Abstract
A network consisting of left dorsal lateral prefrontal cortex (LDLPFC) and dorsal anterior cingulate cortex (dACC) has been implicated in top-down attentional control. Few studies have systematically investigated how this network is altered in psychopathology, despite evidence that depression and anxiety are associated with attentional control impairments. fMRI and dense-array ERP data were collected in separate sessions from 100 participants during a color-word Stroop task. fMRI results guided ERP source modeling to characterize the time course of activity in LDLPFC (300-440 ms) and dACC (520-680 ms). At low levels of depression, LDLPFC activity was indirectly related to Stroop interference and only via dACC activity. In contrast, at high levels of depression, dACC did not play an intervening role, and increased LDLPFC activity was directly related to decreased Stroop interference. Specific to high levels of anxious apprehension, higher dACC activity was related to more Stroop interference. Results indicate that depression and anxious apprehension modulate temporally and functionally distinct aspects of the frontocingulate network involved in top-down attention control.
Keywords: depression, anxious apprehension, dACC, LDLPFC, attention, color-word Stroop
Attentional difficulties are highlighted as key diagnostic criteria for both depression and anxiety in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR, APA 2000). Contributing to cognitive misattributions, individuals with depression often demonstrate an attentional bias that favors negative information, and neutral information is interpreted in a negative manner (Gotlib & Krasnoperova, 1998; Gotlib, Krasnoperova, Yue, & Joorman, 2004). These attentional abnormalities may intensify and prolong symptoms of sadness and worry due to the negatively biased misinterpretation of events and information that is commonly observed in depression and anxiety (Gotlib et al., 2004), leading to a downward spiral of maladaptive thoughts. Individuals with anxiety demonstrate an attentional bias to threat-related information (Compton, Heller, Banich, Palmieri, & Miller, 2000; Nitschke & Heller, 2002). Once threatening stimuli are attended to, it is difficult for individuals with anxiety to disengage their attention and shift to less anxiety-provoking thoughts. These attentional control difficulties often affect daily life function. In clinical settings, it is typical to hear clients with depression and/or anxiety complain of “difficulties attending to a conversation or lecture” or, “problems focusing on reading or homework”. Attentional control problems and related executive function deficits can greatly interfere with interpersonal relationships and daily life activities such as job performance (Jaeger, Berns, Uzelac, & Davis-Conway, 2006). In turn, these difficulties fuel cycles of self-deprecation, sadness, and worry. Cognitive and neural mechanisms associated with the attentional problems that accompany symptoms of depression and anxiety are not well-understood.
Complicating the characterization of these phenomena, depression and anxiety frequently co-occur (Engels et al., 2010; Kessler, Dupont, Berglund, Wittchen, 1999). Based on DSM-IV-TR criteria, it can often be difficult, or even impossible, to distinguish whether an individual's attentional problems are related to depression, anxiety, or both. Further developing clinical assessment methods that have high diagnostic sensitivity and specificity is crucial to advancing treatment for these debilitating disorders. If differential patterns of attentional control difficulties can be identified in depression and anxiety, it will inform evidenced-based treatments for depression and anxiety that involve training individuals to use attentional control methods, such as cognitive control therapy (Siegle, Ghinassi, & Thase, 2007) and mindfulness-based cognitive behavioral therapy (Segal, Williams, & Teasdale 2002).
The effectiveness of attentional control training/cognitive control training for individuals with depression has been demonstrated in treatment outcome studies (Siegle et al., 2007) as well as in experimental research. Hertel (1994) showed that individuals with depression who were coached to use attentional control strategies achieved performance on an attention task that was comparable to that of individuals without depression. These findings indicated that individuals with depression have sufficient attentional resources; their attentional problems arise due to a failure to control these resources (Hertel, 1994). Fundamental attentional control functions that are involved with maintaining focus on the task at hand, rather than getting distracted by threatening or negative task-irrelevant information, or getting caught up in a ruminative loop, may be interrupted in depression (Hertel, 2007) and anxiety (Eysenck, Derakshan, Santos, & Calvo, 2007).
Several dimensions of psychopathology, including anhedonic depression, anxious apprehension (worry), and anxious arousal (panic or autonomic arousal), are accompanied by unique patterns of abnormal activity in regions of the brain involved in attentional control (e.g., Engels et al., 2007, 2010; Nitschke, Heller, & Miller, 2000) In order to examine associated neural mechanisms of attentional disruption that accompany depression and anxiety symptoms, it is strategic to partition anxiety according to these theoretical and methodological distinctions. This is particularly relevant because worry or anxious apprehension is a key feature of generalized anxiety disorder (GAD), and GAD is the most common anxiety disorder to precede and co-occur with depression (Kessler et al., 1996; Kessler, Zhao, Blazer, & Swartz, 1997),
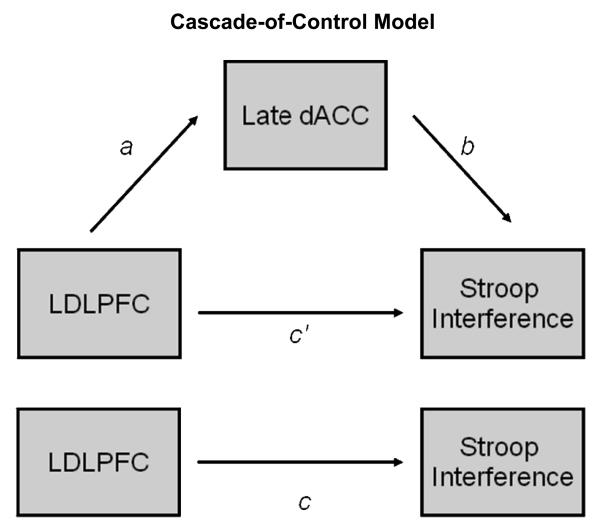
Banich (2009) identified a network of brain regions involved in top-down attentional control, including left dorsolateral prefrontal cortex (LDLPFC) and dorsal anterior cingulate cortex (dACC). Present analyses addressed the possibility that depression and anxious apprehension differentially influence this network. According to Banich's (2009) cascade-of-control model, during attentionally demanding tasks LDLPFC imposes a top-down attentional set for task-relevant processing while late-stage or response-related aspects of selection are implemented by dACC. Hence, a temporal course in which LDLPFC is activated first, followed by dACC, is a key component of Banich's cascade-of-control model. Furthermore, the model posits that the less top-down control exerted by LDLPFC, the more activity should be observed in dACC, as it will need to resolve any remaining aspects of selection before a response can be emitted.
A recent source analysis study investigating the time course of activity in LDLPFC and dACC during an attentional control task (color-word Stroop) in a nonclinical, undergraduate sample (Silton et al., 2010) provided support for this model1. Results indicated that LDLPFC activity preceded dACC activity. Moreover, measures of performance (Stroop interference) were directly related to later dACC activity, but not LDLPFC activity. The “Stroop interference effect” refers to a typical response pattern involving longer reaction time (RT) following incongruent stimuli (the word “red” in blue ink) than congruent (the word “red” in red ink) or neutral stimuli (a non-word or a non-color word, such as “XXXX” or “bond” in red ink). The extent to which dACC activation influenced Stroop performance depended on the degree of earlier LDLPFC activity, showing an interdependent relationship among these brain regions. Consistent with the cascade-of-control model, when LDLPFC activity was high, dACC activity did not affect performance. This pattern of activity was attributed to adequate top-down control imposed by LDLPFC. When LDLPFC activity was low, high dACC activity was associated with better performance and longer reaction time, suggesting that, as predicted, dACC was compensating for the lack of top-down LDLPFC control. When LDLPFC and dACC activity were both low, a higher error rate and shorter reaction time were observed, indicating a lack of dACC compensatory action.
