Abstract
Clinical evaluation of a novel fully automated chemiluminescence immunoassay for determination of immunoglobulin G avidity to human cytomegalovirus (HCMV) showed 92.8% sensitivity and 84.7% specificity in detecting a recent (≤90 days) primary HCMV infection. The assay appears useful for accurately diagnosing recent primary HCMV infections.
Human cytomegalovirus (HCMV) is the leading infectious agent causing mental retardation and deafness in newborns (14). Since symptomatic congenital infections are mostly due to intrauterine transmission following primary maternal infection (4), differential diagnosis of primary versus recurrent infection (or persistence of HCMV-specific immunoglobulin M [IgM] antibody) is crucial for correct counseling and management of pregnancy. The determination of IgG avidity has been shown to help in distinguishing primary from nonprimary HCMV infection (1, 8, 10), as low avidity values have been shown to be associated to recent primary HCMV infections. Indeed, determination of IgG avidity has become a key step in the algorithm for the interpretation of a positive IgM result in pregnant women (10).
The aim of the study was the clinical evaluation of a fully automated chemiluminescence immunoassay for the determination of HCMV-specific IgG avidity (Liaison CMV IgG avidity assay) developed by DiaSorin, Saluggia, Italy.
For this purpose, 413 sera obtained from 212 subjects were examined. The sera had been characterized previously and divided into the following groups: group A, including 167 sequential serum samples from 78 patients collected ≤90 (n = 112), 91 to 180 (n = 38), and >180 (n = 17) days after the onset of primary HCMV infection; group B, including 56 serum samples from 17 pregnant women with persistent HCMV-specific IgM antibody; group C, including 87 sequential serum samples from 14 solid organ transplant recipients with recurrent HCMV infection; and group D, including 103 HCMV IgG-positive, IgM-negative serum samples from 49 pregnant women and 54 transplanted patients with past HCMV infection.
Diagnosis of primary HCMV infection was based on the following criteria: seroconversion, decreasing levels of HCMV-specific IgM antibody, increasing levels of IgG antibody avidity, and detection of HCMV and HCMV products in blood. Dating of the onset was based on the presence of abnormal laboratory findings and/or clinical symptoms (10). Persistent IgM antibody was defined as stable IgM values for ≥3 months in the absence of serologic, virologic, or clinical data indicative of a recent primary HCMV infection in the mothers and of congenital infection in the relevant newborn babies. Congenital HCMV infection was diagnosed within the first 2 weeks of life by virus isolation from urine and virus detection in blood (7, 12). Recurrent HCMV infection in transplanted patients was diagnosed by quantitative determination of pp65 antigenemia in peripheral blood leukocytes (5).
Sera examined in the present study had been previously characterized for HCMV-specific IgG and IgM antibody by in-house-developed enzyme-linked immunosorbent assays (ELISAs) (6, 11). Sensitivity and specificity have been shown to be 99.8 and 100% for the IgG (6) assay and 100 and 98.9% (11) for the IgM assay, respectively. The HCMV IgG ELISA (6) was used for IgG avidity determination with minor modifications. Briefly, microtiter wells, coated overnight with nuclear HCMV and control antigen, were incubated in duplicate with test sera diluted 1:500. Following 1 h of incubation, one pair of duplicate wells was washed with washing buffer and another pair with washing buffer containing 6 M urea. Optimally diluted horseradish peroxidase-conjugated goat anti-human IgG was then added for 1 h. Finally, the chromogen-substrate solution was added for 30 min, and color development was stopped with 4N sulfuric acid. Optical density (OD) was read at 492 nm, and the avidity index (AI) was determined as follows: (net OD in the presence of urea/net OD in the absence of urea) × 100. In each test run, control sera of high, intermediate, and low AI were included.
