. Of these 49 died and 116 withdrew from the study after visit 10 without having complete data on weight, height, waist and hip circumferences from at least 2 unique visits during the study period of V10-V20. The remaining study group of 1208 women included 266 HIV uninfected and 942 HIV-infected.
This final study population contributed a total of 8,745 person-visits between visits 10 to 20. For each subject, the index visit was defined as the participant’s first visit with complete weight, height, waist and hip circumference data during the V10-V20 study period. The index visit occurred at V10 for 879 participants (73%) and at V11 for 156 (13%). The last visit was defined as the participant’s latest visit with complete data during the V10-V20 study period.
Antiretroviral (ARV) therapy use was categorized at each study visit. Regimens that met the International AIDS Society and the DHHS/Kaiser19 definition of HAART were classified as HAART. Use of non-HAART regimens were classified as either monotherapy or combination therapy. In analyses, we classified ARV therapy as: no therapy (No ART), monotherapy or combination therapy, and HAART. Use of non-nucleoside reverse transcript inhibitor (NNRTI) agents and protease inhibitor (PI) agents were also categorized as per the DHHS/Kaiser guidelines.19
Race was self-categorized as African-American (Hispanic and non-Hispanic), White (Hispanic and non-Hispanic) or Other (categories other than African-American or White). Ethnicity was categorized as Hispanic or Other. HIV-infected individuals were also analyzed according to CD4 cell counts and HIV RNA levels. Viral loads <400 c/ml were defined as equal to 400 c/ml before taking the log; a sensitivity analysis demonstrated similar results using 200 and 400 c/ml.
Statistical Analysis
The main outcomes for this study were body mass index (BMI) and waist-to-hip ratio (WHR). Categorical characteristics were expressed as percentages within HIV serostatus groups and compared in cross-sectional visit univariate analyses using Pearson’s chi-square test. Analysis of variance methods or t-tests were used to test the equality of means of continuous measures in cross-sectional visits across HIV serostatus groups.
Generalized linear models evaluated longitudinal changes in BMI and WHR over time and associations with covariates across all visits. The following co-variates are treated as time-dependent variables: age, calendar time, current smoker, years of smoking, CD4 count, Log HIV RNA, ARV Therapy, WHR and BMI. Robust covariance methods were used to handle repeated measures from the same individual. Analyses were conducted using SAS software (Version 8, SAS Institute, Cary, NC). In general, separate analyses were performed for HIV-infected and HIV-uninfected women.
Results
Demographic and clinical characteristics at the index visit
The study group consisted of 942 HIV-infected women and 266 HIV-uninfected women. Age, race, ethnicity, education and smoking history were similar in the two serogroups (Table 1). The race category of Other was almost entirely Hispanic women who do not categorize themselves as African-American or White. Women in both groups had a similar mean number of semiannual visits during the study period (7.2 visits). The mean follow-up time in years between index and last visit was somewhat greater for the HIV-infected women (4.2 vs 4.0 y, p = 0.033). Mean BMI was higher among the HIV-uninfected than –infected women (30.0 vs 28.0 kg/m2, p = 0.0003) but the mean BMI for both groups was in the overweight range or higher. Waist and hip circumferences at the index visit were significantly smaller among the HIV-infected women while WHR was significantly larger. There was no significant difference in the prevalence of menopause between the two groups but estrogen use, such as hormonal contraception, was more common among the HIV-infected vs –uninfected women (14% versus 9 %, p = 0.047).
Table 1.