These findings are relevant for psychopathology, as the Stroop (1935) task has been used to investigate cognitive impairments in depression in top-down control. A number of studies examining performance on the color-word Stroop task in depressed individuals have shown a range of attentional difficulties evidenced by increased RT, increased errors, and greater interference (Biringer et al., 2005; Dunkin et al., 2000; Duncan & Owen, 2000; Holmes & Pizzagalli, 2008; Paradiso et al., 1997; Ravnkilde et al., 2002; Stordal et al., 2004; Videbech et al., 2004). Impaired Stroop performance has been reported for individuals with Major Depressive Disorder (MDD; Videbech et al., 2004), recurrent Major Depressive Episodes (MDEs; Stordal et al., 2004), remitted depression (Biringer et al., 2005; Paradiso et al., 1997), and failure to respond to antidepressant medication (Dunkin et al., 2000). Recent research has revealed that abnormal focal LDLPFC and dACC activity is related to depression during Stroop performance (Holmes & Pizzagalli, 2008; Killgore, Gruber, & Yurgelun-Todd, 2007; Wagner et al., 2006), but precisely how depression influences the timing and relations among relevant brain regions remains an open question.
Research investigating the relationship between anxiety and color-word Stroop performance has used diverse definitions and types of anxiety, as well as various paradigms, perhaps contributing to a lack of consistency in results. In an early study, a state manipulation of anxiety adversely affected performance accuracy in an incongruent but not a congruent condition (Hochman, 1967). Fox (1993) compared behavioral performance for high and low trait-anxious participants on incongruent, neutral, and threatening words in a spatially “separated” Stroop task (attend to a central color patch and ignore the word in the periphery). High trait-anxious participants showed interference effects for both incongruent and threat words presented in the periphery, suggesting a general disruption in the ability to maintain attentional focus that is not limited to threatening information. Other studies involving the color-word Stroop task have not found RT condition differences as a function of anxiety (Gehring, Himle, & Nisenson, 2000; Hajcak, McDonald, & Simons, 2003).
The brain regions that were shown to work in conjunction to exert attentional control in a nonclinical sample, specifically, LDLPFC and dACC (Silton et al., 2010) appear to be differentially affected in depression and anxiety. Depression has been more commonly linked to reduced LDLPFC activity than has anxiety (Herrington et al., in press; Holmes & Pizzagalli, 2008; Fitzgerald et al., 2006; Rogers et al., 1998). Both depression and anxiety have been associated with altered dACC activity (Engels et al., 2010; Hajcak et al., 2003). Anxiety has typically been associated with increased dACC activity (Olvet & Hajcak, 2008; Paulus, Feinstein, Simmons, & Stein, 2004), whereas both dACC hyperactivity and hypoactivity have been reported in depression (Holmes & Pizzagalli 2008; George et al., 1997; Kilgore et al., 2007). These mixed findings may be in part due to unassessed, comorbid anxiety.
Limited research has focused on how psychopathology affects frontocingulate networks. Mayberg's (1997) proposed limbic-cortical network model of depression specifies “ventral” and “dorsal” components. Relevant to the present study, Mayberg proposed that the attentional impairments observed in depression are related to abnormalities in the dorsal components, which include DLPFC, dACC, inferior parietal cortex, and striatum. Consistent with this model, Holmes and Pizzagalli (2008) showed a reduction in both LDLPFC and dACC activity as measured by event-related brain potential (ERP) source analyses during a color-word Stroop task at 620 ms post-stimulus presentation in individuals with depression compared to controls. Reporting different findings using functional magnetic resonance imaging (fMRI) methods, Wagner et al. (2006) observed that individuals with depression had hyperactive LDLPFC but no changes in dACC activity relative to healthy controls during Stroop performance. With implications for translational research, an fMRI study showed post-treatment changes in LDLPFC function after individuals with depression received cognitive control therapy (Siegle et al., 2007). Decreased LDLPFC activity was observed for an easy cognitive control task condition and increased LDLPFC activity was observed for a more difficult condition. These depressed individuals demonstrated improved performance on this cognitive-control task following treatment. These findings support other studies in pointing to changes in patterns of DLPFC and ACC activation as a function of depression, although the precise nature and direction of these changes remains to be determined.
The present study used ERP source analysis to evaluate how the frontocingulate network described in Banich's cascade-of-control model of top-down attentional control is affected by depression and anxiety. Source analysis techniques are an ideal method to study the timing of network function, as they provide information regarding the time course of regional brain activity. MMModerated mediation analyses were used to evaluate the hypothesis that depression and anxiety would influence the time course of early LDLPFC activity and later dACC activity in different ways during a task that requires top-down attentional control (the color-word Stroop task). It was predicted that depression would be related to reduced earlier LDLPFC activity and that later dACC activity would be related to either 1) compensatory behavior, as evidenced by increased activity and normal Stroop performance, or 2) a lack of compensatory behavior, as indicated by decreased activity and poor Stroop performance. Anxious apprehension was hypothesized to be associated with increased dACC activity only. It was uncertain whether this pattern of network activity would affect performance. Increased dACC activity was shown to mediate Stroop performance only when LDLPFC activity was low and associated top-down attentional control was poor (Silton et al., 2010). Since anxious apprehension is not expected to affect LDLPFC activity, it is uncertain whether potential changes to subsequent downstream dACC activity (but not earlier LDLPFC activity) would affect performance.
Method
Participants and Selection Procedures
Participants (N = 100) were recruited from introductory psychology classes via group questionnaire screening sessions as well as from the community via advertisements placed in local newspapers and through recruitment efforts at the campus-run community psychology clinic. Participants (45% female, 80% Caucasian) were paid volunteers ages 18-35 (M = 20.2, SD = 3.6). Participants were right-handed as determined by the Edinburgh Handedness Inventory (Oldfield, 1971) and were native English speakers. Since psychoactive medications are known to affect cognitive function and related regional brain activity (Brody et al., 2001; Mayberg et al., 2000), participants were screened by self-report to be free of such medications. Participants were also screened for abnormal color vision, loss of consciousness >10 minutes, claustrophobia, recent drug/alcohol use, excessive caffeine intake, and lack of sleep. Participants were given a laboratory tour, were informed of the study procedures, and provided written consent. DSM-IV-TR diagnoses were used to select participants from a larger project in order to ensure that individuals who had a lifetime history of clinically-defined depression were included in present analyses. The participant selection method described here was employed prior to running subsequent analyses.
The Structured Clinical Interview for Axis I Disorders, Non-patient edition (SCID-NP, First, Spitzer, Gibbon, & Williams, 1997), was administered to all participants to assess Axis I disorders. Lifetime DSM-IV-TR diagnoses were determined by the interviewer and reviewed by a consensus team consisting of a second interviewer and a clinical faculty supervisor (GAM) reviewing a written case summary detailing each criterion symptom on the scale: 1 = absent, 2 = features (at least 2 symptoms), 3 = provisional (1 short of full DSM-IV-TR criteria), and 4 = definite. SCID-NP data were used to select 34 participants who had a lifetime history of a provisional or definite depressive disorder, 18 of whom had a lifetime history of one or more provisional or definite anxiety disorder(s). Participants with anxiety disorders had diagnoses primarily of GAD (n = 7), as well as of OCD (n = 5), Social Phobia (n = 5), Specific phobia (n = 4), PTSD (n = 4), Panic (n = 1), and Anxiety NOS (n = 1). The SCID-NP does not provide information regarding current anxiety disorders, only information regarding lifetime history of anxiety disorders. 66 participants were free of any depressive or anxiety disorders. None of the participants was in a current MDE. Although participants were screened for all Axis I disorders, other disorders were not used as criteria to select participants for the present study. Participants' diagnostic status was not revealed to the research team until after the participants had completed the entire study protocol. These participant selection methods were used to ensure that a range of depression- and anxiety-related psychopathology was represented in the sample, since dimensional analyses of self-reported depression and anxious apprehension were used to examine their moderating effects (described below).
