Abstract
The development of JAK2 inhibitors followed the discovery of activating mutation of JAK2 (JAK2V617F) in patients with classic Philadelphia-negative myeloproliferative neoplasms (Ph-negative MPNs). It is now known that mutations activating the JAK-STAT pathway are ubiquitous in Ph-negative MPNs, and that deregulated JAK-STAT pathway plays a central role in the pathogenesis of these disorders. JAK2 inhibitors thus are effective in both patients with and without the JAK2V617F mutation. Clinical trials conducted in patients with myelofibrosis have demonstrated that these drugs lead to substantial improvements in systemic symptoms, splenomegaly, leukocytosis and thrombocytosis. Results of one randomized clinical trial suggest that JAK2 inhibition may also lead to improved survival. There are still significant challenges to be overcome, as these drugs do not improve bone marrow fibrosis and do not lead to significant reduction in the allele burden of JAK2V617F. In this manuscript we review the rationale for using JAK2 inhibitors in Ph-negative MPNs and results of more recent clinical trials with these drugs.
Keywords: Polycythemia vera, Essential thrombocythemia, Myelofibrosis, JAK2 inhibitor, JAK2 V617F
Introduction
Philadelphia-negative myeloproliferative neoplasms (Ph-negative MPN) are a group of neoplasms which share some common features including increased tendency to thrombosis and hemorrhage and an increased risk of transforming to acute myeloid leukemia (AML).1 The classic Ph-negative MPN include polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF). MF can also develop secondary to PV (post-PV MF) and ET (post-ET MF).2
In 2005, four independent groups reported on the presence of an activating mutation of the JAK2 gene (JAK2V617F) in approximately 90% of patients with PV and 60% of patient with ET and MF.3–6 The JAK2V617F mutation leads to constitutive activation of the JAK2 tyrosine kinase (TK) and increased signaling through the JAK-STAT (Signal Transducer and Activator of Transcription) pathway, causing hyper responsiveness to cytokine signaling, increased cellular proliferation, resistance to apoptosis and DNA damage.3–7 Following discovery of the JAK2V617F, other mutations were discovered in patients with JAK2V617F-negative MPN, including JAK2 exon 12 mutations (in 3% of PV patients), MPL W515K/L (in 5–10% of patients with ET and MF) and CBL mutations (in 6% of MF patients).8,9 These mutations share the common theme of leading to deregulation of the JAK-STAT pathway, further underscoring the importance of this pathway in the pathogenesis of Ph-negative MPN.10
JAK2V617F formed the rationale for the development of TK inhibitors (TKI) for treating patients with Ph-negative MPN, akin to imatinib and other successfully developed TKI for treatment of malignant neoplasms. The first clinical trials started in mid-2007, and in November 2011, the US Food and Drug Administration (FDA) approved the first JAK2 inhibitor (Ruxolitinib; Jakafi®) for treatment of MF, which is also the first drug to be approved for this disorder.11 While these drugs do not eradicate the neoplastic clone, as is seen with imatinib in chronic myelogenous leukemia, they lead to significant improvements in splenomegaly, systemic symptoms and may possibly improve survival of patients with MF. Thus, there is great benefit to be gained from these compounds. In this article we review the rationale for using these drugs and most recent clinical results.
The JAK kinases
The JAK family of TK includes JAK1, JAK2, JAK3 and TYK2. They were discovered in 1989 and were named after the two-faced Roman god Janus.12 Structurally, JAK kinases share some common domains (figure 1).13 The JH1 domain is the TK domain, responsible for the phosphorylating activity of JAK kinases.14 The adjacent JH2 domain, also called the pseudokinase domain, regulates the activity of the JH1 domain.15 Mutant JAK kinases lacking the JH2 domain display increased TK activity and phosphorylation of downstream messengers such as STAT molecules.15 It was thought that the JH2 domain did not have true kinase activity. Recent evidence, however, has proven this not to be true. The JH2 domain is a dual specificity kinase which phosphorylates key residues S523 and Y570, inhibiting JH1 TK activity.16 The V617F mutation locates in the JH2 domain, and it leads to loss of its kinase activity, thus explaining why JAK2V617F causes increased activity of the JAK2 kinase.16
Figure 1.
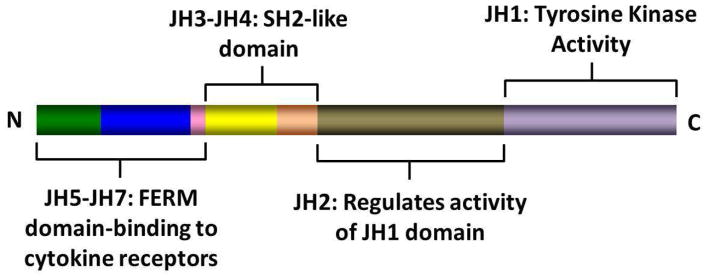
Structure of JAK kinases and domains
JAK kinases associate with the intracellular portion of cytokine receptors that lack intrinsic TK activity, such as receptor for erythropoietin (EPOR), thrombopoietin (MPL) and interferon (IFNR).17 Binding of the putative ligand leads to receptor dimerization and activation of associated JAK kinases, followed by activation of multiple intracellular signaling pathways.17 The various JAK kinases bind to different cytokine receptors. Animal models and reports of patients with germline JAK mutations have proven very instructive in our understanding of the specificity of these kinases. JAK1 mutations are essential for signaling through both type-1 (IFN-α/β) and type II (IFN-γ) receptors and also receptors containing glycoprotein 130 (gp130; e.g. receptors for interleukin 6 [IL-6] and related cytokines) and the common γc chain (e.g. receptors for IL-2, IL-7, IL-9 and IL-15).18 JAK2 is essential for erythropoiesis, as mice embryo devoid of JAK2 die during embryonic life due to failure of developing hematopoiesis.19,20 Accordingly, JAK2 is associated with receptors for EPO, thrombopoietin (TPO) and receptors using the common β chain (e.g. IL-3 receptor, granulocyte-macrophage colony stimulating factor [GM-CSF] receptor] which are essential for granulopoiesis.19,20 The JAK3 TK is associated with receptors of the IL-2 receptor family that share the common γc chain.21,22 JAK3 deficiency impairs development of both T- and B-lymphocytes, as well as NK cells, and JAK3 mutations have been described in patients with severe combined immunodeficiency, a form of primary immunodeficiency.21–23 TYK2 mediates signaling of receptors for type-I IFN (IFN-α/β) and IL-12.24,25
JAK kinases as central molecules in the pathogenesis of Ph-negative MPNs
The first hints for a role of JAK kinases in MPNs came from studies conducted in the Drosophila melanogaster fruit fly. It was found that an activating mutation (E695K) in the JH2 domain of the protein encoded by the Hopscotch gene, the JAK equivalent in Drosophila, led to an increased proliferation of hemocytes (fly blood cells) and a clinical picture reminiscent of a leukemia.26 Increased kinase activation was demonstrated, as well as increased phosphorylation of downstream target STAT92E.