Characteristics of Study Group at Index Visit
| HIV− N = 266 |
HIV+ N = 942 |
P value a | |
|---|---|---|---|
| Mean Age (years), [range] | 40.7, [23–66] | 41.6, [21–79] | 0.10 |
| Race† (%) | |||
| African-American | 62 | 59 | 0.33* |
| Other | 26 | 25 | |
| White | 12 | 16 | |
| Hispanic Ethnicity (%) | 25 | 26 | 0.81* |
| Number of study visits per participant | |||
| Mean [range] | 7.2 [2–11] | 7.2 [2–11] | 0.99 |
| Follow-up time, mean (years) | 4.0 | 4.2 | 0.033 |
| BMI, kg/m2 | |||
| Mean | 30.0 | 28.0 | 0.0003 |
| IQR | 23.8 – 35.3 | 23.3 – 31.1 | |
| Median | 28.7 | 26.6 | |
| Height (cm), mean [range] | 161.8, [139.7–175.3] | 160.9, [132.1–185.4] | 0.036 |
| Waist circumference (cm), mean [range] | 93.7, [62.0–172.0] | 91.1, [60.8–151.0] | 0.0341 |
| Hip Circumference (cm), mean, [range] | 106.3, [79.1–169.0] | 101.3, [73.6–155.4] | <0.0001 |
| WHR, mean, [range] | 0.877, [0.724–1.121] | 0.899, [0.682–1.164] | <0.001 |
| Education (%) | |||
| < High School | 33 | 38 | 0.37* |
| High School | 34 | 31 | |
| > High School | 33 | 31 | |
| Years of Smoking, mean | 15.9 | 14.9 | 0.26 |
| Current smoking (%) | 59 | 53 | 0.14* |
| Menopause (n, %) | 46 (17) | 209 (22) | 0.09 |
| Any Estrogen use, V8-V20 (%) | 9 | 14 | 0.047 |
| HCV Antibody + (n,%) | 78 (30) | 392 (42) | 0.0004* |
| Illicit drug use (n, %) | |||
| Opiate use in past 6 months | 41(15) | 123 (13) | 0.31 |
| Any opiate use | 76 (29) | 228 (24) | 0.15 |
| Cocaine use in past 6 months | 14 (5) | 32 (3) | 0.20 |
| Any cocaine use | 86 (32) | 235 (25) | 0.02 |
| CD4 cells/mm3, mean [IQR] | 1057 [781–1266] | 421 [234–569] | <0.0001 |
| Nadir CD4 < 50 at/before index visit | 116 (12) | ||
| Log HIV PCR, mean [IQR] | 3.2 [1.9 – 4.2] | ||
| ARV use (n, %) | |||
| No ARV use ≥ 1 year before Index visit | 191 (20) | ||
| HAART use at Index visit | 450 (48) | ||
| PI use at Index visit ‡ | 372 (40) | ||
| NNRTI use at Index visit ‡ | 194 (21) | ||
P values are Satterthwaite unless otherwise indicated. Boldface indicates p value <0.05.
Fisher exact test
Chi-square
Race was self-categorized as African-American (Hispanic and non-Hispanic), White (Hispanic and non-Hispanic) or Other (all other races other than African-American or White)
PI and NNRTI use include both HAART and non-HAART regimens
As expected, HCV infection was more common in the HIV-infected group (42% versus 30%, p = 0.0004). Of the 370 HCV-Ab positive participants with HCV viral load data available, 370 (100%) had detectable HCV levels. Illicit drug use prevalence rates were similar between HIV-infected and –uninfected women. A history of any cocaine use, however, was less prevalent among the HIV-infected women than –uninfected: 25% versus 32% (p = 0.02).
Among the HIV-infected women, the mean CD4 count at the index visit was 421 cells/ml, and the mean log viral load was 3.2. Twelve percent had a nadir CD4 < 50 cells/ml measured during a WIHS visit at or before their index visit. Twenty percent had not used any ARV therapy for at least one year prior to the index visit and 48% were using HAART at the index visit. At the index visit, 40% of the HIV-infected participants were using PI-based regimens and 20% were using NNRTI-based regimens, including both HAART and non-HAART regimens.