To provide dimensional measures of depression, anxious apprehension, and anxious arousal, participants completed the Mood and Anxiety Symptom Questionnaire Anhedonic Depression 8-item depressed-mood subscale (MASQ-AD-8 Nitschke et al., 2001; Watson et al., 1995a, 1995b) and the Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990; Molina & Borkovec, 1994). These measures have been shown to provide effective discrimination among these dimensions (for review, see Nitschke et al., 2001) and to distinguish brain regions involved in each (e.g., Engels et al., 2010). Although participants completed other questionnaires as part of a larger study, only the MASQ-AD-8 and PSWQ were analyzed in the present study. With regard to construct validity, the MASQ-AD-8 predicts current MDE and lifetime MDD (Bredemeier et al., in press). Similarly, the PSWQ is an excellent predictor of GAD (Behar, Alcaine, Zuellig, & Borkovec, 2003). See Table 1 for further information about demographics, and questionnaire scores for the depression, anxiety, and comorbid diagnostic categories for the present sample.
Table 1.
Demographics and Questionnaire Scores by Diagnostic Group
| Comorbid n = 18 |
Depression n = 16 |
No Diagnosis n = 66 |
Full Sample N = 100 |
|
|---|---|---|---|---|
| Age | 23.72 (5.15) | 21.12 (5.44) | 19 (.89) | 20.19 (3.60) |
| Gender 1 | 12/6 | 7/9 | 26/30 | 45 /55 |
| PSWQ | 57.44 (18.92) | 39.38 (15.05) | 40.55 (14.22) | 43.40 (16.51) |
| MASQ-AA | 27.72 (7.01) | 24.00 (6.55) | 24.18 (6.90) | 24.79 (6.93) |
| MASQ-AD-8 | 17.72 (6.24) | 17.06 (4.80) | 14.39 (3.95) | 15.42 (4.74) |
| GAF | 67.89 (10.64) | 81.44 (6.50) | 87.76 (5.02) | 83.17 (9.97) |
Number of women / number of men.
Three ANOVAs were conducted to examine a) whether participants with diagnosed depression scored higher than those without diagnoses on the MASQ-AD-8, b) whether participants with comorbid diagnoses scored higher than those without diagnoses on both questionnaire measures, and c) whether participants with comorbid diagnoses scored higher than the participants with only depression diagnoses on the PSWQ, but not the MASQ-AD-8. The participants with comorbid and pure depression were expected to have comparable levels of depression as measured by the MASQ-AD-8 and to vary only on anxiety levels as measured by the PSWQ. The results were as expected. The participants with depression diagnoses scored higher than those without diagnoses on the MASQ-AD-8 (F(1,80) = 5.41, p = .02). The participants with comorbid diagnoses scored higher than those without diagnoses on the PSWQ (F(1,82) = 17.22, p <.001) and MASQ-AD-8(F(1,82) = 7.60, p = .01). The participants with comorbid diagnoses did not differ from those with only depression diagnoses on the MASQ-AD-8 (F(1,32) = .12, p = .74), but they scored higher than those with only depression diagnoses on the PSWQ (F(1,32) = 9.33, p = .01). Given that the diagnosis-based categorical groups were formed using a different measure (the SCID-NP) than the dimensional questionnaires, these analyses provided evidence for convergent validity.
Stimuli and Experimental Design
In brief overview, ERP and fMRI data were collected from all participants during a task requiring top-down attentional control (color-word Stroop task). The fMRI data were used to guide placement of ERP sources, and information regarding the time course of LDLPFC and dACC activity was extracted for neural network analyses involving dimensional depression and anxious apprehension variables.
Participants completed a color-word Stroop task and an emotion-word Stroop task. Both tasks were administered during an fMRI session and again during an EEG session. The order of presentation of the two Stroop tasks within a session was counterbalanced across participants, as was the order of the EEG and fMRI sessions, with the SCID session in-between for most participants. The emotion-word Stroop data do not address present goals and will not be considered further here. The color-word Stroop task consisted of blocks of color-congruent or color-incongruent words alternating with blocks of neutral words, with 256 trials in 16 blocks (4 color-congruent, 4 color-incongruent, 8 neutral). Half the trials in congruent and incongruent blocks were neutral, to prevent the development of word-reading strategies. There were eight orders of stimulus presentation for each Stroop task, designed specifically to control stimulus order effects. Each participant received one of the eight orders.
Each trial consisted of one word presented in one of four ink colors (red, yellow, green, blue). Trials began with the presentation of a word for 1500 ms, followed by a fixation cross for 275 to 725 ms (onset to onset ITI 2000 +/− 225 ms). Word presentation and response recording were controlled by STIM software (James Long Company, Caroga Lake, NY). In the fMRI session, words were presented in capital letters using Tahoma 72-point font via back projection onto a screen outside the scanner bore and a mirror fixed to the head coil, providing a vertical span of 2.9 degrees and a horizontal span of 6.1-16.4 degrees. In the ERP session, the same words were presented in Tahoma 72-point font on a CRT monitor 1.35 m from the participants' eyes, providing a vertical span of 1.5 degrees and a horizontal span of 3.2 - 8.7 degrees. Participants responded with the middle and index fingers of both hands, with each task using the same mapping of color to button. There was a color-to-key-mapping acquisition phase of 32 practice trials. In addition to the 16 word blocks, there were four fixation blocks – one at the beginning, one at the end, and two in the middle of the session. In the fixation condition, a brightened fixation cross was presented for 1500 ms.
MRI Recording, Data Reduction, and Analysis
fMRI data were analyzed from a subset of 30 of the 66 participants without a psychopathology history, providing guidance for the ERP source analysis that was carried out for all 100 participants. Two-tailed t-tests showed that the 30 participants who were used in fMRI analyses did not differ from the other 36 participants without anxiety or depression diagnoses in terms of age, t(65) = 1.11 , p = .27, gender balance, χ2(1, n = 66) = 2.03, p = .15, Global Assessment of Function (GAF), t(65) = .26 , p =.80, or Stroop interference effect, t(65) = −.78, p = .44. Participants without lifetime depression and/or anxiety diagnoses were used for fMRI analyses because the purpose of the study was to understand how network activity in depression and anxiety differs from typical network activity observed in healthy individuals.
The MR technologist and experimenter assisted the participant in correct placement of earplugs and protective headphones. MR data were collected using a research-dedicated 3T Siemens Allegra. Three hundred and seventy functional images were acquired using a gradient-echo echo-planar imaging (EPI) sequence (TR 2000 ms, TE 25 ms, flip angle 80°, FOV=22 cm). Thirty-eight oblique axial slices (slice thickness 3 mm, in-plane resolution 3.4375×3.4375 mm, .3 mm gap between slices) were acquired parallel to the anterior and posterior commissures. After the EPI sequence, a 160-slice MPRAGE structural sequence was acquired (slice thickness 1 mm, in-plane resolution 1×1 mm) for registering each participant's functional data to standard space.