Experimental studies demonstrated that the JAK2V617F oncogenic mutation leads to increased cellular proliferation and resistance to apoptosis.4 Expression of JAK2V617F in Ba/F3 cells expressing EPOR leads to increased cell proliferation and hyper responsiveness to EPO.6 Several animal models of JAK2V617F-positive MPNs have been published.27–37 Mice harboring hematopoietic stem cells and progenitor cells expressing JAK2V617F develop a PV-like disease with bone marrow hypercellularity, increased hematocrit, splenomegaly and some mice eventually develop a clinical picture compatible with MF.29,35
The phenotype acquired with the JAK2V617F mutation is secondary to activation of intracellular oncogenic signaling pathways. Central among these is the JAK-STAT pathway. JAK2V617F phosphorylates latent cytoplasmic transcription factors STAT3 and STAT5.4,6 This leads to STATs dimerization and translocation to the nucleus where they induce expression of several genes relevant to the neoplastic phenotype, including CCND1, BCLXL and BIRC5A.38,39 The role of STATs as central mediators of JAK2 aberrant signaling in MPNs has been demonstrated in recent publications. In a PV-mouse model, inducible deletion of STAT5 through a Cre-recombinase mediated mechanism essentially abolished all signs and symptoms of the disease, with normalization of the red cell mass, splenomegaly and bone marrow cellularity.40 Rescuing the STAT5 deletion with expression of only one allele restored disease features, underscoring the important role of STAT5 in the pathophysiology of MPNs. Other reports have confirmed that STAT5 is an essential mediator of JAK2V617F-mutated disease.41 Besides STATs, JAK2V617F can activate the oncogenic pathways PI3K-Akt-mTOR (phosphatydil-inositol-3 kinase-Akt-mammalian target of rapamycin) and Ras-Raf-MEK-ERK (MAPK, mitogen activated protein kinases).42–44 Patients with JAK2V617F-negative MPNs also have increased STAT5, Akt and Erk phosphorylation, demonstrating that activation of these pathways is a common feature of all patients with Ph-negative MPNs.10
JAK kinases may also contribute to oncogenesis by inducing epigenetic changes in the genome. It has been demonstrated that activated JAK can be found in the nucleus of hematopoietic cells, where it phosphorylates histone H3 at residue Y41.45 This inhibits binding of Heterochromatin Protein 1α (HP1α), which is responsible for gene silencing through epigenetic mechanisms. This epigenetic deregulation induced by JAK2 increases expression of oncogene LMO2, and inhibiting JAK2 increased HP1α phosphorylation and decreased LMO2 expression.45 Nuclear JAK2 has been demonstrated in the CD34+ cells of patients with Ph-negative MPNs.46 Thus, JAK2 may regulate gene expression not only through activation of oncogenic molecules, such as STAT5, but also through epigenetic deregulation.
More recently, the role of cytokines has gained greater importance in the pathophysiology of Ph-negative MPNs, particularly MF. Several pro-inflammatory and pro-fibrotic cytokines (e.g. transforming growth factor-β, IL-1b, IL-2, IL-6, IL-8, IL-12, IL-15, tumor necrosis factor-α [TNF-α]) have been found to be elevated in patients with MF and PV.47,48 Cells that are responsible for cytokine production include neoplastic megakaryocytes, monocytes and bone marrow stromal cells.49,50 These cytokines are associated with many of the clinical features of Ph-negative MPNs, including bone marrow fibrosis, osteosclerosis, constitutional symptoms, hematopoietic stem cell mobilization and transfusion-dependent anemia.47 In one recent report, increased levels of cytokines IL-8, IL-2R, IL-12, IL-15 and IP-10 (IFN-γ inducible protein 10) were found to be associated with decreased overall survival in patients with MF.47 Several of these cytokines are dependent on the JAK-STAT for intracellular signaling, and STAT3 activation increases autocrine production of pro-inflammatory cytokines such as IL-6.51 Additionally, increased cytokine signaling may lead to resistance to JAK2 inhibitors. Knock-down of the JAK2V617F gene with small interfering RNA inhibited proliferation of JAK2V617F positive cells or CD34+ cells from patients with MPNs.52 However, addition of IL-3 and TPO impeded growth inhibition and increased STAT5 activation. In another study, co-culture of JAK2V617F cells with bone marrow stromal cells blocked JAK2 inhibition by the compound atiprimod.53 This protective effect of stromal cells was due to their production of pro-inflammatory cytokines IL-6 and IP-10.
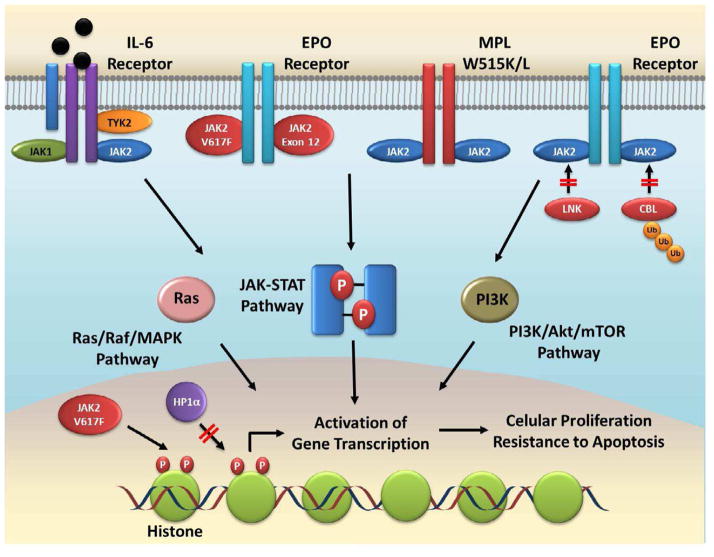
In conclusion, the following picture emerges from our current understanding of the pathophysiology of Ph-negative MPNs (Figure 2). These disorders are caused by mutations that lead to chronic, persistent activation of the JAK-STAT pathway in hematopoietic stem cells. Mutations can either directly activate the JAK2 kinase (e.g. JAKV617F, JAK2 exon 12 mutation) or indirectly (e.g. MPL mutation, CBL mutation). Activation of the JAK-STAT pathway leads to increased cellular proliferation, resistance to apoptosis, genetic instability and acquisition of further mutations. Epigenetic effects of JAK activation and the balance between STAT1 and STAT5 activation are likely related to the different disease phenotypes associated with these various mutations.54 Chronic JAK-STAT activation also leads to increased production of pro-inflammatory cytokines, which further contribute to disease pathogenesis and activation of the pathway. While the JAK2V617F mutation is not detected in all patients with Ph-negative MPNs, activation of JAK kinases (mutated or wild-type) remains at the center of the pathogenesis of probably most patients with these disorders.10 Thus, the development of drugs with ability to inhibit chronic JAK-STAT signaling is an important goal towards achieving effective therapeutic agents for these patients.
Figure 2. Central role of JAK kinases in Pathogenesis of Ph-negative MPNs.