Mean anthropometric values over time
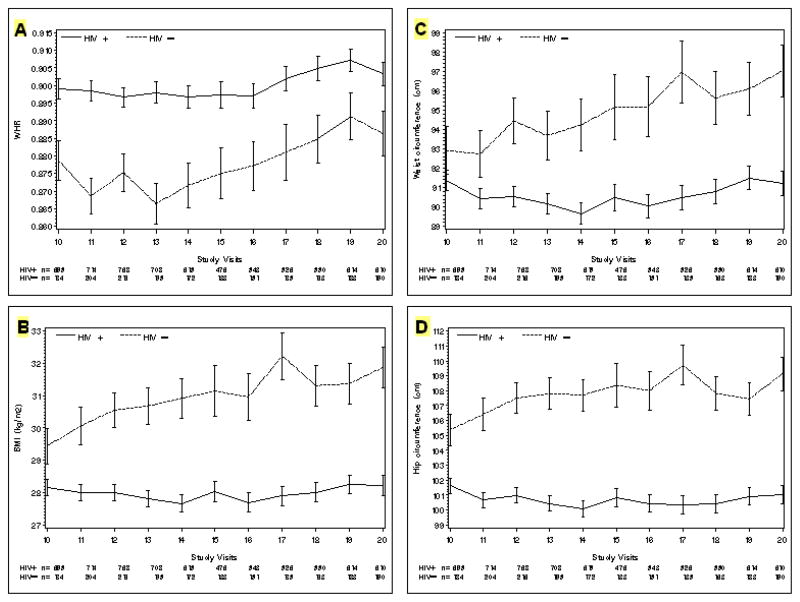
Figure 1 shows patterns of mean BMI, waist and hip circumference, and WHR by HIV status during the five year study period. The sample sizes varied from visit to visit by approximately 20% for each HIV serogroup, with the peak at V12 and the minimum at V17 for both serogroups. At V10, the beginning of the study period, the mean BMI was significantly smaller in the HIV-infected women compared to the –uninfected women (28.1 kg vs. 29.4 kg, p = 0.032), yet mean WHR was larger in the HIV-infected women compared to the –uninfected women (0.90 vs 0.88, p = 0.0014).
Figure 1.
Mean [+/− standard error] at each study visit of the following anthropometric measures:
A: Waist-to-Hip ratio
B: Body Mass Index (kg/m2)
C: Waist circumference (cm)
D: Hip circumference (cm)
During the study period, the mean BMI of HIV-infected women remained significantly smaller than the mean BMI of HIV-uninfected women at every study visit, with differences ranging from −1.29 kg/m2 to −4.33 kg/m2 (pooled p <0.024 or smaller). Mean BMI of the HIV-infected women did not significantly increase over time (28.15 kg/m2 at V10 versus 28.22 kg/m2 at V20, p = 0.10). In contrast, mean BMI of the HIV-uninfected women increased over time by a total of 2.43 kg or 8.2% (29.44 kg/m2 at V10 to 31.87 kg/m2 at V20, p <0.0001). For the subset of women who had both an index visit at V10 and a last visit at V20 (HIV-infected, n = 469; HIV-uninfected, n = 136), the change in mean BMI over 5 years was very small for the HIV-infected women, while the HIV-uninfected women had a clear increase (HIV+: −0.05 kg/m2 vs HIV-: + 0.44 kg/m2, p <0.0001).
WHR was significantly larger among the HIV-infected women at all study visits, compared to the HIV-uninfected women (differences ranged from +0.016 to +0.032, p = 0.024 or less), despite smaller mean BMI values. Among women with complete data at both V10 and V20, the mean change in V20-V10 WHR was not significant for either serogroup.
We analyzed mean waist and hip circumferences separately to see which measure might explain WHR patterns. Mean waist circumference was similar in both serogroups at the beginning of the study period (HIV− vs HIV+: 92.9 vs 91.4 cm, p = 0.26) while hip circumference was significantly smaller among the HIV-infected women (HIV− vs HIV+: 105.4 vs 101.6 cm, p =0.002). Over time waist and hip circumferences each remained stable among HIV-infected women. In contrast, consistent increases over time occurred among the HIV-uninfected women for both waist (+4.1 cm or 4.4% increase) and hip circumference (+3.76 cm or 3.5% increase).
