Abstract
A soybean histone-type protein kinase was used to prepare 32P-labeled histone H1 as substrate for purification and characterization of a phosphoprotein phosphatase (EC 3.1.3.16) from soybean hypocotyls. The phosphatase has been purified 169-fold by ammonium sulfate fractionation, ethanol precipitation, and chromatography on Sephadex G-150, DEAE-Sephadex A-25 and Sephadex G-100. The activity of the phosphoprotein phosphatase is distinct from that of acid and alkaline phosphatases (EC 3.1.3.1) as well as from that of nucleotidases. The final enzyme preparation does not contain histone protease activity, although it can be detected during the early stages of purification. The protease(s) apparently can attack phosphorylated histone H1, indicating that phosphorylation does not protect the protein against proteolytic degradation.
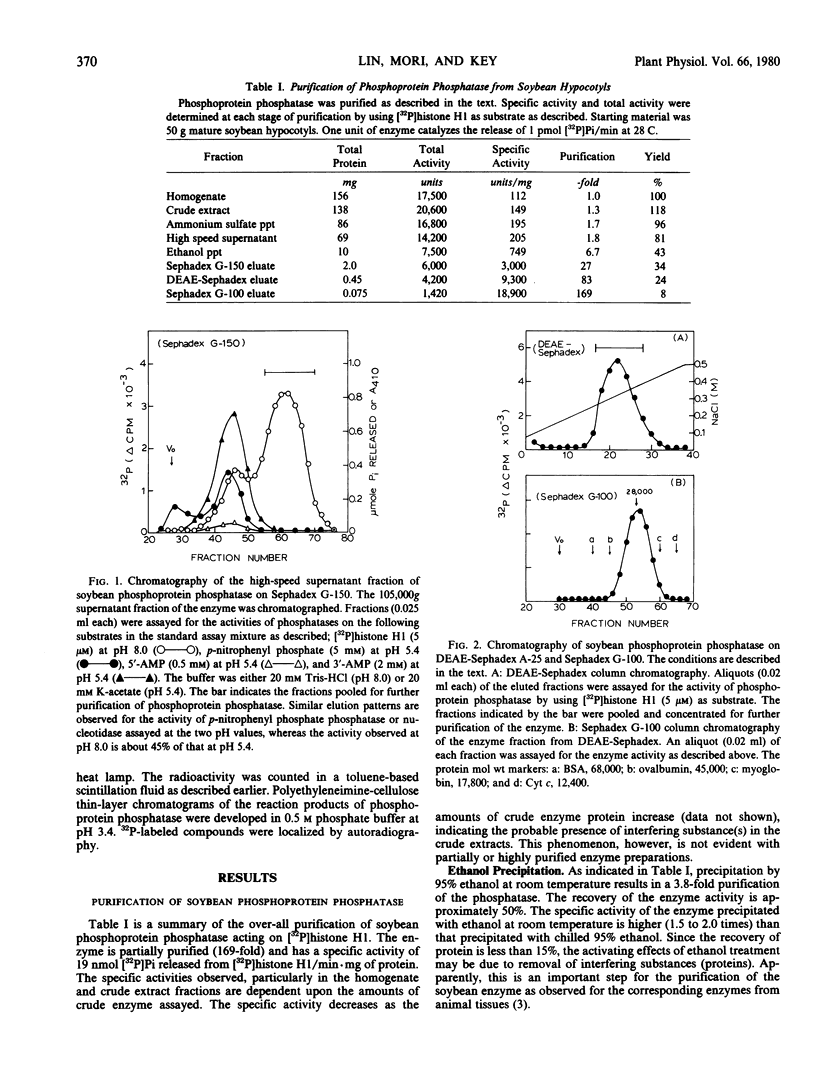
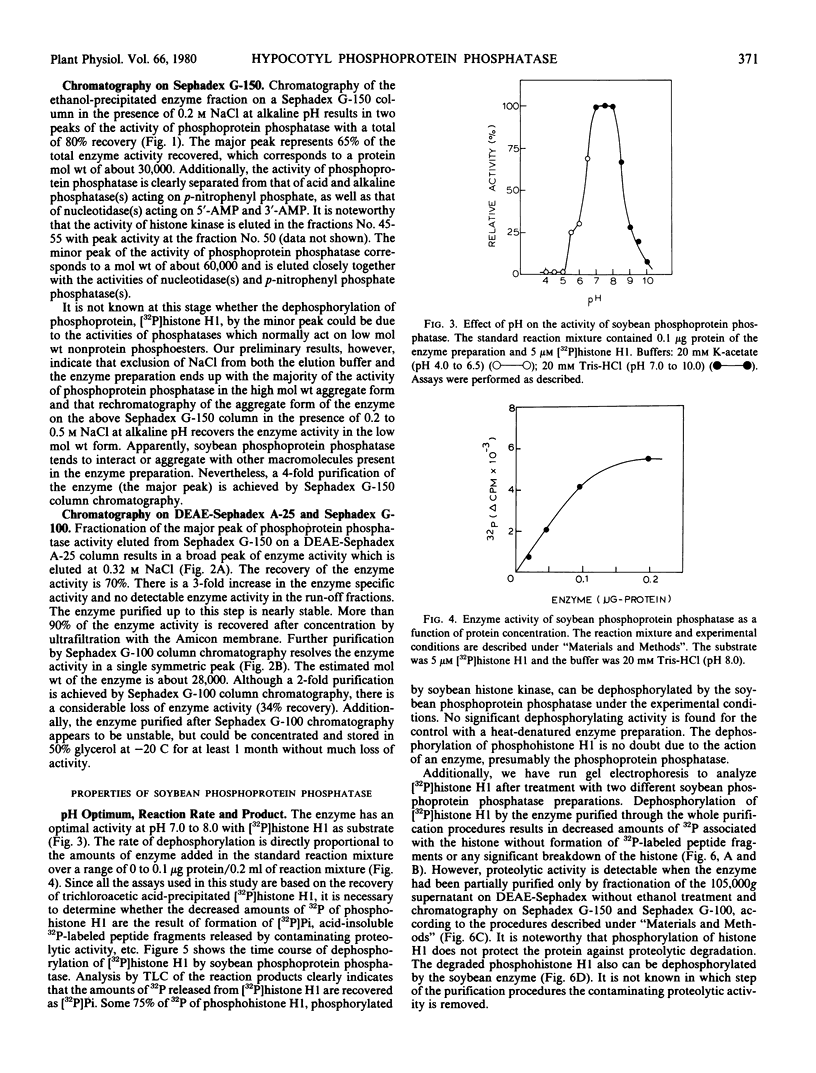
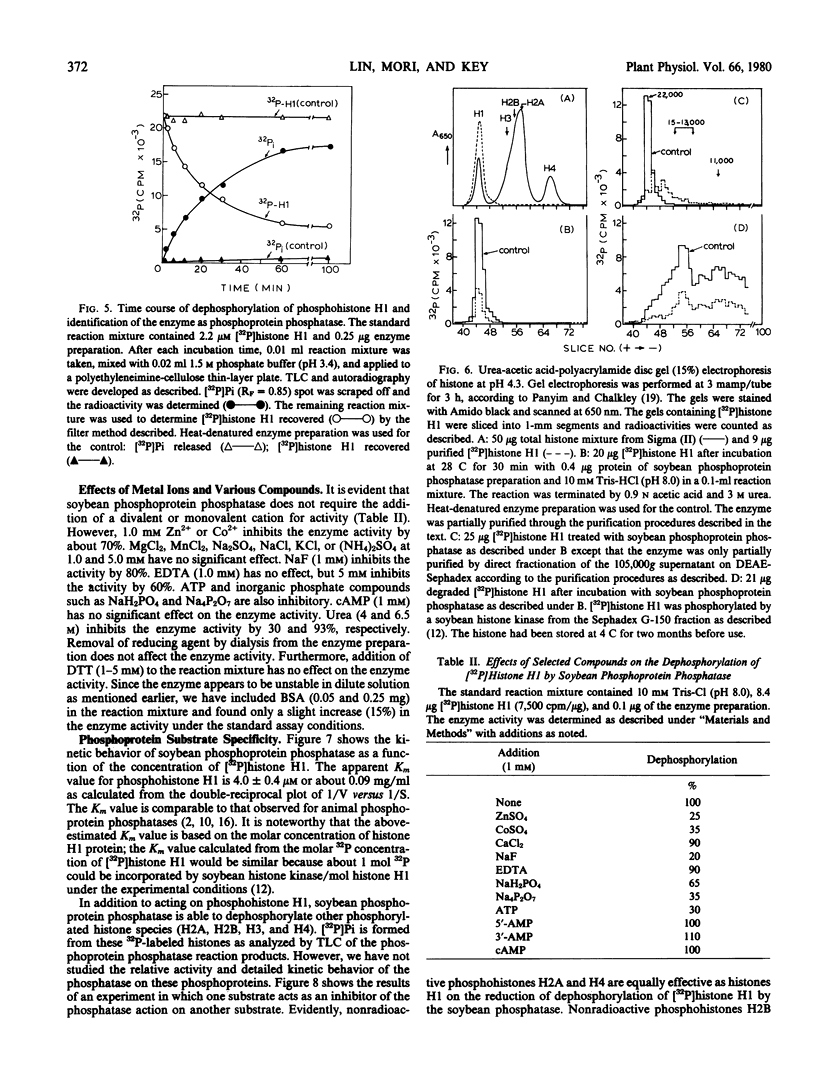
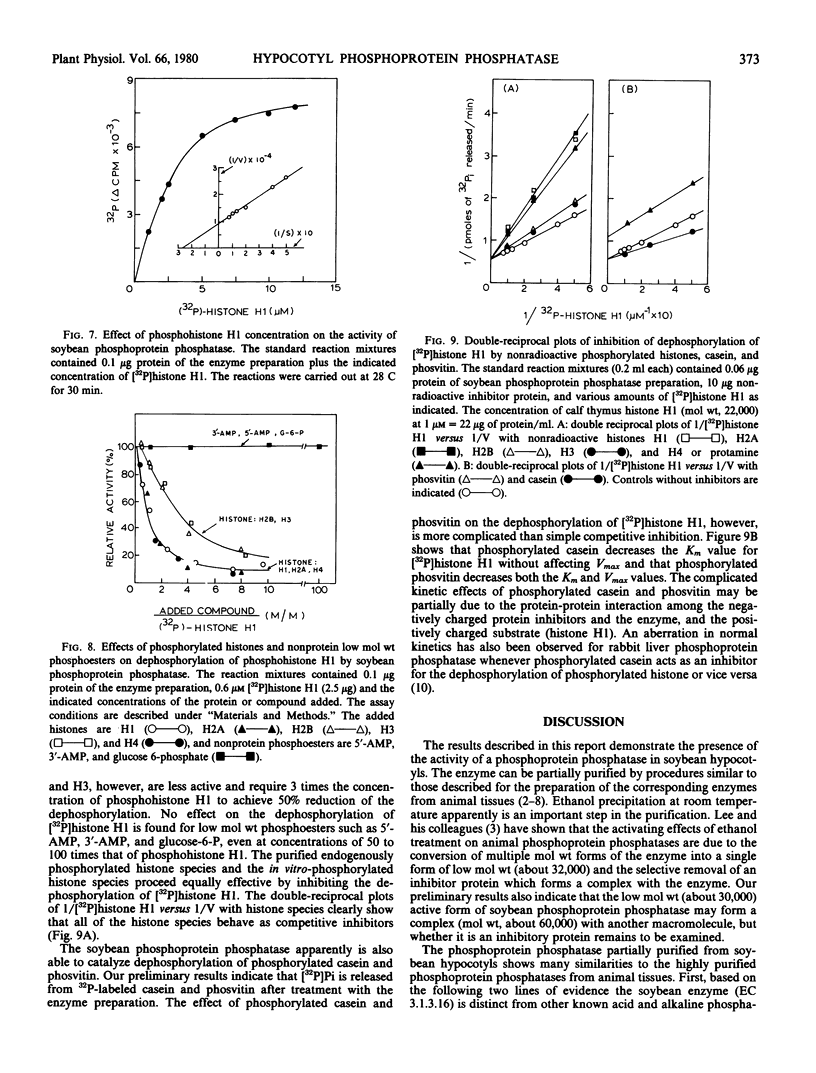
The amounts of 32P released from [32P]histone H1 are proportionally recovered as [32P]Pi, indicating that the dephosphorylation is due to the action of phosphoprotein phosphatase. The enzyme shows maximal activity at pH 7 to 8 and has a specific activity of 19 nanomoles of [32P]Pi released from [32P]histone H1 per minute per milligram of protein. The apparent Km for phosphohistone H1 is 4.0 ± 0.4 micromolar. The estimated molecular weight of the enzyme is approximately 30,000 by gel filtration. The enzyme activity does not depend upon the addition of reducing agent and metal ion. Zn2+, Co2+, NaF, pyrophosphate, or ATP at 1 millimolar, however, inhibits the enzyme activity by about 70%. The enzyme activity is unaffected by cyclic nucleotides and plant growth substances but is inhibited by polyamines. All the phosphorylated histone species and protamine, not low molecular weight phosphoesters, act as competitive inhibitors for the dephosphorylation of [32P]histone H1.