JAK-STAT signaling is activated in most, if not all, patients with the classic Ph-negative MPNs. This can be due to direct (JAK2 exon 12, JAK2V617F) or indirect (MPLW515L/K, LNK and CBL mutations) activation of the JAK2 kinase. The presence of pro-inflammatory cytokines such as IL-6 also contributes to STAT activation. Besides STATs, JAK2 kinases can also activate other oncogenic pathways such as the PI3K and MAPK pathways. Nuclear JAK2 leads to epigenetic deregulation by phosphorylating histone proteins and blocking binding of epigenetic regulators such as HP1α. The final consequence of activation of these pathways is expression of genes associated with cellular proliferation and resistance to apoptosis.
Results of JAK2 inhibitors in Myelofibrosis
Several JAK2 inhibitors are in currently development for therapy of Ph-negative MPNs (Table 1). They differ in their specificity against JAK2 kinases, and their main therapeutic benefits in patients with MF are reduction of splenomegaly and improvement in systemic symptoms.
Table 1.
JAK2 inhibitors for therapy of Ph-negative MPNs
| Compound | Disease | Target | Responses | Toxicity |
|---|---|---|---|---|
| Ruxolitinib60,61 | MF | JAK1, JAK2 | Splenomegaly-28–44% Improvement in MF-related symptoms, leukocytosis, thrombocytosis, overall survival |
Anemia, Thrombocytopenia, Dizziness Headache |
| Ruxolitinib77 | PV/ET | JAK1, JAK2 | PV-ORR 100% (CR 62%) ET-ORR 62% (CR 41%) |
Anemia, Thrombocytopenia, Dizziness Headache |
| SAR30250365 | MF | JAK2 | Splenomegaly-39% Improvement in MF-related symptoms, leukocytosis, thrombocytosis, JAK2V617F allelic burden |
Anemia, Thrombocytopenia, Diarrhea, Nausea, Vomiting, Elevated Pancreatic enzymes |
| CYT38768 | MF | JAK1, JAK2, TYK2 | Splenomegaly-31% Improvement in MF-related symptoms Transfusion independence-46% |
Thrombocytopenia, first dose effect, peripheral neuropathy |
| Pacritinib70 | MF | JAK2, FLT3 | Splenomegaly-39% Improvement in MF-related Symptoms |
Diarrhea, Nausea, Vomiting Can be safely used in patients with cytopenias |
| LY278454472 | MF | JAK2V617F | Splenomegaly-41% Improvement in MF-related symptoms, bone marrow fibrosis |
Tumor lysis syndrome, creatinine increase |
| NS-01873 | MF | JAK2, SRC Kinases | NA | NA |
| BMS-91145374 | MF | JAK2 | NA | NA |
CR, complete response, ET, essential thrombocythemia; MF, myelofibrosis; NA, not available; ORR, overall response rate; PV, polycythemia vera
Ruxolitinib
Ruxolitinib (formerly known as INCB018424; Incyte, Wilmington, DE) is an orally available, dual JAK1- and JAK2-inhibitor that has been recently approved for therapy of patients with intermediate or high risk MF, either primary or post-PV/ET. In pre-clinical evaluation it was demonstrated that ruxolitinib inhibited both JAK1 (half-maximal concentration [IC50]=3.3nM) and JAK2 (IC50=2.8nM), while sparing JAK3 (IC50=322nM).55 Ruxolitinib inhibited proliferation of JAK2V617F positive cell lines and CD34+ cells from patients with MPNs, and this was associated with decreased phosphorylation of STAT3.55 In mouse models, ruxolitinib led to spleen reduction, weight gain and improved cytokine profile.
A phase I/II clinical recruited 153 patients with MF to be treated with ruxolitinib at different doses and schedules.56 The dose limiting toxicity (DLT) was thrombocytopenia, and maximum tolerated doses were 25 mg twice daily or 50 mg once daily. However, a dose adjusted schedule whereupon patients started with 15 mg twice daily (10 mg if platelet count between 100×109/L and 200×109/L) with further monthly dose increments if there was no response or side effect led to the best balance between efficacy and toxicity. A total of 61 among 140 patients had a clinical improvement (CI) in spleen size by the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) response criteria (i.e. ≥50% spleen size reduction by physical exam).57 This was demonstrated to correspond to a 35% decrease in spleen volume by magnetic resonance imaging (MRI). Responses were observed in JAK2V617F-positive and - negative patients. After 1 year, 73% of patients who started with 15 mg twice daily and 78% of patients who started with 25 mg twice daily maintained their response. There was substantial improvement in systemic symptoms, including night sweats, pruritus, fatigue, bone pain and abdominal pain, as well as improved ability to walk.56 Improvement in symptoms was accompanied by a decrease in pro-inflammatory cytokine levels (e.g. TNF-α, IL-6, IL-1ra). Reduction in intracellular phosphorylated STAT3 was observed after treatment with ruxolitinib, consistent with on-target activity.
More recently, two reports have focused on longer follow-up of patients who were enrolled in the phase I/II trial.58,59 In one report, the outcomes of 107 patients with MF who were enrolled and treated at M.D. Anderson Cancer Center (MDACC) was compared to an historical cohort of 310 patients identified from three large MPN databases.58 The historical cohort was matched to the MDACC cohort based on eligibility criteria for the phase I/II trial. In the MDACC cohort, after a median follow-up of 32 months, 54% of patients remained on study. Median duration of spleen response had not been reached at the time of report. Most common reasons for discontinuation were death (12.1%) and progressive disease (11.2%). Compared to the historical cohort, therapy with ruxolitinib significantly improved survival outcomes, with a hazard ratio (HR) of 0.61 (95% confidence interval [CI] 0.41–0.89; p=0.022) for overall survival (OS).58 This effect was more pronounced in the subgroup of patients with a high risk IPSS (HR=0.50; 95% CI 0.31–0.81; p=0.006).58 In contrast, the report from the Mayo Clinic on 51 patients treated with ruxolitinib at that center did not reveal an improvement in OS compared to a cohort of 410 patients with MF from all IPSS risk categories treated with standard therapy (unadjusted p=0.43; p=0.58 after adjusting for the Dynamic International Prognostic Scoring System-Plus [DIPSS-Plus] score).59 The rate of treatment discontinuation was very high in the Mayo Clinic cohort (51% at 1 year, 89% at 3 years), and this may have affected the results obtained.59 Most common reasons for discontinuation in the Mayo cohort were patient withdrawal of consent (29.4%), physician decision (23.5%) and disease progression (19.6%).