Predictors of BMI and WHR
Robust covariance univariate analysis of demographic, clinical and anthropometric data from all of the participants’ study visits (up to 6,819 observations among the HIV-infected women and 1,926 observations among the HIV-uninfected women) allowed us to estimate the effect of demographic and clinical variables on BMI and WHR (univariate data not shown). We then fit a robust covariance linear regression model (see Table 2A and B) for each HIV serostatus group, including all the variables analyzed in the univariate model. We excluded BMI from the final WHR models and WHR from the final BMI models because of the high degree of correlation between these two variables. NNRTI use had a minimal unadjusted association with WHR and was excluded from the WHR model due to co-linearity problems of having 3 measures of ARV use in the model. PI use had a minimal unadjusted association with BMI and was similarly excluded from the final BMI model.
Table 2.
| Table 2A: Multivariate Analysis of BMI Predictors, Excluding WHR | ||||||
|---|---|---|---|---|---|---|
| HIV + Women | HIV− Women | |||||
| Predictor | Estimate (kg/m2) | Standard error | p value | Estimate (kg/m2) | Standard error | p value |
| Age (per decade) | −0.20 | 0.26 | ns* | −0.16 | 0.73 | ns |
| Calendar time (y) | −0.032 | 0.019 | 0.09 | 0.20 | 0.039 | <.0001 |
| Race | ||||||
| White | −2.64 | 0.60 | <.0001 | −0.91 | 1.60 | ns |
| Other | −0.72 | 0.52 | ns | −0.047 | 1.22 | ns |
| African-American (ref) | ||||||
| Education | ||||||
| >High School | −0.39 | 0.53 | ns | −0.32 | 1.32 | ns |
| High School | 0.017 | 0.52 | ns | 1.30 | 1.26 | ns |
| <High School (ref) | ||||||
| HCV Antibody | ||||||
| positive | −0.98 | 0.48 | 0.041 | 0.68 | 1.22 | ns |
| negative (ref) | ||||||
| Current Smoker | ||||||
| Yes | −0.38 | 0.11 | 0.0006 | −1.12 | 0.34 | 0.0018 |
| No (ref.) | ||||||
| Year of Smoking (per 10 years) | −0.35 | 0.19 | 0.070 | −0.25 | 0.44 | ns |
| CD4 count (per 100 cells/mm3 increase) | 0.096 | 0.016 | <.0001 | |||
| Log HIV RNA PCR | −0.095 | 0.041 | 0.020 | |||
| ARV Therapy | ||||||
| HAART | 0.086 | 0.084 | ns | |||
| No Therapy (ref) | ||||||
| NNRTI use | −0.060 | 0.080 | ns | |||
| Table 2B: Multivariate Analysis of WHR Predictors, Excluding BMI | ||||||
|---|---|---|---|---|---|---|
| HIV + Women | HIV− Women | |||||
| Predictor | Estimate (WHR unit per decade) | Standard error | p value | Estimate (WHR unit per decade) | Standard error | p value |
| Age (per decade) | 0.019 | 0.0031 | <.0001 | 0.012 | 0.0065 | 0.065 |
| Calendar time (y) | −0.00004 | 0.00027 | ns | −0.00037 | 0.00048 | ns |
| Race | ||||||
| White | 0.017 | 0.0064 | 0.009 | 0.015 | 0.014 | ns |
| Other | 0.016 | 0.0055 | 0.003 | 0.019 | 0.010 | 0.071 |
| African-American (ref.) | ||||||
| Education | ||||||
| >High School | −0.0054 | 0.0056 | ns | −0.031 | 0.011 | 0.006 |
| High School | −0.0078 | 0.0054 | ns | 0.00077 | 0.011 | ns |
| <High School (ref.) | ||||||
| HCV Antibody | ||||||
| positive | 0.0070 | 0.0051 | ns | 0.0095 | 0.010 | ns |
| negative (ref.) | ||||||
| Current Smoker | ||||||
| Yes | −0.0017 | 0.0024 | ns | +0.0029 | 0.0059 | ns |
| No (ref.) | ||||||
| Years of Smoking (per 10 years) | −0.0034 | 0.0021 | ns | 0.0079 | 0.0044 | 0.074 |
| CD4 count (per 100 cells/mm3 increase) | 0.0013 | 0.00036 | 0.0002 | |||
| Log HIV RNA PCR | 0.00044 | 0.00097 | ns | |||
| ARV Therapy | ||||||
| HAART | 0.00084 | 0.0020 | ns | |||
| No Therapy (ref) | ||||||
| PI use | 0.0035 | 0.0017 | 0.044 | |||
ns signifies non-significant p values >0.10 and ref indicates the reference group
Independent predictors of a smaller BMI among HIV-infected women included White race compared to African-American (−2.64 kg/m2, p<0.0001), HCV seropositive status (−0.98 kg/m2, p = 0.041), current smoking (−0.38 kg/m2, p =0.0006), higher viral load (−0.095 kg/m2 per log HIV RNA PCR, p = 0.020),and lower CD4 count (−0.096 kg/m2 for every 100 cell/mm3 decrease, p<0.0001). Age, ARV therapy category, including mono- or combination therapy (data not shown), and NNRTI use were not independently associated with BMI. Similar results were seen when WHR was included in the model (data not shown).