The Liaison CMV IgG avidity assay evaluated here does not require sample dilution and is completed in 50 min. In the first incubation step, test sera are reacted with HCMV antigen coated onto magnetic particles in two separate wells. After washing, beads are reacted with a chaotropic solution or with buffer, respectively. An anti-human IgG monoclonal antibody coupled to aminobutylethylisoluminol is then added. After washing, the chemiluminescence reaction is triggered and light is quantitated as relative light units. Relative light units are then converted into IgG international units per milliliter, and AI is calculated by dividing the international units of the sample treated with chaotropic solution by the international units of the untreated sample. According to instructions provided by the manufacturer, AIs of ≤0.20, 0.21 to 0.30, and >0.30 indicate low, moderate, and high avidity, respectively. In particular, an AI of ≤0.2 suggests the possibility of primary infection acquired less than 3 months prior to sample collection.
Sera were tested under code at DiaSorin. When testing was completed, codes were broken and results analyzed in Pavia. Mean AI values of different groups of sera examined were compared by analysis of variance. Tukey's multiple comparison test was performed as posttest.
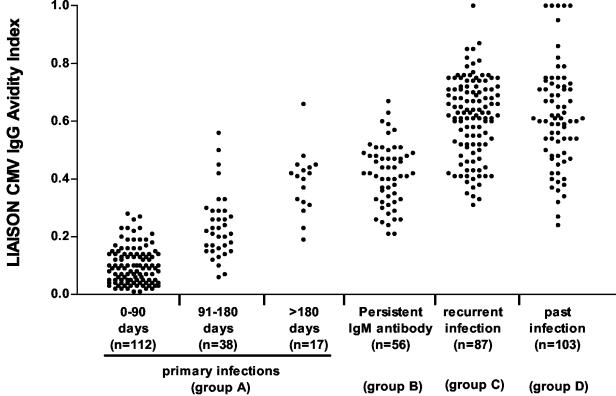
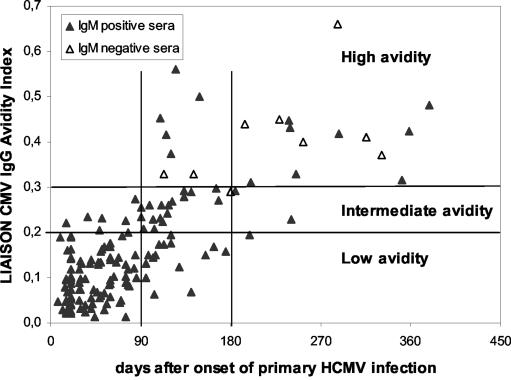
Overall results are shown in Fig. 1, while detailed results are reported in Table 1. In particular, in group A, consisting of sequential sera from subjects with primary infection, a progressive and significant (P < 0.01) increase in mean AI values was observed in sera collected during the 3-month time intervals. However, while the great majority of sera collected at <3 and >6 months showed low and high AI, respectively, sera collected 3 to 6 months after the onset showed a wide range of AI. Maturation of HCMV-specific IgG as determined by the Liaison assay together with IgM results obtained in sequential serum samples are shown in Fig. 2. Nearly all sera (145 of 146, 99.3%) with low or intermediate AI were positive for HCMV-specific IgM, while 13 of 21 (61.9%) sera with high AI were still IgM positive.
FIG. 1.
Liaison CMV IgG AI values according to type of HCMV infection. n, number of serum samples in each group.
TABLE 1.
AI results obtained on different groups of sera examined by the Liaison CMV IgG avidity assay
| Group of sera | No. of sera | No. of sera (%) with AI classified as:
|
Mean AI ± SD (95% CI) | Pa | ||
|---|---|---|---|---|---|---|
| Low (<0.20) | Moderate (0.21-0.30) | High (>0.30) | ||||
| A (primary infection) | ||||||
| 0-90 days | 112 | 104 (92.8) | 8 (7.2) | 0 | 0.10 ± 0.06 (0.08-0.11) | <0.001 |
| 91-180 days | 38 | 13 (34.2) | 18 (47.4) | 7 (18.4) | 0.24 ± 0.1 (0.20-0.27) | <0.01 |
| >180 days | 17 | 1 (5.9) | 2 (11.8) | 14 (82.3) | 0.39 ± 0.1 (0.33-0.44) | NS |
| B (persistent IgM antibody) | 56 | 0 | 10 (17.9) | 46 (82.1) | 0.41 ± 0.1 (0.39-0.44) | <0.001 |
| C (recurrent infection) | 87 | 0 | 0 | 87 (100) | 0.61 ± 0.1 (0.59-0.64) |
|
| D (past infection) | 103 | 0 | 2 (1.9) | 101 (98) | 0.62 ± 0.2 (0.57-0.66) | |
| Total | 413 | 118 | 40 | 255 | ||
Determined by Tukey's multiple comparison test. NS, not significant.