Ruxolitinib was further evaluated in two phase III trials for patients with MF. In the trial conducted in North America and Australia (COMFORT-I), 309 patients with MF and Int-2/High IPSS risk were randomized among ruxolitinib and placebo in a 1:1 fashion.60 Starting dose was 15 mg twice daily for those patients with a platelet count between 100–200×109/L, and 20 mg twice daily for those patients with platelet count higher than 200×109/L. The primary endpoint was the percentage of patients with a reduction in spleen volume by MRI ≥ 35% at 24 weeks. After a median follow-up of 32 weeks, the primary endpoint had been reached in 41.9% of patients in the ruxolitinib group versus 0.7% of patients in the placebo group (p<0.0001).60 Patients receiving ruxolitinib had a mean decrease in spleen volume of 31.6% vs. an increase of 8.1% in patients receiving placebo. Similar to phase I/II trial data, there were significant improvements in symptoms related to MF.60 The Myelofibrosis Symptom Assessment Form Total Symptom Score (TSS; sum of scores for itching, night sweats, bone/muscle pain, abdominal discomfort, pain under the ribs on the left and early satiety) decreased ≥50% in 46% of patients in the ruxolitinib arm versus 5.3% of patients in the placebo arm (p<0.001). The efficacy of ruxolitinib in decreasing splenomegaly and improving systemic symptoms was independent of the presence of the JAK2V617F mutation. There was reduction in pro-inflammatory cytokines and an increase in leptin and EPO levels. After a median follow-up of 51 weeks, a planned data cutoff analysis for safety revealed that ruxolitinib improved survival compared to placebo (HR 0.5; p=0.04).60
In the companion trial COMFORT-II, 219 patients with Int-2/High risk MF were randomized 2:1 to receive ruxolitinib or best available therapy.61 The primary endpoint was percentage of patients with a ≥35% reduction in spleen volume by MRI at 48 weeks. In the ruxolitinib arm, 28% of patients reached the primary endpoint, versus 0% in control arm (p<0.001).61 Responses were durable, and after 12 months of follow-up 80% of responding patients receiving ruxolitinib still maintained response. There was also significant improvement in quality of life and symptoms associated with MF, including fatigue, insomnia and appetite loss in patients receiving ruxolitinib, while a worsening of these symptoms were observed in patients in the best available therapy group.61 There was no improvement in OS in patients receiving ruxolitinib, but the study was underpowered to detect such a difference.
Ruxolitinib is a relatively well tolerated medication. Most non-hematological side effects are grade 1–2 in severity, and treatment discontinuations due to side effects were uncommon in both phase III trials (11% and 8%).60,61 Side effects that occurred more frequently with ruxolitinib compared to placebo in the COMFORT-I study included ecchymosis (18.7% vs. 9.3%), headache (14.8% vs. 5.3%) and dizziness (14.8% vs. 6.6%). These side effects were all grade 1–2 in severity, with the exception of one case of grade 3–4 dizziness.60 Due to its mechanism of action, cytopenias are a common side effect of ruxolitinib. Compared to placebo, grade 3–4 anemia occurred in 25% more patients in the ruxolitinib arm of the COMFORT-I phase III trial, while grade 3–4 thrombocytopenia was documented in an excess of 12% of cases.60 Severe (grade 3–4) neutropenia was uncommon (7% in one trial).60 Data from both phase III trials demonstrated a clear time-dependent pattern of anemia severity during therapy with ruxolitinib: hemoglobin drops approximately 1.5 g/dL during the first 8–12 weeks of therapy, reaching a mean nadir value of 9.4 g/dL. By 24 weeks of therapy, hemoglobin increases to a new mean steady state of 10.1 g/dL. This pattern was independent of transfusions and dose modifications. Most cases of grade 3–4 thrombocytopenia happened during the first 8 weeks of therapy with ruxolitinib and were managed with dose reductions and treatment interruptions. The monthly prevalence of thrombocytopenia decreased to placebo levels with 24 weeks of therapy.60 Grade 3–4 bleeding episodes happened in 3.9% of patients being treated with ruxolitinib and 3.3% of patients in the placebo arm. Despite its high incidence, few patients (only one in each trial) discontinued ruxolitinib due to cytopenias.60,61
Patients who discontinue ruxolitinib have a rapid relapse of symptoms, usually within 7 days of therapy interruption.60 One report has stated that patients who discontinue therapy with ruxolitinib may develop a cytokine rebound syndrome, with acute spleen enlargement and a shock-like syndrome.62 In the COMFORT-I trial, adverse events after treatment interruption were not more commonly seen with ruxolitinib. Grade 3 events occurred in 16% of patients in the ruxolitinib arm compared to 13% of patients in the placebo arm.60 No clear pattern suggestive of a withdrawal effect was seen. Severe adverse events occurred in only 3 patients in each treatment arm (6.1% [ruxolitinib] vs. 5.6% [placebo]).60 Eleven percent of patients in each arm of the COMFORT-I study discontinued therapy due to side effects.
The FDA approved ruxolitinib for the treatment of patients with intermediate and high risk MF. Despite this, ruxolitinib is still being evaluated in clinical trials. Alternative dose schedules for patients with thrombocytopenia (50–100×109/L) are being studied (NCT01348490, NCT01317875), as well as a sustained release formulation (NCT01340651). Combination trials with lenalidomide (NCT01375140) and panobinostat (NCT01433445) have recently started.
SAR302503
SAR302503 (formerly known as TG101348; Sanofi S.A., Paris, France) is an orally available TKI that has selective activity versus JAK2 (IC50=3nM).63,64 SAR302503 has limited activity against JAK1 (IC50=105 nM) and no activity against JAK3 (IC50=996nM). Pre-clinical evaluation of SAR302503 demonstrated it to be a potent inhibitor of JAK2V617F oncogenic signaling and had in vivo activity in a mouse model of JAK2V617F-positive MPN.63,64
SAR302503 has been evaluated in a phase I/II clinical trial which recruited 59 patients with MF, and the majority (86%) was JAK2V617F-positive.65 The MTD was determined to be 680 mg once daily, and DLT included asymptomatic grade 3–4 hyperamylasemia and hyperlipasemia. After 6 cycles of therapy, a spleen response by IWG-MRT criteria was seen in 39% of patients, and the spleen response was 45% in the MTD cohort.65 Median time to response was 113 days, and mean duration of spleen response was 315 days. SAR302503 led to normalization of leukocytosis and thrombocytosis in 56% and 90% of cases, respectively. The drug led to improvements in systemic symptoms, including cough, early satiety, fatigue, night sweats and pruritus.65 No decrease in pro-inflammatory cytokines was observed in this trial, suggesting that this drug has more of an anti-proliferative than an anti-cytokine effect.65 A decrease in JAK2V617F allele burden has been reported with this drug. At baseline, the median allele burden was 20% (range 3–100%), and it decreased to 9% (range 0–100%; p=0.03) after 24 cycles of treatment.65 A similar effect was seen in patients who presented with baseline JAK2V617F burden greater than 20% (baseline median 60% [range 23–100%]; post 24 cycles of therapy, median 21% [range 6–100%]; p=0.03).65
Toxicities of SAR302503 include cytopenias, gastrointestinal toxicity and elevation of pancreatic enzymes. In the phase I/II study, anemia was reported in 43.2% of patients, and it was grade 3–4 in 35% of cases.65 Thrombocytopenia occurred in 40.7% (grade 3–4 23.7%) and neutropenia in 4.4% (grade 3–4 1%). Among non-hematological adverse events, diarrhea (all grades 64%; grade 3–4 10%), nausea (all grades 69.5%, grade 3–4 3.4%) and vomiting (all grades 57.6%, grade 3–4 3.4%) were the most commonly reported. In the MTD cohort of patients (N=40), 70% of patient required dose reductions during the first 6 cycles of therapy, more commonly due to gastrointestinal toxicity or cytopenias (32.5% of cases).65
SAR302503 is highly effective for treating patients with MF, but has a relative high incidence of side effects at the MTD. A lower starting dose may provide similar efficacy with more manageable toxicity. Currently, a randomized trial of SAR302503 versus placebo for patient with Int-2/High risk MF is recruiting, and patients randomized to the drug will receive SAR302503 at doses of 400 or 500 mg once daily (NCT01437787).