Image processing and analyses relied primarily on tools from the FMRIB Software Library (FSL) analysis package (http://www.fmrib.ox.ac.uk/fsl). Each fMRI time series was first motion-corrected using Motion Correction using FMRIB's Linear Image Registration Tool (MCFLIRT; Jenkinson, Bannister, Brady, & Smith, 2002), and “spikes” (artifactual sudden intensity shifts) were corrected using the AFNI tool 3dDespike (http://afni.nimh.nih.gov/afni). Participants demonstrated less than 3.3 mm absolute motion or 2 mm relative motion (participants with motion exceeding this threshold were excluded from analysis, beyond the 30 control participants relied on in the present analysis). After motion correction and despiking, each time series was corrected for geometric distortions caused by magnetic field inhomogeneity. Remaining preprocessing steps, single-subject statistics, and group statistics were implemented by FMRI Expert Analysis Tool (FEAT). The first three volumes of each dataset were discarded to allow the MR signal to reach a steady state. Each time series was then temporally filtered with a nonlinear high-pass filter (to remove drift in signal intensity), mean-based intensity-normalized by the same single scaling factor, and spatially smoothed using a 3D Gaussian kernel (full-width-half-maximum 5 mm) prior to analysis.
Regression analyses were performed on each participant's time series using FMRIB's Improved Linear Model (FILM). Statistical maps were generated via multiple regression computed for each intracerebral voxel (Woolrich, Ripley, Brady, & Smith, 2001). An explanatory variable (EV) was created for each trial block type (color-congruent, color-incongruent, neutral, rest), with the fixation condition the unmodeled baseline. Each EV was convolved with a gamma function to better approximate the temporal course of the blood-oxygen-dependent (BOLD) hemodynamic response (e.g., Aguirre, Zarahn, & D'Esposito, 1998; Miezin, Maccotta, Ollinger, Petersen, & Buckner, 2000). Each EV yielded a per-voxel effect-size parameter (β) estimate (PE) map representing the magnitude of activity associated with that EV. The β values for the incongruent word condition were contrasted with those for the congruent word condition, resulting in a per-voxel contrast parameter estimate map for each subject. These functional activation maps as well as the corresponding structural MRI map were registered into Montreal Neurological Institute (MNI) stereotaxic space using FMRIB's Nonlinear Image Registration Tool (FNIRT) with FSL's default configuration file and a warp resolution of 10 mm.
Inferential statistical analyses were carried out using FMRIB's Local Analysis of Mixed Effects (FLAME). To identify regions associated with the Stroop interference effect, significantly activated voxels were identified for the incongruent minus congruent contrast via a one-sample t-test, yielding a 3D functional z-map image. Monte Carlo simulations via AFNI's AlphaSim program (Ward, 2000) estimated the overall significance level (probability of a false detection) for thresholding, using a gray-matter mask to limit the number of voxels under consideration. These simulations provided a z-value (z = 3.0902, p = .01) and cluster size (34) combination for thresholding that resulted in an overall familywise error rate of .05. Clusters that survived this thresholding are reported in Table 2. Center of Mass coordinates for clusters in hypothesized regions of interest were used to place regional sources in the ERP source model.
Table 2.
fMRI center of mass coordinates (MNI)
| Region | Cluster Size |
Mean Z |
X | Y | Z |
|---|---|---|---|---|---|
| Left Frontal Orbital Cortex | 545 | 3.76 | −31 | 19 | −8 |
| Right Frontal Orbital Cortex | 749 | 3.61 | 36 | 21 | −6 |
| Left Inferior Temporal Gyrus | 36 | 3.36 | −53 | −56 | −15 |
| Left Intracalcarine Cortex | 111 | 3.39 | −8 | −79 | 2 |
| Right Thalamus | 1126 | 3.61 | 0 | −16 | 7 |
| Right Caudate | 67 | 3.28 | 11 | 11 | 7 |
| Left Putamen | 37 | 3.31 | −23 | −2 | 9 |
| Right Inferior Frontal Gyrus | 42 | 3.35 | 41 | 35 | 13 |
| Left Precentral Gyrus | 1195 | 3.72 | −40 | 11 | 34 |
| Right Anterior Cingulate Gyrus | 182 | 3.50 | 7 | 21 | 27 |
| Left Lateral Occipital Cortex | 1376 | 3.77 | −35 | −57 | 44 |
| Left Precuneus Cortex | 443 | 3.61 | −5 | −63 | 45 |
| Paracingulate Gyrus | 335 | 3.54 | 0 | 14 | 51 |
| Right Superior Parietal Lobule | 132 | 3.49 | 39 | −50 | 48 |
Electrophysiological Recording, Data Reduction, and Analysis
Participants were seated in a comfortable chair in a quiet room connected to the adjacent equipment room by intercom. EEG was recorded with a custom-designed Falk Minow 64-channel cap with equidistantly spaced Ag/AgCl electrodes. After placement of the electrode cap, electrode positions were digitized for later topographic and source-localization analyses. An additional electrode was placed below each eye; these and nearby electrodes in the cap provided a basis for off-line eye-blink artifact correction of the EEG data implemented in BESA 5.1.8 (Berg & Scherg, 1994). The left mastoid served as the online reference for all other sites, including EOG. Impedances were below 20 Ω, appropriate given the high input impedance of the amplifiers. Half-power amplifier bandpass was .1 to 100 Hz, with digitization at 250 Hz.
The following steps were done separately for each participant. Muscle and other artifact was manually removed using BESA 5.1.8. A series of steps were taken to remove and/or correct eye blinks and movements. Electrodes above and below both the right and left eyes and near the left and right external canthi were used to measure vertical and horizontal eye movements. Pairs of channels were used to compute bipolar derivations used to identify epochs that included either a horizontal or vertical saccade. The saccades were marked as artifact periods and removed from the data. A typical blink was identified in the data. Using the pattern search function in BESA 5.1.8, the data were scanned to identify other blink periods. Stimulus-locked averages were calculated for the experimental conditions (congruent, incongruent, and neutral) for each participant. Only trials with correct responses that occurred 350 ms to 1400 ms after stimulus onset were included in the individual participant averages. All participants included in the sample had a minimum of 16 trials for each condition average. Following these steps, the surrogate multiple source eye correction (MSEC) algorithm was used to correct blink artifacts for each participant (Berg & Scherg, 1994). In the MSEC method, using all EEG channels, sources of brain and artifact activity (e.g., blink) are simultaneously modeled, and only the modeled blink activity is removed from each EEG channel. The Berg and Scherg (1994) method reduces distortion of brain activity by accounting for the EEG signal during the estimation of eye activity (see Silton et al., 2010, for additional details about the blink removal process and application of the MSEC method).