Independent predictors of a larger WHR among HIV-infected women included age in years (+0.019 increase in WHR per decade, p<0.0001), White and Other race compared to African-American (+0.017, p = 0.0009; and +0.016, p = 0.0003 respectively), CD4 count (+0.0013 for every 100 cell/mm3 increase, p =0.0002) and PI use (+0.0035, p = 0.044). ARV therapy category, including mono- and combination therapy (data not shown), viral load, HCV status and smoking status were not independently associated with WHR. When BMI was included in the model, results were similar except HCV status was marginally significant as a WHR predictor (+0.0097, p =0.0.051).
Among HIV-uninfected women, calendar time in years (+0.20 kg/m2 per year, p<0.0001), and current smoking (−1.12 kg/m2, p =0.0018) were independent predictors of BMI. When WHR was included in the model, results were similar (data not shown).
Education beyond high school was the only independent predictor of WHR among HIV-uninfected women (−0.031 decrease in WHR per decade, p = 0.006). When BMI was included in the model, age in years (+0.012 increase in WHR per decade, p = 0.029), Other race vs African-American race (+0.019, p =0.032), years of smoking (+0.0096 increase in WHR per 10 years of smoking, p = 0.012) and BMI (+0.0045 increase in WHR per kg/m2, p<0.0001) all independently predicted larger WHR, while calendar time in years (−0.0012 per year, p = 0.007) and college education (−0.030, p =0.002) were independent predictors of smaller WHR.
Discussion
Findings from this large observational cohort study of HIV-infected women in the United States indicate no change in waist and hip circumference, WHR and BMI among HIV-infected women over a 5 year period, in contrast to the consistent gains in BMI and waist and hip circumference seen in HIV-uninfected women. The stable pattern of these measures among HIV-infected women does not indicate increasing obesity or ongoing fat loss among the HIV-infected participants, many of whom received HAART. Of note, WHR was larger in the HIV-infected women compared to the HIV-uninfected women, despite the fact that HIV-infected women had smaller BMI, waist and hip circumferences. In addition, the mean BMI of all of the women, both HIV-infected and –uninfected, was consistently in the overweight range or higher.
Linear regression analysis of demographic and clinical predictors of BMI and WHR revealed differences between HIV serogroups. Among HIV-infected women, White race vs. Arican-American, HCV seropositivity, current smoking, lower CD4 and higher viral load predicted smaller BMI. Similarly, predictors of larger WHR among HIV-infected women included White or Other race (vs. African-American), age, and higher CD4. Although PI use was also independently associated with a larger WHR, HAART itself was not associated with either BMI or WHR. Among the HIV-uninfected women, there were fewer significant predictors of either BMI or WHR, perhaps in part due to smaller sample size: current smoking was associated with smaller BMI, and calendar time independently predicted larger BMI while higher education independently predicted smaller WHR.