CYT387
The compound CYT387 (YM Biosciences, Mississauga, Canada) is an orally available JAK1 (IC50=11nM), JAK2 (IC50=17nM) and TYK2 (IC50=17nM) inhibitor.66 Pre-clinical activity of CYT387 was demonstrated against both JAK2V617F- and MPL-mutated cell lines.66,67
A phase I/II clinical trial of CYT387 reported on 166 patients with MF (primary=65%; post-PV=22%; post-ET=14%) treated with CYT387 at different doses and schedules.68 The MTD was 300 mg once daily, and DLTs were headache and hyperlipasemia. After a median follow-up of 10.4 months, the response rate on splenomegaly by IWG-MRT criteria was 31%.68 Responses were rapid, occurring at a median of 15 days post-treatment start. There was also significant improvement in MF-related symptoms, including bone pain (49% improvement), cough (49%), fever (100%), night sweats (80%) and pruritus (74%). An improvement in erythropoiesis and transfusion dependency has been seen with this drug. Among 68 patients (41%) who were transfusion-dependent at baseline, according to IWG-MRT criteria, 46% achieved transfusion independence and a Hb level ≥ 8 g/dL.68 Median time to transfusion independence was 84 days. Median duration has not been reached.
Similar to other compounds of this class, CYT387 can induce thrombocytopenia (all grades 33%, grade 3–4 17%).68 Neutropenia and anemia have also been reported, albeit at much lower rates (grade 3–4 3% and 1%, respectively). The first dose effect is a transient dizziness and lightheadedness which may be accompanied by hypotension and occurs after the patient takes the first dose of the drug.68 It has been reported in 20% of patients, and is usually grade 1 in severity. Peripheral neuropathy (grade 1–2 only, 20%), nausea (grade 1–2 17%), and diarrhea (grade 1–2 18%) have also occurred with this medication.
The compound CYT387 is highly effective for inducing spleen responses and improvement in constitutional symptoms, while possessing a relative favorable side effect profile, similar to other JAK2 inhibitors. The reported rates of transfusion independence are encouraging and need to be further explored in additional trials. Despite this, it should be mentioned that the IWG-MRT criteria for transfusion independence are suboptimal, since in the COMFORT-I trial a transfusion-independence rate of 47% was reported in patients in the placebo, underscoring the need for better and improved criteria.60
Other JAK2 Inhibitors in Clinical Development
The JAK2 inhibitor pacritinib (formerly known as SB1518; Cell Therapeutics, Inc., Seattle, WA) has selective activity against JAK2 (IC50=19 nM) and is in clinical trials at the moment.69 Phase I studies determined the MTD to be 500 mg/day, but pharmacokinetic analysis recommended a dose of 400 mg/day since no increase in plasma level is seen above this dosage. A phase II study has recruited 31 patients, and recently presented data demonstrated that pacritinib improves splenomegaly (39% by physical examination; 55% had a ≥ 25% reduction in spleen volume by MRI) and constitutional symptoms (31–78% improvement in Myelofibrosis Symptom Assessment Form scores by the 7–10th cycle).70 Adverse events consisted mostly of diarrhea (all grades 87%; grade 3–4 10%), nausea (all grades 45%; grade 3–4 6%) and vomiting (all grades 29%; grade 3–4 3%). Pacritinib appears to induce few treatment-related cytopenias. Rates of grade 3–4 thrombocytopenia and anemia are low (14% and 5%, respectively) and the drug could be safely used in patients with platelet count < 100×109/L.70 Spleen response was not affected by pre-treatment platelet counts (35% [platelet < 150×109/L] vs. 39% [all patients]).
LY2784544 (Eli Lilly, Indianopolis, IN) is an orally available, selective inhibitor of JAK2V617F (IC50=55nM), harboring no activity against wild type JAK2 (IC50=2.26μM).71 Preclinical studies demonstrated that LY2784544 inhibited JAK2V617F and STAT5 phosphorylation, induced cell cycle arrest and apoptosis, and reduced growth of Ba/F3-JAKV617F-GFP tumor cells xenografts in mice.71 At the same time, LY2784544 did not inhibit proliferation of cells expressing wild type JAK2 (IC50=1.356μM).71 Preliminary results of a phase I clinical trial of LY2784544 have been presented in abstract form.72 Nineteen patients (MF=18, PV=1) were recruited, and the MTD was reported to be 120 mg. DLTs included hyperuricemia and creatinine increase. There were 4 cases of tumor lysis syndrome, 3 of them with clinical consequences.72 Regarding response rates, of 17 evaluable patients, 41% had a ≥50% reduction in splenomegaly.72 A ≥50% reduction in MPN-related symptoms was observed in 59% of patients. Additionally, very preliminary observations suggest that an improvement in bone marrow fibrosis could be obtained, as 3 of 5 patients with follow-up bone marrow biopsies had a reduction in the severity of marrow fibrosis.72 The study is ongoing and alternative dose schedules in order to decrease toxicity will be explored.
NS-018 (Nippon Shinyaku Co., Ltd, Kyoto, Japan) is a JAK2-specific inhibitor that also has activity against Src family kinases.73 In vitro, NS-018 inhibits JAK2 (IC50=0.72 nM), has limited activity against JAK1, JA3 and TYK2, and can inhibit several Src-family kinases including SRC, FYN and YES.73 NS-018 inhibits JAK2 phosphorylation in Ba/F3 cells expressing EPOR and JAK2V617F and demonstrated activity in a mouse model of JAK2V617F-induced MPN, with resolution of splenomegaly, extramedullary hematopoiesis and leukocytosis.73 NS-018 is being tested in phase I/II clinical trial in patients with MF (NCT01423851).
BMS-911543 (Bristol-Myers Squibb, New York, NY) is a JAK2 kinase selective inhibitor (IC50=1 nM) that has virtually no activity against other JAK family members and other target kinases.74 BMS-911543 inhibited proliferation of cells expressing JAK2V617F, and had no effect was seen in cell lines dependent on JAK3 for proliferation. The drug inhibited proliferation of primary CD34+ cells from patients with MPNs (IC50=0.15–0.9μM), while having limited affect against control CD34+ cells (IC50=1.5μM).74 A phase I/II clinical trial in patients with MF will provide further information on the clinical activity of BMS-911543 (NCT01236352).
Clinical results in Polycythemia Vera and Essential Thrombocythemia
Most clinical studies with JAK2 inhibitors have focused on patients with MF, who usually have few available therapeutic options. Patients with PV and ET usually have a more benign clinical course than MF, and are typically managed with phlebotomies (for PV), aspirin and cytoreductive agents such as hydroxyurea and pegylated interferon-α2a. The role of JAK2 inhibitors for these patients is unclear. However, a fraction of patients with PV/ET who need cytoreductive therapy will be either intolerant or resistant to currently available agents.75,76 A phase I/II trial evaluated ruxolitinib in this population of patients.77 Inclusion criteria were a diagnosis of ET or PV with need for cytoreduction and intolerance or resistance to hydroxyurea, the most commonly employed agent for this purpose. Additional inclusion criteria were hematocrit > 45% and/or phlebotomy-dependence (for PV) and platelet count > 650×109/L (for ET).