FIG. 2.
Maturation of HCMV-specific IgG avidity in 167 sequential sera from 78 subjects with primary HCMV infection. Horizontal bars indicate low, intermediate, and high AI ranges. Vertical bars indicate <90-, 91- to 180-, and >180-day intervals after the onset of primary infection.
In group B, consisting of 56 sera with persistent IgM antibody, 46 (82.1%) gave high and 10 (17.9%) gave moderate AI, respectively, whereas no serum gave low AI (Table 1). Mean AI (0.41 ± 0.1) was comparable to that observed in sera collected >6 months after the onset of primary infection (0.39 ± 0.1) but significantly lower than those observed in patients with recurrent or past infection (0.61 ± 0.1 and 0.62 ± 0.2, respectively). Of the 10 sera giving intermediate AI, five belonged to the same pregnant woman. Her samples were collected over a period of 193 days, and AI varied from 0.21 to 0.29 while IgM index ranged from 1.5 to 2.5 over the same period of time. The remaining five samples with intermediate AI were from four pregnant women from whom 10 additional samples were examined, all giving high (0.31 to 0.51) AI. All 10 sera with intermediate AI by the Liaison assay gave high AI when tested by the in-house method. No woman with persistent IgM antibody delivered an HCMV-infected infant, as documented by the absence of congenital infection.
All of the 87 sequential samples from transplanted patients with recurrent HCMV infection (group C) showed high AI. Finally, of the 103 sera of group D, 101 (98.%) gave high AI, with only two sera showing moderate AI (Table 1). The two sera with moderate (0.27 and 0.24) AI belonged to two pregnant women. One of them was referred to our institute at 6 weeks' gestation because of a borderline IgM positivity. A possible preconceptional primary HCMV infection was diagnosed, and the woman was counseled accordingly (13). No data were available from the other pregnant woman in whom a high AI was detected by the in-house method. Mean AI values observed in group C and group D serum samples were comparable to each other (P > 0.05) but significantly higher (P < 0.001) than those observed in sera from patients of groups A and B (Table 1).
As shown in Table 1, the Liaison CMV IgG avidity assay showed 92.8% sensitivity in detecting a recent (<90 days) HCMV infection, as 104 out of 112 sera collected less than 90 days after the onset gave an AI of ≤ 0.20. On the other hand, 255 out of 301 sera collected from subjects with primary infection of >90 days, or persistent IgM, or recurrent or past HCMV infection gave an AI of >0.3, thus providing an overall specificity of 84.7%. If a single 0.30-AI cutoff had been employed, sensitivity would have increased to 100% (112 of 112) without affecting specificity.
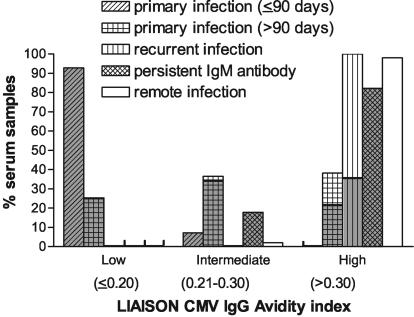
Figure 3 shows the percentages of IgM-positive sera in each group of sera. These data allow us to speculate that if the Liaison assay with a 0.30-AI cutoff had been used as the first line assay instead of IgM determination, in this series of sera no case of recent primary HCMV infection would have been missed, whereas additional viral assays (i.e., assays for HCMV detection in blood) would have been avoided in a fair number of subjects with an AI of >0.30 and IgM positivity unrelated to a recent primary HCMV infection. Prospective studies are warranted to assess the reliability of such an approach (see below).