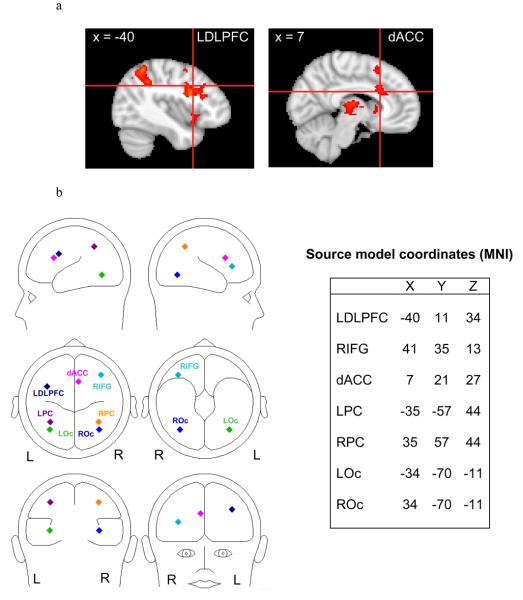
Source modeling was carried out using BESA 5.1.8. The source model (see Figure 3b for full model) was created by placing a priori regional sources based on Center of Mass coordinates for fMRI activation clusters obtained from the 30 psychopathology-free participants as discussed above. Fourteen candidate locations survived thresholding (Table 2). If all 14 clusters were placed as sources in the model, the model would have overfit the data. Rather, the selection of sources from among these clusters was based on relevant color-word Stroop fMRI research (Michel et al., 2004). Four of these 14 clusters (LDLPFC, dACC, right inferior gyrus, left parietal cortex) were used in the source model (see Figure 3a for fMRI images). Although analyses for the present study primarily involved LDLPFC and dACC, the full source model included right inferior gyrus (RIFG), left parietal cortex (LPC), and right parietal cortex (RPC) in order to account for variance that is thought to be contributed by these sources based on available literature. The LDLPFC and dACC locations were very close to the locations proposed by the cascade-of-control model (Banich, 2009). Similar studies that have used nonverbal stimuli have also implicated LDLPFC and dACC, suggesting that tasks that involve top-down attentional control recruit these brain regions across stimulus types (Fan et al., 2003; Liu, Banich, Jacobson, & Tanabe, 2004).
Figure 3.
a) LDLPFC and dACC activation for incongruent vs. congruent stimuli, Z= 3.0902, p = .01, cluster size = 34 (corrected p < .05). Cross-hairs placed at center of intensity. b) Functional magnetic resonance imaging (fMRI) Montreal Neurological Institute (MNI) coordinates for source model containing 7 regional sources used in brain electrical source analysis (BESA) source modeling. LDLPFC = Left dorsolateral prefrontal cortex; RIFG = Right inferior frontal gyrus; dACC = Dorsal anterior cingulate cortex; LPC = Left parietal cortex; RPC = Right parietal cortex; LOc = Left occipital cortex; ROc = Right occipital cortex.
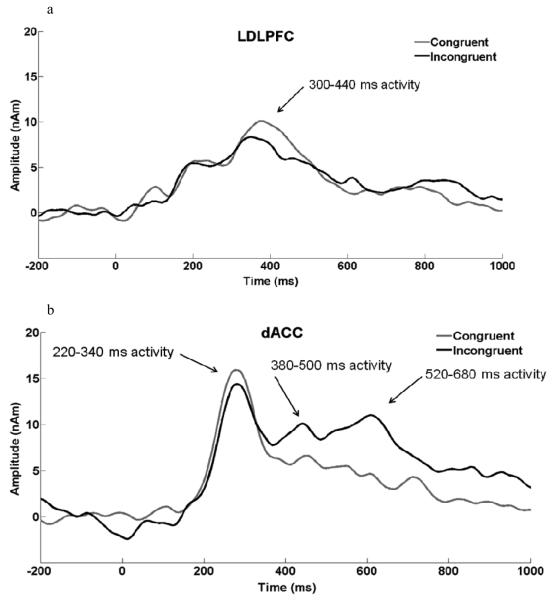
Prior to placing these sources in the model, blink activity was modeled as described above. Next, bilateral visual cortex sources (LOc, ROc) thus were localized based on ERP data from correct trials in the neutral condition. The neutral condition involved the largest number of trials and was selected to maximize the signal-to-noise ratio for localization. A grand average computed from per-subject waveform averages for neutral-trial blocks from all psychopathology-free participants (n = 66) was used for localizing the visual sources. The epoch used for the localization was 100 ms to 188 ms, spanning primary and secondary visual cortex responses. The LOc and ROc sources were constrained to be symmetrical (see Figure 3b for LOc/ROc coordinates). Finally, the LDLPFC, dACC, RIFG, and LPC sources were placed in the model along with a contralateral RPC source. Since magnitude of source activity, rather than orientation of source activity, was the primary variable of interest, all dipoles were converted to regional sources. The ERP data were digitally filtered .1-12 Hz, and the source model was applied separately in each Stroop condition (congruent, incongruent) for each participant. Prestimulus baseline activity (-200 ms to 0 ms) was removed from the source waveforms after the model was fit to each participant. Scoring windows were based on visual inspection of the source waveforms as well as taking into consideration findings from relevant scalp- and source-ERP color-word Stroop research. One window for LDLPFC (300 ms to 440 ms) and two windows for dACC (220 ms to 340 ms, 520 ms to 680 ms) were identified. Source component amplitude was calculated by averaging data points 24 ms before and 24 ms after peak latency. With the exception of determining the location and temporal scoring window of the sources, all source-analysis steps described above were performed separately for each of the 100 participants.
Moderated Mediation Analyses
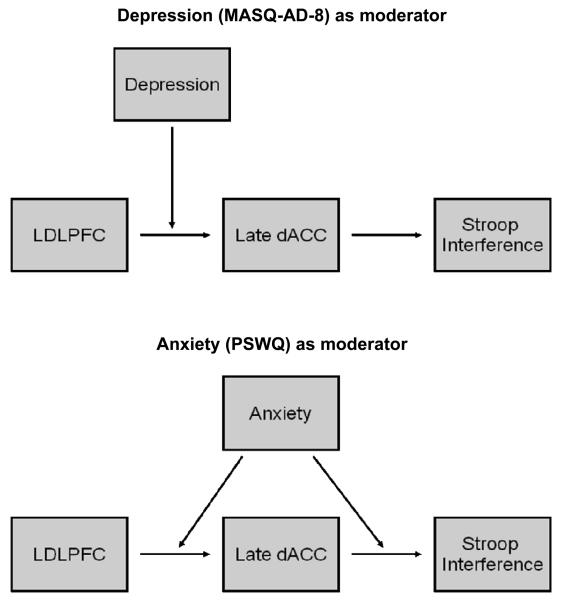
Moderated mediation analyses (Preacher, Rucker, & Hayes, 2007) were used to evaluate the hypothesis that high levels of depression would interfere with the relationship between LDLPFC and dACC previously observed in the nonclinical sample (Silton et al., 2010). In the context of a mediation model, a moderator variable is an additional variable that is not part of the causal sequence that modifies the relationship between two variables (e.g., independent and dependent variables). Moderator effects are also referred to as interactions. Continuous psychopathology variables (MASQ-AD-8 and PSWQ scores) were assigned as moderators to evaluate whether the relationships between LDLPFC and dACC and between dACC and Stroop interference depended on levels of psychopathology (Figure 1 provides a graphic representation of these moderated mediation models). A series of linear regressions were used to test the moderated mediation models. Five participants were considered outliers on source measures (3 SD from the mean for at least one component) and were omitted from subsequent analyses (resulting n = 95). The SPSS macro (MODMED) described in Preacher et al. (2007; http://www.comm.ohio-state.edu/ahayes) was used to conduct the moderation analyses.
Figure 1.
Moderation models for cascade-of-control model.
Results
Behavioral Performance
RT analyses were conducted to confirm that the Stroop interference effect was obtained in the present sample. A MANOVA with Condition (congruent RT, incongruent RT) and Gender confirmed slower RT for incongruent than for congruent trials, Condition F (1,94) = 214.10, p < .001 (congruent mean = 631 ms, SD = 95 ms; incongruent mean = 791 ms, SD = 138 ms). The Stroop effect did not vary by gender. Participants made more errors during the incongruent than the congruent condition, F(1,94) = 48.66, p <.001 (congruent mean = .68 errors, SD = .95, incongruent mean = 2.37 errors; SD = 2.22). The depression and anxiety measures were not significantly correlated with congruent or incongruent RT, errors, or Stroop interference.