A total of 73 patients were recruited (PV=34; ET=39). The MTD was 10 mg twice daily (PV) and 25 mg twice daily (ET).77 The JAK2V617F mutation was present in 100% of PV patients and 67% of ET patients. In PV, therapy with ruxolitinib led to a decrease in hematocrit with phlebotomy independence in 97% of patients, and all continued to maintain response at last follow-up.77 A ≥50% decrease in spleen size was seen in 80% of patients, as well as normalization of leukocytosis (73%) and thrombocytosis (69%) and improvements in pruritus, night sweats and bone pain. The overall response rate (ORR) by European LeukemiaNet criteria78 was 97%, including a complete remission (CR) rate of 50% and a partial response rate of 47%.77 As expected from the ruxolitinib toxicity profile, most common side effects were anemia (grade 1– 2 only, 74%), thrombocytopenia (all grades 29%, grade 3–4 6%) and leukopenia (grade 1–2 only 15%). A downward trend in the JAK2V617F allele burden was seen in 4 patients with PV. Responses did not correlate with JAK2V617F allele burden. In patients with ET, ruxolitinib normalized platelet count after a median of 15 days in 49% of patients, and in 79% the platelet count went down <600×109/L.77 Four patients had palpable splenomegaly and had achieved a non-palpable splen (N=3) or a ≥50% reduction in size (N=1). The ORR was 90%, with a CR rate of 26% and a PR rate of 64%.77 The most frequent side effects in the ET cohort were anemia (grade 1–2 only, 74%) and weight increase (grade 1–2 only 23%).
Despite these excellent results, there is still uncertainty regarding the role of JAK2 inhibitors in the routine management of patients with PV and ET. Currently, there is a randomized, open-label phase III trial evaluating ruxolitinib (10 mg twice daily) against best available therapy in patients with hydroxyurea-resistant or –intolerant patients with PV (RESPONSE trial; NCT01243944). The primary endpoint is the proportion of patients who achieve a response (absence of phlebotomy and ≥35% reduction in spleen volume as determined by MRI or CT) at 32 weeks of therapy. Results of this trial will help to determine if ruxolitinib is superior to alternative agents in this population of patients with PV.
Resistance to JAK2 inhibitors
Currently, the exact mechanisms of resistance to JAK2 inhibitors in patients with MF and other Ph-negative MPNs remain largely unknown. In chronic myelogenous leukemia, resistance to imatinib is often (40–50%) associated with point mutations in the kinase domain of BCR-ABL1. These mutations interfere with the binding of imatinib to BCR-ABL1, or change the oncoprotein conformation to an active state that imatinib cannot bind. In vitro exposure of JAK2V617F-positive cells to JAK2 inhibitors can induce mutations associated with resistance to JAK2 inhibitors.79 The most relevant mutations include Y931C, G935R, R938L, I960V and E985K. These mutations interfere with binding of JAK2 inhibitors to the kinase domain of JAK2, and confer resistance to all available JAK2 inhibitors.79 The gatekeeper mutation M929I in JAK2 is homologous to the T315I mutation described in BCR-ABL1, but it only alters sensitivity to ruxolitinib, with no cross resistance to other JAK2 inhibitors.79 No studies have been published so far demonstrating the presence of JAK2 mutations associated with resistance to JAK2 inhibition in patients treated with these compounds.
Besides mutation of JAK2, other mechanisms may operate that are associated with resistance to these drugs. It is known that JAK2 inhibition does not seem to eradicate the malignant clone, as evidenced by the lack of significant reduction in JAK2V617F allele burden in most clinical trials in MF, absence of improvement in fibrosis and by the rapid relapse in symptoms and splenic enlargement once therapy is discontinued. This persistence of malignant cells suggests maintenance of JAK2 signaling in the setting of chronic JAK2 inhibition. To further elucidate this, in one study the authors generated mutant JAK2/MPL cells that had persistent JAK2 signaling in the presence of JAK inhibitors (e.g. ruxolitinib, SAR302503).80 Evaluation of intracellular signaling pathways revealed that JAK2 was activated in trans by other JAK kinases, such as JAK1 and TYK2, and there was increased heterodimerization of JAK2 with other JAK kinases.80 Increased expression of JAK2 was found in JAK2/MPL persistent cells, as well as in samples from patients treated with JAK2 inhibitors who did not have a response to the drug, indicating that the cells remained dependent on JAK2 for survival.80 This increased expression was secondary to epigenetic abnormalities and changes in histone methylation in the JAK2 gene locus. Targeting JAK2 degradation by other pathways such as Heat Shock Protein 90 inhibition may lead to signaling inhibition and may be potentially used as therapeutic agents in conjunction with JAK2 inhibitors.81
Conclusions
Results of clinical trials have demonstrated that JAK2 inhibitors are a significant addition to roster of therapeutic agents in MF. These drugs can significantly decrease spleen size, improve systemic symptoms, decrease leukocytosis and thrombocytosis and improve patients’ quality of life. Results of a randomized trial and a retrospective analysis compared to a historical cohort suggest that these drugs may also improve the survival of patients with MF, which has not been achieved with any conventional drug therapy so far in this disease.58,60 These benefits, however, come at the cost of side effects, mainly cytopenias which can lead to transfusion requirements in a percentage of patients. Fortunately, in most situations drug-induced cytopenias can be transient and are managed with drug interruptions and/or dose reduction. Better understanding of intrinsic and acquired mechanisms of resistance to these drugs is needed, as no major reduction in JAK2V671F allele burden has been observed during therapy with these drugs, and symptoms recur rapidly once the medication is stopped, indicating that JAK2 inhibitors do not eradicate the malignant clone. Future studies conducted with these compounds as single agents and in combination therapy will help to further improve on the results obtained with JAK2 inhibitors at present time.
KEY POINTS.
Activation of JAK-STAT pathway is at the center of pathogenesis of most cases of Ph-negative MPNs
Activation of JAK-STAT in these disorders is dependent on JAK2, which can be activated either directly or indirectly through activating mutations of JAK2 and related molecules
Therapy with JAK2 inhibitors in myelofibrosis is associated with significant improvements in splenomegaly, constitutional symptoms, leukocytosis and thrombocytosis
No significant improvement in bone marrow fibrosis and JAK2 allelic burden is usually seen with these drugs
Cytopenias are the most common side effects observed with JAK2 inhibitors
Footnotes
Conflicts of interest: FPSS has received research funding from Novartis; SV has received research support from Incyte
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thiele J, Kvasnicka HM, Tefferi A, et al. Primary Myelofibrosis. In: Swerdlow SH, Campo E, Harris NL, et al., editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 44–47. [Google Scholar]
- 2.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 3.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 4.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 5.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 6.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Plo I, Nakatake M, Malivert L, et al. JAK2 stimulates homologous recombination and genetic instability: potential implication in the heterogeneity of myeloproliferative disorders. Blood. 2008;112:1402–12. doi: 10.1182/blood-2008-01-134114. [DOI] [PubMed] [Google Scholar]
- 8.Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–68. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472–6. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- 10.Anand S, Stedham F, Gudgin E, et al. Increased basal intracellular signaling patterns do not correlate with JAK2 genotype in human myeloproliferative neoplasms. Blood. 2011;118:1610–21. doi: 10.1182/blood-2011-02-335042. [DOI] [PubMed] [Google Scholar]
- 11. [Accessed on 04/10/2012];FDA Approval of Ruxolitinib. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm280155.htm.