The finding that BMI and waist circumference increased over time among the HIV-uninfected women is consistent with the well-established observation that weight and waist circumference are now increasing in young and middle-aged adults in the general population in the USA.20–22 The increase of approximately 4% in waist circumference seen among the HIV-uninfected women in our study over a 5-year period is of similar magnitude to the 2.8% increase over a 3-year period recently described among midlife American women.21 The lack of such an increase among the HIV-infected women in our analysis is therefore quite striking, but is consistent with earlier studies from WIHS23,24 as well as studies from other cohorts12,25,26 that have noted a static weight pattern over time in HIV-infected women. A recent study from the MACS cohort, however, found that HIV-infected men had more rapid increases in waist circumference over five years than did HIV–uninfected men, unrelated to PI or NNRTI exposure. The men in this cohort were predominantly Caucasian and were older than the women in our study group, and both factors may explain some of the differences from our findings. 27
Some authors have raised concerns that obesity has become more common than wasting among HIV-infected USA populations.10 Although most of the women in our study were overweight or obese at the beginning of the study period, our study indicates that obesity did not increase among urban HIV-infected women followed for 5 years, but did increase in comparable women not infected with HIV.
The HIV-infected women in this study had only mild HIV-related immunosuppression, with a mean index visit CD4 cell count of over 400 cells/mm3. This suggests that even in the setting of mild immunosuppression, HIV infection itself interferes with the usual age-associated increases in weight and waist circumference seen in the U.S.,20 and is consistent with recent findings of Mwamburi et al..28 While higher CD4 was an independent predictor of larger BMI and WHR, the order of magnitude is not clinically significant.
The findings of a disproportionately large WHR in the setting of HIV infection is consistent with the observations of others that used direct measures of fat and found a relative preservation of trunkal fat.29 However, in another study from WIHS, Tien et al, using self-report confirmed by circumference measurements, found a similar incidence of central hypertrophy among both HIV-infected and -uninfected women.24 In our study, in contrast, self-report was not incorporated into the endpoint definitions and the effect of ARV therapy category was analyzed. Although linear regression analysis did not indicate an independent effect of ARV therapy category (HAART vs nonHAART vs no therapy), we found PI use was an independent predictor of a larger WHR. This could be due an effect on waist circumference, hip circumference, or both and is compatible with previous findings of an association between PI use and trunk fat conservation.30
Several limitations of the present analysis warrant discussion. As with any observational cohort, participants were are not assigned to therapy in a randomized fashion. As HIV-infected and –uninfected participants were similar in height (within 1 centimeter), we assume that the women were similar in body habitus in the past and that differences in anthropometrics at the index visit reflect the impact of HIV and/or ARV therapy. Some fat changes, however, may have already occurred among HIV-infected women prior to our study period. It is also possible that aging caused some HIV-infected women to gain fat over time while ARV use caused others to lose fat, leading to an overall lack of a mean change in longitudinal anthropometric measures. The multivariate analyses, however, do not indicate any age-related increase or decrease in BMI, and ARV therapy category is not associated with either an increase or a decrease in BMI or WHR, making this explanation unlikely.
This study did not assess the role of diet or physical activity, nor the transition to menopause. Although DEXA and CT/MRI scans are the method of choice to measure body composition in HIV-infected patients, these methods were neither economically nor logistically feasible in this large multicenter cohort study. Anthropometric measurements correlate well with DEXA in assessing central fat, including among obese women.31,32 These inexpensive measurements can easily be done in clinical settings, and are a sound alternative to DEXA and CT, especially when these measurements are performed after intensive training and certification of the examiners, as in this study. We used NHANES III methodology and standardized training of all clinicians by a single trainer to optimize precision. Several large cohort studies, such as the Study of Women’s Health Across North America (SWAN)21 and others33 have also used anthropometric measurements, greatly facilitating comparisons across studies.
This study has several strengths. The WIHS study, the largest cohort of HIV-infected women in the US, includes HIV-uninfected participants as a control. The predominantly African-American composition of the cohort is representative of the racial distribution of AIDS among women in the United States.34 Moreover, the study group has a high prevalence of obesity (33%), similar to prevalence of obesity among African-American women in the general US population (39%),35 and higher than other HIV cohort studies of lipodystrophy.