FIG. 3.
Prevalence of low, intermediate, and high AI values according to type of HCMV infection. The shaded area in each column indicates the percentage of HCMV-specific IgM-positive sera.
Presently, four commercial assays are used for the determination of HCMV-specific IgG avidity, i.e., those produced by Radim (Pomezia, Italy), Bouty (Sesto S. Giovanni, Italy), Dade-Behring (Deerfield, Ill.), and bioMérieux (Marcy l'Etoile, France). Radim, Bouty, and Dade-Behring assays are based on the ELISA technology, i.e., they are performed in microplates and are completely manual, whereas the bioMérieux assay is a semiautomated assay by which each serum is reacted in two single cartridges (one without and the other with the dissociating solution) containing all reagents.
Recently, a comparative study between the bioMérieux and Dade-Behring assays, with a panel of well-characterized sera from recent or past HCMV infection, showed that the bioMérieux assay provided 100 and 82.5% ability to detect (sensitivity) and to exclude (specificity), respectively, a recent (<90 days) primary HCMV infection, whereas the detection and the exclusion ability were 97.9 and 30.4%, respectively, for the Dade-Behring assay (2). The present study indicates, albeit indirectly, that the Liaison and the bioMérieux assays perform comparably. However, the same study reported above (2) showed that when 80 unclassified HCMV IgM-positive single sera from pregnant women were tested by the bioMérieux and an in-house (reference) assay, as many as 30% discrepant results were obtained. These data, and the fact that most of the studies so far performed have been retrospectively conducted, point to the need for prospective studies specifically designed to define the actual performance of commercially available assays with respect to the clinical value of AI.
Prospective studies should assess in routine practice (i) the reliability (specificity and sensitivity) of avidity determination as an aid for the interpretation of positive IgM results and (ii) the advantages and disadvantages of using avidity as first line assay instead of the IgM assay. The rationale for the latter suggestion relies on the consideration that in the great majority of laboratories, serology is the only diagnostic approach available and, thus, interpretation of positive IgM results can eventually rely on an additional serological assay, such as the avidity test. In addition, by using avidity as first line assay, the potential risk (feared by some investigators) of missing a recent primary infection without detectable IgM could be overcome. On the other hand, the opposite situation, i.e., absence of IgG in the presence of IgM, which may occur very early during the infection, does not represent a limitation to the suggested approach. In fact, should this event occur, an additional blood sample would be requested to demonstrate IgG seroconversion.
An additional important consideration is that there would be no need to use highly sensitive (and, forcibly, less-specific) IgM assays. The issue of the sensitivity of the HCMV IgM assay to be used for routine screening in pregnant women is a debated one. Lazzarotto et al. (9) reported that a highly sensitive assay such as the AxSYM CMV IgM assay was required in order to detect low levels of IgM in sera containing IgG antibody of low avidity. In fact, of the 134 sera which were IgM positive only by AxSYM, 13 (9.7%) were shown to contain IgG of low-moderate avidity according to the Radim assay. However, no information was provided concerning these IgM-positive sera, and the outcomes of the pregnancies were not reported. Therefore, until the performance of commercial avidity assays will not be assessed in ad hoc studies, caution should be adopted in favoring the use of IgM assays of high sensitivity.
In addition, whichever screening approach is adopted, the time of pregnancy at which first HCMV serology is performed may represent per se an objective limitation, as high avidity values observed in samples collected after 12 weeks of gestation do not rule out a primary infection during pregnancy (3).
As a final consideration, we must remind that the combined performance of both serologic and virologic assays, together with an appropriate follow-up, remain the key tools for the correct interpretation of positive IgM antibody results.
Acknowledgments
We thank Giuliana Casali for skillful technical assistance and Linda D'Arrigo for revision of the English. This work was partially supported by Ministero della Salute, IRCCS Policlinico San Matteo, Ricerca Corrente, grant no. 80513, and by Ricerca Finalizzata (convenzione 126).