- 12.Wilks AF. Two putative protein-tyrosine kinases identified by application of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989;86:1603–7. doi: 10.1073/pnas.86.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giordanetto F, Kroemer RT. Prediction of the structure of human Janus kinase 2 (JAK2) comprising JAK homology domains 1 through 7. Protein Eng. 2002;15:727–37. doi: 10.1093/protein/15.9.727. [DOI] [PubMed] [Google Scholar]
- 14.Wilks AF, Harpur AG, Kurban RR, et al. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol. 1991;11:2057–65. doi: 10.1128/mcb.11.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saharinen P, Silvennoinen O. The pseudokinase domain is required for suppression of basal activity of Jak2 and Jak3 tyrosine kinases and for cytokine-inducible activation of signal transduction. J Biol Chem. 2002;277:47954–63. doi: 10.1074/jbc.M205156200. [DOI] [PubMed] [Google Scholar]
- 16.Ungureanu D, Wu J, Pekkala T, et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat Struct Mol Biol. 2011;18:971–6. doi: 10.1038/nsmb.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 18.Rodig SJ, Meraz MA, White JM, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–83. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 19.Neubauer H, Cumano A, Muller M, et al. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 20.Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–95. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 21.Nosaka T, van Deursen JM, Tripp RA, et al. Defective lymphoid development in mice lacking Jak3. Science. 1995;270:800–2. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 22.Thomis DC, Gurniak CB, Tivol E, et al. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:794–7. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 23.Russell SM, Tayebi N, Nakajima H, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 24.Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549–60. doi: 10.1016/s1074-7613(00)00054-6. [DOI] [PubMed] [Google Scholar]
- 25.Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561–71. doi: 10.1016/s1074-7613(00)00055-8. [DOI] [PubMed] [Google Scholar]
- 26.Luo H, Rose P, Barber D, et al. Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol Cell Biol. 1997;17:1562–71. doi: 10.1128/mcb.17.3.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akada H, Yan D, Zou H, et al. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood. 2010;115:3589–97. doi: 10.1182/blood-2009-04-215848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bumm TG, Elsea C, Corbin AS, et al. Characterization of murine JAK2V617F-positive myeloproliferative disease. Cancer Res. 2006;66:11156–65. doi: 10.1158/0008-5472.CAN-06-2210. [DOI] [PubMed] [Google Scholar]
- 29.Lacout C, Pisani DF, Tulliez M, et al. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108:1652–60. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Spensberger D, Ahn JS, et al. JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood. 2010 doi: 10.1182/blood-2009-12-259747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marty C, Lacout C, Martin A, et al. Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood. 2010 doi: 10.1182/blood-2009-12-257063. [DOI] [PubMed] [Google Scholar]
- 32.Mullally A, Lane SW, Ball B, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010;17:584–96. doi: 10.1016/j.ccr.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shide K, Shimoda HK, Kumano T, et al. Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia. 2008;22:87–95. doi: 10.1038/sj.leu.2405043. [DOI] [PubMed] [Google Scholar]
- 34.Tiedt R, Hao-Shen H, Sobas MA, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–40. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- 35.Wernig G, Mercher T, Okabe R, et al. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107:4274–81. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing S, Wanting TH, Zhao W, et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111:5109–17. doi: 10.1182/blood-2007-05-091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaleskas VM, Krause DS, Lazarides K, et al. Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS One. 2006;1:e18. doi: 10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 39.Gu L, Chiang KY, Zhu N, et al. Contribution of STAT3 to the activation of survivin by GM-CSF in CD34+ cell lines. Exp Hematol. 2007;35:957–66. doi: 10.1016/j.exphem.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Yan D, Hutchison RE, Mohi G. Critical requirement for Stat5 in a mouse model of polycythemia vera. Blood. 2011 doi: 10.1182/blood-2011-03-345215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walz C, Ahmed W, Lazarides K, et al. Essential role for Stat5a/b in myeloproliferative neoplasms induced by BCR-ABL1 and Jak2V617F in mice. Blood. 2012 doi: 10.1182/blood-2011-12-397554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Shami A, Naccache PH. Granulocyte-macrophage colony-stimulating factor-activated signaling pathways in human neutrophils. Involvement of Jak2 in the stimulation of phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:5333–8. doi: 10.1074/jbc.274.9.5333. [DOI] [PubMed] [Google Scholar]
- 43.Bouscary D, Pene F, Claessens YE, et al. Critical role for PI 3-kinase in the control of erythropoietin-induced erythroid progenitor proliferation. Blood. 2003;101:3436–43. doi: 10.1182/blood-2002-07-2332. [DOI] [PubMed] [Google Scholar]
- 44.Mizuguchi R, Noto S, Yamada M, et al. Ras and signal transducer and activator of transcription (STAT) are essential and sufficient downstream components of Janus kinases in cell proliferation. Jpn J Cancer Res. 2000;91:527–33. doi: 10.1111/j.1349-7006.2000.tb00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dawson MA, Bannister AJ, Gottgens B, et al. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461:819–22. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rinaldi CR, Rinaldi P, Alagia A, et al. Preferential nuclear accumulation of JAK2V617F in CD34+ but not in granulocytic, megakaryocytic or erythroid cells of patients with Philadelphia-negative myeloproliferative neoplasia. Blood. 2010 doi: 10.1182/blood-2010-08-302265. [DOI] [PubMed] [Google Scholar]
- 47.Tefferi A, Vaidya R, Caramazza D, et al. Circulating Interleukin (IL)-8, IL-2R, IL-12, and IL-15 Levels Are Independently Prognostic in Primary Myelofibrosis: A Comprehensive Cytokine Profiling Study. J Clin Oncol. 2011;29:1356–63. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- 48.Vaidya R, Sulai N, Rozell SA, et al. Comprehensive Plasma Cytokine Profiling in Polycythemia Vera: Comparison with Myelofibrosis and Clinical Correlates [abstract] Blood. 2011;118:Abstract 3850. [Google Scholar]
