Abstract
Twenty different legume species (20 genera) were examined for α-galactosidase and hemagglutinin activities. Although all of the species contained enzyme activity, only 13 of 20 contained hemagglutinin activities and none displayed a hemagglutinin activity comparable to the previously described α-galactosidase-hemagglutinins.
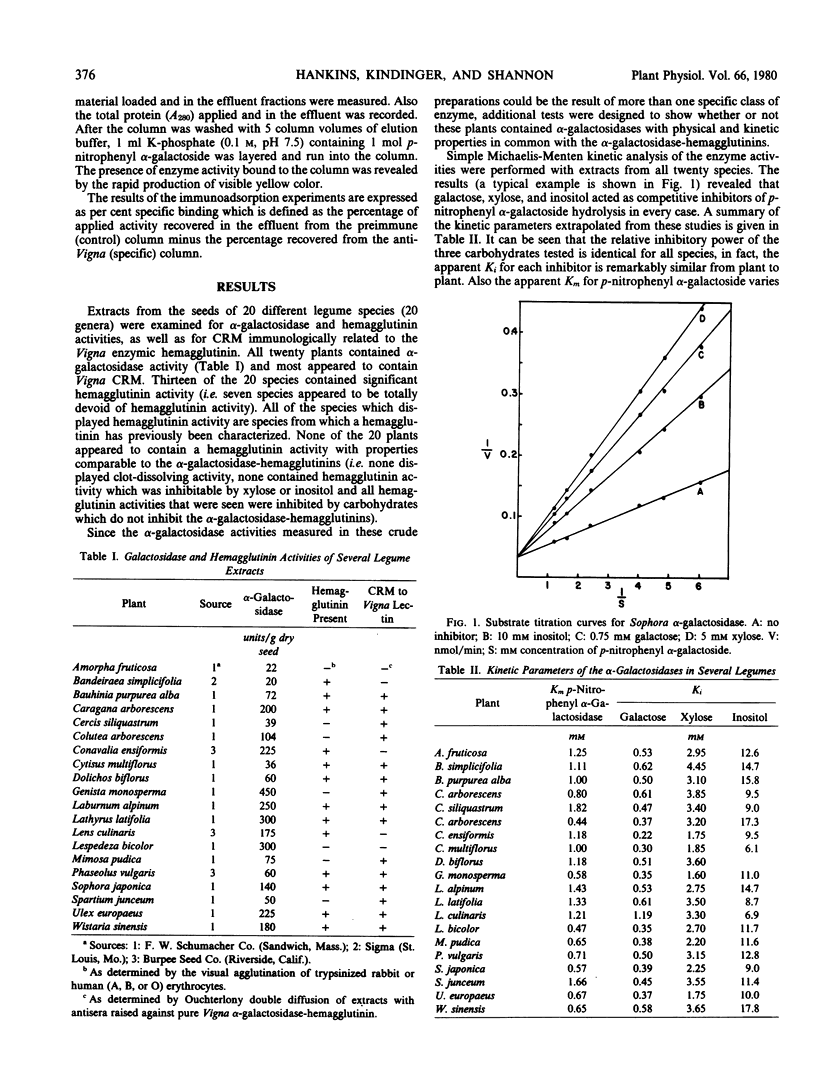
The α-galactosidase activities in the 20 species possessed remarkably similar kinetic behavior and carbohydrate specificities. All were inhibited by galactose, xylose, and inositol (very similar Ki values from plant to plant) and had very similar Km values for the substrate, p-nitrophenyl α-galactoside.