REFERENCES
- 1.Blackburn, N. K., T. G. Besselaar, B. D. Schoub, and K. F. O'Connell. 1991. Differentiation of primary cytomegalovirus infection from reactivation using the urea denaturation test for measuring antibody avidity. J. Med. Virol. 33:6-9. [DOI] [PubMed] [Google Scholar]
- 2.Bodéus, M., D. Beulné, and P. Goubau. 2001. Ability of three IgG-avidity assays to exclude recent cytomegalovirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 20:248-252. [DOI] [PubMed] [Google Scholar]
- 3.Bodéus, M., M. Van Ranst, P. Bernard, C. Hubinont, and P. Goubau. 2002. Anticytomegalovirus IgG avidity in pregnancy: a 2-year prospective study. Fetal Diagn. Ther. 17:362-366. [DOI] [PubMed] [Google Scholar]
- 4.Fowler, K. B., S. Stagno, R. F. Pass, W. J. Britt, T. J. Boll, and C. A. Alford. 1992. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N. Engl. J. Med. 326:663-667. [DOI] [PubMed] [Google Scholar]
- 5.Gerna, G., E. Percivalle, M. Torsellini, and M. G. Revello. 1998. Standardization of the human cytomegalovirus antigenemia assay by means of in vitro-generated pp65-positive peripheral blood polymorphonuclear leukocytes. J. Clin. Microbiol. 36:3585-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerna, G., M. G. Revello, M. Palla, E. Percivalle, M. Torsellini. 1992. Microneutralization as a reference method for selection of the cut-off of an enzyme-linked immunosorbent assay for detection of IgG antibody to human cytomegalovirus. Microbiologica 15:177-182. [PubMed] [Google Scholar]
- 7.Gerna, G., M. G. Revello, E. Percivalle, M. Zavattoni, M. Parea, and M. Battaglia. 1990. Quantification of human cytomegalovirus viremia by using monoclonal antibodies to different viral proteins. J. Clin. Microbiol. 28:2681-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grangeot-Keros, L., M. J. Mayaux, P. Lebon, F. Freymuth, G. Eugene, R. Stricker, and E. Dussaix. 1997. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J. Infect. Dis. 175:944-946. [DOI] [PubMed] [Google Scholar]
- 9.Lazzarotto, T., C. Galli, R. Pulvirenti, R. Rescaldani, R. Vezzo, A. La Gioia, C. Martinelli, S. La Rocca, G. Agresti, L. Grillner, M. Nordin, M. Van Ranst, B. Combs, G. T. Maine, and M. P. Landini. 2001. Evaluation of the Abbott AxSYM cytomegalovirus (CMV) immunoglobulin M (IgM) assay in conjunction with other CMV tests and a CMV IgG avidity assay. Clin. Diagn. Lab. Immunol. 8:196-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Revello, M. G., and G. Gerna. 2002. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin. Microbiol. Rev. 15:680-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Revello, M. G., E. Percivalle, M. Zannino, V. Rossi, and G. Gerna. 1991. Development and evaluation of a capture ELISA for IgM antibody to the human cytomegalovirus major DNA binding protein. J. Virol. Methods 35:315-329. [DOI] [PubMed] [Google Scholar]
- 12.Revello, M. G., M. Zavattoni, F. Baldanti, A. Sarasini, S. Paolucci, and G. Gerna. 1999. Diagnostic and prognostic value of human cytomegalovirus load and IgM antibody in blood of congenitally infected newborns. J. Clin. Virol. 14:57-66. [DOI] [PubMed] [Google Scholar]
- 13.Revello, M. G., M. Zavattoni, M. Furione, D. Lilleri, G. Gorini, and G. Gerna. 2002. Diagnosis and outcome of preconceptional and periconceptional primary human cytomegalovirus infections. J. Infect. Dis. 186:553-557. [DOI] [PubMed] [Google Scholar]
- 14.Stagno, S. 2001. Cytomegalovirus, p. 389-424. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant. W. B. Saunders Co., Philadelphia, Pa.