- 49.Emadi S, Clay D, Desterke C, et al. IL-8 and its CXCR1 and CXCR2 receptors participate in the control of megakaryocytic proliferation, differentiation, and ploidy in myeloid metaplasia with myelofibrosis. Blood. 2005;105:464–73. doi: 10.1182/blood-2003-12-4415. [DOI] [PubMed] [Google Scholar]
- 50.Rameshwar P, Narayanan R, Qian J, et al. NF-kappa B as a central mediator in the induction of TGF-beta in monocytes from patients with idiopathic myelofibrosis: an inflammatory response beyond the realm of homeostasis. J Immunol. 2000;165:2271–7. doi: 10.4049/jimmunol.165.4.2271. [DOI] [PubMed] [Google Scholar]
- 51.Huang WL, Yeh HH, Lin CC, et al. Signal transducer and activator of transcription 3 activation up-regulates interleukin-6 autocrine production: a biochemical and genetic study of established cancer cell lines and clinical isolated human cancer cells. Mol Cancer. 2010;9:309. doi: 10.1186/1476-4598-9-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jedidi A, Marty C, Oligo C, et al. Selective reduction of JAK2V617F-dependent cell growth by siRNA/shRNA and its reversal by cytokines. Blood. 2009;114:1842–51. doi: 10.1182/blood-2008-09-176875. [DOI] [PubMed] [Google Scholar]
- 53.Manshouri T, Estrov Z, Quintas-Cardama A, et al. Bone marrow stroma-secreted cytokines protect JAK2(V617F)-mutated cells from the effects of a JAK2 inhibitor. Cancer Res. 2011;71:3831–40. doi: 10.1158/0008-5472.CAN-10-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen E, Beer PA, Godfrey AL, et al. Distinct clinical phenotypes associated with JAK2V617F reflect differential STAT1 signaling. Cancer Cell. 2010;18:524–35. doi: 10.1016/j.ccr.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quintas-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109–17. doi: 10.1182/blood-2009-04-214957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–27. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tefferi A, Barosi G, Mesa RA, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT) Blood. 2006;108:1497–503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- 58.Verstovsek S, Kantarjian HM, Estrov Z, et al. Comparison of Outcomes of Advanced Myelofibrosis Patients Treated with Ruxolitinib (INCB018424) to Those of a Historical Control Group: Survival Advantage of Ruxolitinib Therapy [abstracts] Blood. 2011;118:Abstract 793. [Google Scholar]
- 59.Tefferi A, Litzow MR, Pardanani A. Long-term outcome of treatment with ruxolitinib in myelofibrosis. N Engl J Med. 2011;365:1455–7. doi: 10.1056/NEJMc1109555. [DOI] [PubMed] [Google Scholar]
- 60.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 62.Tefferi A, Pardanani A. Serious adverse events during ruxolitinib treatment discontinuation in patients with myelofibrosis. Mayo Clin Proc. 2011;86:1188–91. doi: 10.4065/mcp.2011.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wernig G, Kharas MG, Okabe R, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell. 2008;13:311–20. doi: 10.1016/j.ccr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Lasho TL, Tefferi A, Hood JD, et al. TG101348, a JAK2-selective antagonist, inhibits primary hematopoietic cells derived from myeloproliferative disorder patients with JAK2V617F, MPLW515K or JAK2 exon 12 mutations as well as mutation negative patients. Leukemia. 2008;22:1790–2. doi: 10.1038/leu.2008.56. [DOI] [PubMed] [Google Scholar]
- 65.Pardanani A, Gotlib JR, Jamieson C, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29:789–96. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tyner JW, Bumm TG, Deininger J, et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood. 2010;115:5232–40. doi: 10.1182/blood-2009-05-223727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pardanani A, Lasho T, Smith G, et al. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia. 2009;23:1441–5. doi: 10.1038/leu.2009.50. [DOI] [PubMed] [Google Scholar]
- 68.Pardanani A, Gotlib J, Gupta V, et al. An Expanded Multicenter Phase I/II Study of CYT387, a JAK- 1/2 Inhibitor for the Treatment of Myelofibrosis [abstract] Blood. 2011;118:Abstract 3849. [Google Scholar]
- 69.Verstovsek S, Deeg HJ, Odenike O, et al. Phase 1/2 Study of SB1518, a Novel JAK2/FLT3 Inhibitor, In the Treatment of Primary Myelofibrosis [abstract] Blood. 2010;116:Abstract 3082. [Google Scholar]
- 70.Deeg HJ, Odenike O, Scott BL, et al. Phase II study of SB1518, an orally available novel JAK2 inhibitor, in patients with myelofibrosis. J Clin Oncol. 2011;29:Abstract 6515. [Google Scholar]
- 71.Ma L, Zhao B, Walgren R, et al. Efficacy of LY2784544, a Small Molecule Inhibitor Selective for Mutant JAK2 Kinase. JAK2 V617F-Induced Hematologic Malignancy Models [abstract] Blood. 2010;116:Abstract 4087. [Google Scholar]
- 72.Verstovsek S, Mesa RA, Rhoades SK, et al. Phase I Study of the JAK2 V617F Inhibitor, LY2784544, in Patients with Myelofibrosis (MF), Polycythemia Vera (PV), and Essential Thrombocythemia (ET) [abstract] Blood. 2011;118:Abstract 2814. [Google Scholar]
- 73.Nakaya Y, Shide K, Niwa T, et al. Efficacy of NS-018, a potent and selective JAK2/Src inhibitor, in primary cells and mouse models of myeloproliferative neoplasms. Blood Cancer Journal. 2011;1:e29. doi: 10.1038/bcj.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Purandare AV, McDevitt TM, Wan H, et al. Characterization of BMS-911543, a functionally selective small-molecule inhibitor of JAK2. Leukemia. 2012;26:280–8. doi: 10.1038/leu.2011.292. [DOI] [PubMed] [Google Scholar]
- 75.Alvarez-Larran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood. 2012;119:1363–9. doi: 10.1182/blood-2011-10-387787. [DOI] [PubMed] [Google Scholar]
- 76.Antonioli E, Guglielmelli P, Pieri L, et al. Hydroxyurea-related toxicity in 3,411 patients with Ph’-negative MPN. Am J Hematol. 2012;87:552–554. doi: 10.1002/ajh.23160. [DOI] [PubMed] [Google Scholar]
- 77.Verstovsek S, Passamonti F, Rambaldi A, et al. Durable Responses with the JAK1/ JAK2 Inhibitor, INCB018424, In Patients with Polycythemia Vera (PV) and Essential Thrombocythemia (ET) Refractory or Intolerant to Hydroxyurea (HU) [abstract] Blood. 2010;116:Abstract 313. [Google Scholar]
- 78.Barosi G, Birgegard G, Finazzi G, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood. 2009;113:4829–33. doi: 10.1182/blood-2008-09-176818. [DOI] [PubMed] [Google Scholar]
- 79.Deshpande A, Reddy MM, Schade GO, et al. Kinase domain mutations confer resistance to novel inhibitors targeting JAK2V617F in myeloproliferative neoplasms. Leukemia. 2012;26:708–15. doi: 10.1038/leu.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhagwat N, Koppikar P, Kilpivaara O, et al. Heterodimeric JAK-STAT Activation As a Mechanism of Persistence to JAK2 Inhibitor Therapy [abstract] Blood. 2011;118:Abstract 122. doi: 10.1038/nature11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weigert O, Lane AA, Bird L, et al. Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. J Exp Med. 2012;209:259–73. doi: 10.1084/jem.20111694. [DOI] [PMC free article] [PubMed] [Google Scholar]