In summary, in this observational cohort we found static longitudinal anthropometric patterns among HIV-infected women over a 5 year period, in contrast to consistent gains in central adiposity seen in HIV-uninfected women over this time. Moreover, WHR was significantly and disproportionately larger while BMI, waist and hip circumference were smaller in HIV infected compared to HIV-uninfected women. We did not find evidence to support either increasing obesity or ongoing fat loss among the HIV-infected participants.
Both waist circumference and waist-to-hip ratio, as noted above, have been described as cardiovascular risk factors in HIV-uninfected women,13,14 and waist circumference may be a more powerful predictor of all-cause mortality than WHR.36,37 However, WHR has also been reported to be a significant predictor of inflammatory indices, including C-reactive protein in HIV-infected women.38 Further studies are needed to assess whether the smaller waist circumference and BMI seen among the HIV-infected women will be protective for morbidity and mortality in the setting of a disproportionately large WHR. Until the cardiovascular implications of these anthropometric patterns have been clarified, it remains prudent to monitor and treat the metabolic complications that arise in the setting of HIV, as per clinical guidelines. 39
Acknowledgments
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases with supplemental funding from the National Cancer Institute, the National Institute on Drug Abuse (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590). Funding is also provided by the National Institute of Child Health and Human Development (UO1-HD-32632) and the National Center for Research Resources (MO1-RR-00071, MO1-RR-00079, MO1-RR-00083).
Reference List
- 1.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 4.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Dube MP, Parker RA, Tebas P, et al. Glucose metabolism, lipid, and body fat changes in antiretroviral-naive subjects randomized to nelfinavir or efavirenz plus dual nucleosides. AIDS. 2005;19:1807–1818. doi: 10.1097/01.aids.0000183629.20041.bb. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein KA, Ward DJ, Moorman AC, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389–1398. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 7.Bacchetti P, Gripshover B, Grunfeld C, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtenstein KA, Delaney KM, Armon C, et al. Incidence of and risk factors for lipoatrophy (abnormal fat loss) in ambulatory HIV-1-infected patients. J Acquir Immune Defic Syndr. 2003;32:48–56. doi: 10.1097/00126334-200301010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Palella FJ, Jr, Cole SR, Chmiel JS, et al. Anthropometrics and examiner-reported body habitus abnormalities in the multicenter AIDS cohort study. Clin Infect Dis. 2004;38:903–907. doi: 10.1086/381684. [DOI] [PubMed] [Google Scholar]
- 10.Amorosa V, Synnestvedt M, Gross R, et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr. 2005;39:557–561. [PubMed] [Google Scholar]
- 11.Ogden CLP, Carroll MDM, Curtin LRP, McDowell MAM, Tabak CJM, Flegal KMP. Prevalence of Overweight and Obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 12.Brown T, Wang Z, Chu H, et al. Longitudinal Anthropometric Changes in HIV-Infected and HIV-Uninfected Men. J Acquir Immune Defic Syndr. 2006 doi: 10.1097/01.qai.0000243052.73321.8e. [DOI] [PubMed] [Google Scholar]
- 13.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 14.Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 15.UNAIDS 2006: Report on the global AIDS epidemic. 2006 [Google Scholar]
- 16.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: an Observational Cohort Brings Clinical Sciences to the Bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. The Third National Health and Examination Survey Reference Manuals and Reports. 2002 CD-ROM. 1996. [Google Scholar]
- 19.Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Department of Health and Human Services and the Henry J. Kaiser Family Foundation. Ann Intern Med. 1998;128:1079–1100. doi: 10.7326/0003-4819-128-12_part_2-199806151-00003. [DOI] [PubMed] [Google Scholar]