Source-Waveform ERP Moderated Mediation Analysis
The ERP source-waveform data analyses (see Figure 4 for waveforms) employed scores from incongruent trials only, to examine the effects of psychopathology within the context of cognitive control mechanisms prompted by Stroop conflict.
Figure 4.
a) Grand-average source waveforms for LDLPFC elicited during the color-word Stroop task for congruent and incongruent conditions, highlighting the 300 to 440 ms scoring window. N = 100. b) Grand-average source waveforms for dACC elicited during the color-word Stroop task for congruent and incongruent conditions, highlighting the 220 to 340 ms and 520 to 680 ms scoring windows. N = 100.
Replication of cascade-of-control model mediation analysis
Prior to proceeding with moderation analyses, the mediation analyses were repeated (see Figure 2), since the sample selection procedures varied from Silton et al. (2010). Figure 2 depicts the mediation model that was tested. The present sample included participants recruited from the community, which broadened the sample, increased the sample size, and included more psychopathology. The mediation analyses for the cascade-of-control model were replicated and are presented in Table 3 (see Model 1). The indirect effect was used to test directly the overall significance of the cascade-of-control model (Preacher & Hayes, 2008). As before, now with a sample expanded to include participants recruited from the community, the indirect effect was significant, and the cascade-of-control model was supported, with relevant LDLPFC activity preceding rather than following relevant dACC activity. Similar to the findings in Silton et al. (2010), the total variance accounted for was 9% (F(2,92) = 4.29, p = .02), which represents a medium effect size (Cohen, 1992; 9% corresponds to r = .30, which is standard for a medium effect size).
Figure 2.
The cascade-of-control model.
Table 3.
Summary of mediation analyses for the cascade-of-control model of Figure 1
| Model | Path A |
Path b |
Path c |
Path c′ |
Indirect Effect (a × b) |
Regression Summary(R2) |
|---|---|---|---|---|---|---|
| 1. Cascade-of-Control (Present study) | .44* | 1.84** | −.89 | −1.70* | .81 b | .09** |
| 2. Cascade-of-Control (Silton et al., 2010) | .34* | 1.86** | −1.41 | −2.00** | .63a | .09** |
Note. Table entries in middle five columns are path coefficients
p < .05
p < .10
Significant point estimate (p < .05), 95% BCa CI = .01 to 1.82, k = 5000
Significant point estimate (p < .05), 95% BCa CI = .11 to 1.87, k = 5000
The influence of psychopathology on the frontocingulate network: Moderated mediation analyses
It was predicted that depression would influence the frontocingulate network that is activated during Stroop performance. Depression was expected to be associated with reduced LDLPFC activity, which in turn would influence subsequent dACC activity and related Stroop performance. Specifically, the interaction of depression with early LDLPFC activity was expected to predict later dACC activity as well as Stroop performance. Given that it was predicted that depression would alter the relationship between early LDLPFC and later dACC activity, the model with depression as a moderator was tested (Figure 1) with two hierarchical regressions. For these two regressions, one-tailed tests were used to evaluate the a priori hypothesis discussed above.
An initial regression tested whether depression influenced the relationship between LDLPFC and dACC during Stroop performance. LDLPFC, MASQ-AD-8, and LDLPFC × MASQ-AD-8 were predictors, and late dACC activity was the DV. Added last, LDLPFC × MASQ-AD-8 was significant, b = −.06, t(91) = −1.99, p = .035 (one-tailed). The results of this analysis showed that the interaction of LDLPFC and depression predicted dACC activity. The omnibus model accounted for 14% of the variance (F(3,91) = 4.82, p < .01), which corresponds to r = .37, representing a medium effect size (Cohen, 1992). The LDLPFC × MASQ-AD-8 interaction accounted for 4% of the total variance for this model. Interactions observed in psychological research typically account for a few percentage points of variance beyond first-order effects (Cohen, Cohen, West & Aiken, 2003).
Next, a regression evaluated whether the interaction of depression and LDLPFC predicted Stroop performance when variance related to dACC was accounted for. LDLPFC, MASQ-AD-8, LDLPFC × MASQ-AD-8, and dACC were predictors, and Stroop interference was the DV. Added last, the interaction was significant, b = −.32, t(90) = −1.64, p = .05 (one-tailed), a finding sufficient for a directional a priori hypothesis. The omnibus model accounted for 15% of the variance (F(4,90) = 3.86, p < .01), which corresponds to r = .39, representing a medium effect size (Cohen, 1992). The LDLPFC × MASQ-AD-8 interaction contributed 3% of the total variance for this model. Overall, these findings indicate that depression and LDLPFC interact to predict Stroop performance.
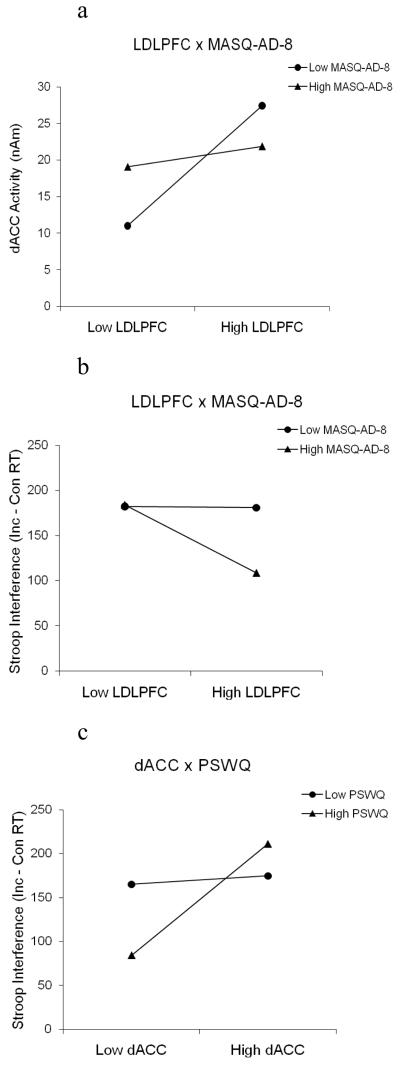
In order to better understand the moderating effects of depression, the interactions for both regressions were plotted (Figure 5a and 5b), and the significance of the slopes was tested (Aiken & West, 1991). In Figures 5a – 5c, “low” and “high” refer to ±1 SD (Aiken & West, 1991). Importantly, the test of simple slopes is not a test of an interaction effect (the interactions are tested in the regression above). Rather, it is a method of describing the nature of the interactive relationship (Aiken & West, 1991). “High” and “low” are defined relative to the present sample, and these terms are used to represent two portions of a dimension rather than classification categories used to distinguish the absence or presence of clinical diagnoses. The t-test for whether a simple slope differed from zero was calculated by dividing the value of the simple slope by its standard error with (n – k – 1) degrees of freedom (where n is the number of cases and k is the number of predictors). The standard error was calculated from the variance-covariance matrix of the regression coefficients. As shown in Figure 5a, LDLPFC activity predicted dACC activity at low levels of depression, t(91) = 3.69, p < .001, but not at higher levels of depression, t(91) = .54, p = .59 (Figure 5a). Furthermore, Figure 5b shows that LDLPFC activity was associated with less Stroop interference at higher levels of depression, t(90) = −2.28, p = .03, but not at low levels of depression, t(90) = −.05, p = .96. That is, at low levels of depression, Stroop interference did not vary as a function of LDLPFC activity. For individuals higher in depression, LDLPFC and dACC were less well coupled, and LDLPFC activity was more tightly linked directly with RT performance.