Besides its action on phosphohistone H1, the soybean enzyme also catalyzes the dephosphorylation of other phosphohistone species (H2A, H2B, H3, and H4), degraded phosphohistone H1, and possibly phosphorylated casein and phosvitin. All these results indicate that the enzyme is a nonspecific phosphoprotein phosphatase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt H., Capulong Z. L., Lee E. Y. Purification and properties of rabbit liver phosphorylase phosphatase. J Biol Chem. 1975 Oct 25;250(20):8038–8044. [PubMed] [Google Scholar]
- Brandt H., Lee E. Y., Killilea S. D. A protein inhibitor of rabbit liver phosphorylase phosphatase. Biochem Biophys Res Commun. 1975 Apr 21;63(4):950–956. doi: 10.1016/0006-291x(75)90661-0. [DOI] [PubMed] [Google Scholar]
- Chen L. J., Walsh D. A. Multiple forms of hepatic adenosine 3':5'-monophosphate dependent protein kinase. Biochemistry. 1971 Sep 14;10(19):3614–3621. doi: 10.1021/bi00795a020. [DOI] [PubMed] [Google Scholar]
- Chou C. K., Alfano J., Rosen O. M. Purification of phosphoprotein phosphatase from bovine cardiac muscle that catalyzes dephosphorylation of cyclic AMP-binding protein component of protein kinase. J Biol Chem. 1977 May 10;252(9):2855–2859. [PubMed] [Google Scholar]
- Detwiler T. C., Gratecos D., Fischer E. H. Rabbit muscle phosphorylase phosphatase. 2. Kinetic properties and behavior in glycogen particles. Biochemistry. 1977 Nov 1;16(22):4818–4823. doi: 10.1021/bi00641a010. [DOI] [PubMed] [Google Scholar]
- Kato K., Bishop J. S. Glycogen synthetase-D phosphatase. I. Some new properties of the partially purified enzyme from rabbit skeletal muscle. J Biol Chem. 1972 Nov 25;247(22):7420–7429. [PubMed] [Google Scholar]
- Keates R. A., Trewavas A. J. Protein kinase activity associated with isolated ribosomes from peas and lemna. Plant Physiol. 1974 Jul;54(1):95–99. doi: 10.1104/pp.54.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal R. L., Vandenheede J. R., Krebs E. G. Purification, properties, and substrate specificities of phosphoprotein phosphatase(s) from rabbit liver. J Biol Chem. 1976 Aug 25;251(16):4850–4858. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li H. C., Hsiao K. J., Sampathkumar S. Characterization of a novel alkaline phosphatase activity which co-purifies with a phosphorylase (phosphoprotein) phosphatase of Mr = 35,000 cardiac muscle. J Biol Chem. 1979 May 10;254(9):3368–3374. [PubMed] [Google Scholar]
- Lin P. P., Key J. L. Histone Kinase from Soybean Hypocotyls: PURIFICATION, PROPERTIES, AND SUBSTRATE SPECIFICITIES. Plant Physiol. 1980 Sep;66(3):360–367. doi: 10.1104/pp.66.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. P., Varner J. E. Cyclic nucleotide phosphodiesterase in pea seedlings. Biochim Biophys Acta. 1972 Aug 28;276(2):454–474. doi: 10.1016/0005-2744(72)91007-8. [DOI] [PubMed] [Google Scholar]
- Lin P. P., Wilson R. F., Bonner J. Isolation and properties of nonhistone chromosomal proteins from pea chromatin. Mol Cell Biochem. 1973 Jun 27;1(2):197–207. doi: 10.1007/BF01659330. [DOI] [PubMed] [Google Scholar]
- Meisler M. H., Langan T. A. Characterization of a phosphatase specific for phosphorylated histones and protamine. J Biol Chem. 1969 Sep 25;244(18):4961–4968. [PubMed] [Google Scholar]
- Murray M. G., Key J. L. 2,4-Dichlorophenoxyacetic Acid-enhanced Phosphorylation of Soybean Nuclear Proteins. Plant Physiol. 1978 Feb;61(2):190–198. doi: 10.1104/pp.61.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai C., Thomas J. A. Properties of a phosphoprotein phosphatase from bovine heart with activity on glycogen synthase, phosphorylase, and histone. J Biol Chem. 1974 Oct 25;249(20):6459–6467. [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Ralph R. K., McCombs P. J., Tener G., Wojcik S. J. Evidence for modification of protein phosphorylation by cytokinins. Biochem J. 1972 Dec;130(4):901–911. doi: 10.1042/bj1300901a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D. D., Rubin P. M. Plant Pyruvate Dehydrogenase Complex: II. ATP-Dependent Inactivation and Phosphorylation. Plant Physiol. 1977 Jan;59(1):1–3. doi: 10.1104/pp.59.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel P. F., Wells R. D. The synthesis and purification of (gamma-32P)-adenosine triphosphate with high specific activity. J Biol Chem. 1973 Dec 10;248(23):8319–8321. [PubMed] [Google Scholar]
- Titanji V. P. Purification and properties of a phosphoprotein phosphatase from rat liver. Biochim Biophys Acta. 1977 Mar 15;481(1):140–151. doi: 10.1016/0005-2744(77)90145-0. [DOI] [PubMed] [Google Scholar]
- Trewavas A. The Phosphorylation of Ribosomal Protein in Lemna minor. Plant Physiol. 1973 Apr;51(4):760–767. doi: 10.1104/pp.51.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]