- 20.Lewis CE, Jacobs DR, Jr, McCreath H, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 2000;151:1172–1181. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 21.Sternfeld B, Wang H, Quesenberry CP, Jr, et al. Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;160:912–922. doi: 10.1093/aje/kwh299. [DOI] [PubMed] [Google Scholar]
- 22.Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med. 1990;150:665–672. [PubMed] [Google Scholar]
- 23.Justman JE, Benning L, Danoff A, et al. Protease inhibitor use and the incidence of diabetes mellitus in a large cohort of HIV-infected women. J Acquir Immune Defic Syndr. 2003;32:298–302. doi: 10.1097/00126334-200303010-00009. [DOI] [PubMed] [Google Scholar]
- 24.Tien PC, Cole SR, Williams CM, et al. Incidence of lipoatrophy and lipohypertrophy in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2003;34:461–466. doi: 10.1097/00126334-200312150-00003. [DOI] [PubMed] [Google Scholar]
- 25.Schwenk A, Kremer G, Cornely O, Diehl V, Fatkenheuer G, Salzberger B. Body weight changes with protease inhibitor treatment in undernourished HIV-infected patients. Nutrition. 1999;15:453–457. doi: 10.1016/s0899-9007(99)00083-0. [DOI] [PubMed] [Google Scholar]
- 26.Yanovski JA, Miller KD, Kino T, et al. Endocrine and metabolic evaluation of human immunodeficiency virus-infected patients with evidence of protease inhibitor-associated lipodystrophy. J Clin Endocrinol Metab. 1999;84:1925–1931. doi: 10.1210/jcem.84.6.5740. [DOI] [PubMed] [Google Scholar]
- 27.Brown T, Chu H, Wang Z, Palella FJ, Kingsley L, Witt MD, Dobs AS. Longitudinal increases in waist circumference are associated with HIV-serostatus, independent of antiretroviral therapy. AIDS. 2007;21:1731–1738. doi: 10.1097/QAD.0b013e328270356a. [DOI] [PubMed] [Google Scholar]
- 28.Mwamburi DM, Wilson IB, Jacobson DL, et al. Understanding the role of HIV load in determining weight change in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:167–173. doi: 10.1086/426591. [DOI] [PubMed] [Google Scholar]
- 29.Mallon PW, Miller J, Cooper DA, Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS. 2003;17:971–979. doi: 10.1097/00002030-200305020-00005. [DOI] [PubMed] [Google Scholar]
- 30.Mulligan K, Anastos K, Justman J, et al. Fat distribution in HIV-infected women in the United States: DEXA substudy in the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2005;38:18–22. doi: 10.1097/00126334-200501010-00004. [DOI] [PubMed] [Google Scholar]
- 31.Kamel EG, McNeill G, Van Wijk MC. Usefulness of anthropometry and DXA in predicting intra-abdominal fat in obese men and women. Obes Res. 2000;8:36–42. doi: 10.1038/oby.2000.6. [DOI] [PubMed] [Google Scholar]
- 32.Stewart KJ, DeRegis JR, Turner KL, et al. Usefulness of anthropometrics and dual-energy x-ray absorptiometry for estimating abdominal obesity measured by magnetic resonance imaging in older men and women. J Cardiopulm Rehabil. 2003;23:109–114. doi: 10.1097/00008483-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Molarius A, Seidell JC, Sans S, Tuomilehto J, Kuulasmaa K. Waist and hip circumferences, and waist-hip ratio in 19 populations of the WHO MONICA Project. Int J Obes Relat Metab Disord. 1999;23:116–125. doi: 10.1038/sj.ijo.0800772. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control. HIV/AIDS among US women: minority and young women at continuing risk. http://www.cdc.gov/hiv/pubs/facts/women.pdf 05/02.2002.
- 35.National Center for Health Statistics. National Health Interview Survey: Early Release of Selected Estimates. www.cdc.gov/nchs/data/nhis/earlyrelease/200612_06.pdf12-12-0006.
- 36.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 37.Zhu S, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am J Clin Nutr. 2002;76:743–749. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 38.Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr. 2005;39:44–54. doi: 10.1097/01.qai.0000159323.59250.83. [DOI] [PubMed] [Google Scholar]
- 39.Schambelan M, Benson CA, Carr A, et al. Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: recommendations of an International AIDS Society-USA panel. J Acquir Immune Defic Syndr. 2002;31:257–275. doi: 10.1097/00126334-200211010-00001. [DOI] [PubMed] [Google Scholar]