Figure 5.
a) LDLPFC × MASQ-AD-8 interaction and tests of simple slopes show that, at low levels of depression, LDLPFC predicts dACC. b) LDLPFC × MASQ-AD-8 interaction, with Stroop interference as the predictor and tests of simple slopes, shows that, at high levels of depression, increased LDLPFC activity is related to less interference. c) dACC × PSWQ interaction and tests of simple slopes show that at high levels of anxiety, increased dACC is related to greater Stroop interference.
Moderated mediation analyses were conducted to evaluate whether anxious apprehension also influenced the frontocingulate network during Stroop performance (see Figure 1, lower panel). Similar in structure to the regression analyses conducted with MASQ-AD-8, two hierarchical regressions were used to test the influence of anxious apprehension on the mechanisms articulated in the cascade-of-control model. Two-tailed significance tests were used for these regressions, because these analyses were implemented to evaluate exploratory hypotheses. First, a regression was conducted to assess whether anxious apprehension influenced the relationship between LDLPFC and dACC. LDLPFC, PSWQ, and LDLPFC × PSWQ were predictors, and late dACC activity was the DV. As predicted, the interaction was not significant. Second, a regression was conducted to evaluate whether anxiety interacted with either LDLPFC or dACC activity to predict Stroop performance. LDLPFC, dACC, PSWQ, LDLPFC × PSWQ, and dACC × PSWQ were predictors, and Stroop interference was the DV. dACC × PSWQ was significant, b = −.11, t(89) = 2.37, p = .02, but LDLPFC × PSWQ was not, b = −.04, t(89) = −.70, p = .48, indicating that anxious apprehension influenced the relationship between dACC and Stroop interference effect, but not the relationship between LDLPFC and dACC. The model including only the significant dACC × PSWQ interaction (the LDLPFC × PSWQ interaction was not included) accounted for 14% of the variance (F(4,90) = 3.79, p < .01), which corresponds to r = .37, a medium effect size (Cohen, 1992). The dACC × PSWQ interaction accounted for 5% of the total variance in this model. In order to interpret the moderating effects of anxiety, the interaction was plotted (Figure 5c). The slope was significant for higher levels of anxiety, t(89) = 3.59, p < .001, but not for low levels of anxiety t(89) = .39, p = .67. At higher levels of anxious apprehension, increased dACC activity was related to greater Stroop interference.
In order to ascertain whether the influence of anxiety on dACC function was specific to anxiety, a final moderation analysis was conducted to evaluate whether depression also modified dACC function during Stroop performance, when variance related to LDLPFC was accounted for. This regression included LDLPFC, dACC, MASQ-AD-8, and dACC × MASQ-AD-8 (only LDLPFC × MASQ-AD-8 was tested previously) as predictors, and Stroop interference was the DV. The interaction was not significant, b = −.06, t(90) = .17, p = .74, indicating that depression and anxious apprehension influence distinct aspects of the frontocingulate network in distinct ways.
Discussion
The present study examined how depression and anxiety influence frontocingulate activity under conditions of high attentional demand. A previous study showed that, ignoring psychopathology, the extent to which dACC activation influenced Stroop performance depended on the degree of earlier LDLPFC activity (Silton et al., 2010). When LDLPFC activity was high, dACC activity did not influence the degree of Stroop interference, whereas, when LDLPFC activity was low, higher dACC activity was associated with reduced Stroop interference. Based on this pattern of activity, it was predicted in the present study that depression would be related to reduced early LDLPFC activity, which in turn was expected to influence subsequent dACC activity and Stroop interference. Anxious apprehension was expected to influence dACC activity, but not LDLPFC activity. It was unclear how this pattern of network activity might affect performance. Results showed that both depression and anxiety affected this frontocingulate network involved in attentional control and did so in different ways.
LDLPFC activity predicted dACC activity only at low levels of depression during Stroop performance, indicating a functional relationship similar to the one observed in Silton et al., (2010), such that earlier LDLPFC activity predicted later dACC activity. At higher levels of depression, however, LDLPFC and dACC activity were less related. As the relationship between LDLPFC and dACC activity weakened with increasing depression, a direct relationship between LDLPFC and performance emerged. In the context of this weakened neural coupling associated with depression, increased LDLPFC activity was associated with reduced Stroop interference (better performance). Although the degree of depression alone does not directly predict performance on the Stroop task, it appears that it does alter the neural circuitry that is employed to meet task demands. This pattern of activity is consistent with the predictions of the cascade-of-control model, such that increased LDLPFC activity is indicative of an increased need for compensatory top-down control.
At higher levels of anxious apprehension, increased dACC activity was related to greater Stroop interference (worse performance), suggesting that, as anxious apprehension increases, cognitive control is implemented increasingly via dACC. Conceivably, anxious apprehension is associated with worries about aspects of performance, which in turn interfere with adaptive conflict resolution, leading to increased recruitment of dACC to aid in response selection.
Very few studies have addressed the relationship between anxiety and dACC function during top-down attentional control. Instead, most research has focused on rostral ACC (rACC, called the “affective” region by Bush et al., 2000) and its role in processing affective information (e.g., Bishop, Duncan, Brett, & Lawrence, 2004; Engels et al., 2007; Mohanty et al., 2007). Evidence suggests that dACC and rACC are distinct regions that contribute to different cortical and subcortical pathways. rACC has been implicated in the evaluation of emotional information and the regulation of emotional responses, whereas dACC is often associated with cognitive function, particularly during tasks that involve conflict resolution (Bush et al., 2000; Mohanty et al., 2007).
Given different roles, it is not surprising that inverse patterns of rACC activity and dACC activity have been associated with anxiety. Lower rACC activity has been related to higher anxiety, possibly indicating less control in the presence of threatening stimuli (Bishop et al., 2004; Engels et al., 2007). In contrast to rACC, present results showed that higher dACC activity was associated with higher anxious apprehension levels. Similar findings have been reported in other studies (Breiter et al., 1996; Bystritsky et al., 2001, Eisenberger, Liberman, & Satpute, 2005; Ursu et al., 2003). Eisenberger et al. (2005) showed that neuroticism, a personality factor that is consistently related to anxiety, was positively correlated with dACC activity but negatively correlated with rACC. Moreover, the Eisenberger et al. study showed that individuals high in neuroticism demonstrated increased dACC activity during conflict trials. These findings suggested that individuals high in anxiety have an abnormal conflict system that is reflected in higher dACC activity, consistent with many studies showing that dACC is engaged in conflict resolution and later aspects of response selection (e.g., Banich, 2009; Botvinick et al., 2004; Silton et al., 2010). Similarly, a study that involved individuals with Obsessive-Compulsive Disorder (an anxiety disorder that commonly involves high levels of worry) showed more dACC during high-conflict trials (Ursu et al., 2003), and Krug and Carter (in press) found that individuals high on trait anxiety had more dACC activity than individuals low on trait anxiety during conflict trials in a facial Stroop task. However, another study that directly investigated the impact of trait and state anxiety on dACC function found that anxiety did not influence dACC during attentional control (Bishop, 2009). Possibly, the letter-search task used in that study did not involve the level of conflict resolution demanded by the Stroop task, which has repeatedly been shown to involve dACC (Botvinick et al., 2004). Although more research is needed to elucidate the various ways that anxiety types may differentially affect dACC and rACC activity and related cognitive function, the bulk of the evidence favors the conclusion that anxious apprehension or worry is associated with more dACC activity.
Present data suggest that performance impairments in anxious individuals during conflict-resolution tasks are related to inadequate dACC-mediated cognitive-control mechanisms that would typically suppress attentional disruption caused by worries or ruminations. Inadequate control mechanisms may lead to further difficulties shifting attention away from such concerns. Inadequate compensatory dACC activity and related difficulties resolving conflict may accentuate problems resolving issues of daily life and thus contribute to a ruminative cycle due a lack of more effective and efficient problem-solving options.