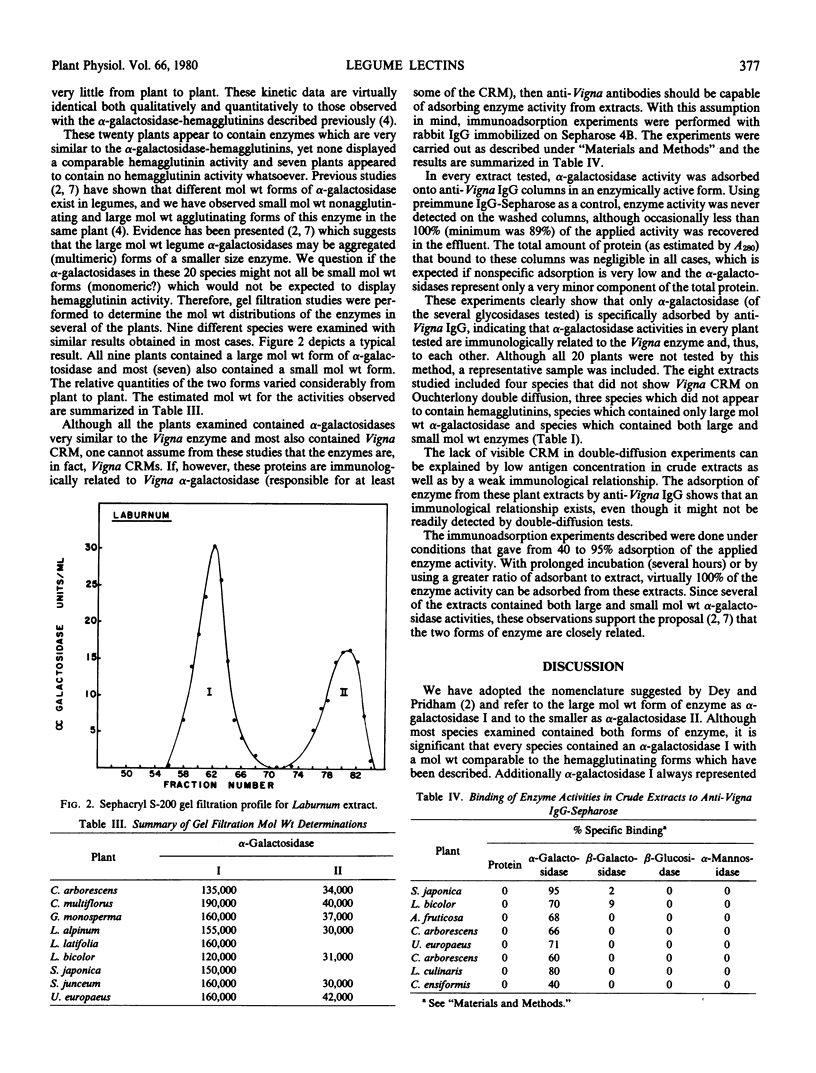
Gel filtration analysis of extracts from nine species suggests that legume α-galactosidase activities may frequently reside in two molecular weight forms. However, all these species contained a large molecular weight enzyme activity with a size comparable to the α-galactosidase-hemagglutinins.
Immunochemical studies reveal that the α-galactosidases in these plants are immunologically related to an α-galactosidase-hemagglutinin and, therefore, are related to one another.
These studies suggest that each of the legume species studied (and perhaps all members of this plant family) contain a homologue from a specific class of α-galactosidase. Although the previously described α-galactosidase-hemagglutinins appear to be members from this enzyme class, these proteins most frequently occur as forms devoid of hemagglutinin activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dey P. M., Pridham J. B. Purification and properties of alpha-galactosidases from Vicia faba seeds. Biochem J. 1969 Jun;113(1):49–55. doi: 10.1042/bj1130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Hankins C. N., Kindinger J. I., Shannon L. M. Legume Lectins: I. Immunological Cross-Reactions between the Enzymic Lectin from Mung Beans and other Well Characterized Legume Lectins. Plant Physiol. 1979 Jul;64(1):104–107. doi: 10.1104/pp.64.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins C. N., Shannon L. M. The physical and enzymatic properties of a phytohemagglutinin from mung beans. J Biol Chem. 1978 Nov 10;253(21):7791–7797. [PubMed] [Google Scholar]
- Harpaz N., Flowers H. M., Sharon N. alpha-D-galactosidase from soybeans destroying blood-group B antigens. Purification by affinity chromatography and properties. Eur J Biochem. 1977 Jul 15;77(2):419–426. doi: 10.1111/j.1432-1033.1977.tb11682.x. [DOI] [PubMed] [Google Scholar]