The present study is apparently the first to explicitly evaluate the influence of depression and anxious apprehension on a frontocingulate network (not solely focal cortical activity) during top-down attentional control. Results showed that depression and anxiety affect the network in different ways, and these different patterns of network activity were generally consistent with the predictions of the cascade-of-control model. This study provides support for models that posit that depression influences a network rather than individual brain regions in isolation (e.g. Heller, 1993; Mayberg, 1997). Unlike previous depression neuroimaging studies that have used non-directional correlation methods such as functional connectivity, the present study provides unique information regarding how depression and anxiety modify specific temporal relationships between network segments involved in attentional control. Medium effect sizes were obtained for the models that were evaluated, and the present study was adequately powered to detect a medium effect size (per Cohen, 1992). Effect sizes for interactions in psychological research are typically within the small to medium range (Cohen et al., 2003). It is rare for studies that have used connectivity methods to explicitly report effect size, so it is difficult to estimate how the effect size obtained in the present study compares to other studies. Because distinct patterns of network activity were related to behavioral outcomes, the medium effect size in the present study suffices to demonstrate functional significance.
Future studies should continue to address how psychopathology influences network activity during cognitive function, as multiple networks may be recruited based on specific task demands, and different types of psychopathology will likely differentiate these various networks and related function. Furthermore, evidenced-based treatment outcome research that incorporates pre- and post-treatment neuroimaging measures may benefit from studying how treatment changes network activity rather than focusing on how treatment influences individual brain regions. Research in this vein may help inform future diagnostic categories and methods that aim to provide reliable identification of psychological disorders, along with furthering the development of effective evidenced-based treatments for depression and anxiety.
Acknowledgments
This research was supported by the National Institute of Mental Health (P50 MH079485, R01 MH61358, T32 MH19554), the National Institute on Drug Abuse (R21 DA14111), and the University of Illinois Beckman Institute, Department of Psychology, and Intercampus Research Initiative in Biotechnology. The authors thank Adrienne Abramowitz, Kirstin Aschbacher, Patrick Berg, Keith Bredemeier, Amanda Bull, Emily Cahill, Laura Crocker, Monica Fabiani, Kara Federmeier, Joscelyn Fisher, Christian Hendershot, Brenda Hernandez, Karsten Hoechstetter, Angela Lawson, Renee Thompson, Edelyn Verona, and Stacie Warren, for their contributions to this project. This manuscript is based on data that was included in Rebecca Levin Silton's doctoral dissertation. A portion of this research was presented at the 2009 meeting of the Society for Research in Psychopathology. A different manuscript that involves a subset of the participants included in this manuscript was published in NeuroImage. That manuscript evaluated the temporal course of dACC and LDLPFC proposed in the cascade-of-control model and did not address the role of psychopathology.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/abn
Silton et al. (2010) did not address the relationship between psychopathology and the temporal course of LDLPFC and dACC. The present study was designed to follow-up questions raised about psychopathology in the Silton et al. study. As a follow-up study, a superset of the Silton et al. sample and similar methodology were used in the present study. Results from Silton et al. guided present hypotheses regarding how depression and anxiety influence the temporal course of the frontocingulate network.
Contributor Information
Rebecca Levin Silton, Department of Psychology, University of Illinois at Urbana-Champaign.
Wendy Heller, Department of Psychology and Beckman Institute Biomedical Imaging Center, University of Illinois at Urbana-Champaign.
Anna S. Engels, Department of Psychology, University of Illinois at Urbana-Champaign
David N. Towers, Department of Psychology, University of Illinois at Urbana-Champaign
Jeffrey M. Spielberg, Department of Psychology, University of Illinois at Urbana-Champaign
J. Christopher Edgar, Department of Psychology and Beckman Institute Biomedical Imaging Center, University of Illinois at Urbana-Champaign.
Sarah M. Sass, Department of Psychology, University of Illinois at Urbana-Champaign
Jennifer L. Stewart, Department of Psychology, University of Illinois at Urbana-Champaign
Bradley P. Sutton, Department of Psychology and Beckman Institute Biomedical Imaging Center, University of Illinois at Urbana-Champaign
Marie T. Banich, Department of Psychology, University of Colorado at Boulder
Gregory A. Miller, Departments of Psychology and Psychiatry and Beckman Institute Biomedical Imaging Center, University of Illinois at Urbana-Champaign.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage Publications, Inc; Thousand Oaks, CA: 1991. [Google Scholar]
- Aguirre GK, Zarahn E, D'Esposito M. The variability of human BOLD hemodynamic response. NeuroImage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. doi:10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Magin R. fMRI studies of stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of Cognitive Neuroscience. 2000a;12:988–1000. doi: 10.1162/08989290051137521. doi:10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer A, Brown C. Prefrontal regions play a predominant role in imposing an attentional “set”: Evidence from fMRI. Cognitive Brain Research. 200b;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. doi:10.1016/S0926-6410(00)00015-X. [DOI] [PubMed] [Google Scholar]
- Banich MT. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009;18:89–94. doi:10.1111/j.1467-8721.2009.01615.x. [Google Scholar]
- Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RSJ, Dolan RJ. Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia. 1993;31:907–922. doi: 10.1016/0028-3932(93)90147-r. doi:10.1016/0028-3932(93)90147-R. [DOI] [PubMed] [Google Scholar]
- Behar E, Alcaine O, Zuellig AR, Borkovec TD. Screening for generalized anxiety disorder using the Penn State Worry Questionanire: a receiver operating characterisitic analysis. Journal of Behavior Therapy and Experimental Psychiatry. 2003;34:25–43. doi: 10.1016/s0005-7916(03)00004-1. doi:10.1016/S0005-7916(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalography and Clinical Neurophysiology. 1994;90:229–241. doi: 10.1016/0013-4694(94)90094-9. doi:10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Biringer E, Lundervold A, Stordal K, Mykletun A, Egeland J, Bottlender R, Lund A. Executive function improvement upon remission of recurrent unipolar depression. European Archives of Psychiatry and Clinical Neuroscience. 2005;255:373–380. doi: 10.1007/s00406-005-0577-7. doi:10.1007/s00406-005-0577-7. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12:92–98. doi: 10.1038/nn.2242. doi:10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–188. doi: 10.1038/nn1173. doi:10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. doi:10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bredemeier K, Spielberg JM, Silton RL, Berenbaum H, Heller W, Miller GA. Screening for clinical depression using the MASQ anhedonic depression scale: A receiver-operator characteristic analysis. Psychological Assessment. doi: 10.1037/a0019915. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arvices of General Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, Lewis RB. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy. Archives of General Psychiatry. 2001;58:631–640. doi: 10.1001/archpsyc.58.7.631. doi:10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- Bystritsky A, Pontillo D, Powers M, Sabb FW, Craske MG, Bookheimer SY. Functional MRI changes during panic anticipation and imagery exposure. NeuroReport. 2001;12:3953–3957. doi: 10.1097/00001756-200112210-00020. doi:10.1097/00001756-200112210-00020. [DOI] [PubMed] [Google Scholar]
- Cheung GW, Lau RS. Testing mediation and suppression effects of latent variables: Bootstrapping with structural equation models. Organizational Research Methods. 2008;11:296–325. doi:10.1177/1094428107300343. [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd Edition Lawrence Erlbaum Associates, Inc; Mahwah, NJ: 2003. [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. doi:10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, Salmon E. Exploring the unity and diversity of the neural substrates of executive functioning. Human Brain Mapping. 2005;25:409–423. doi: 10.1002/hbm.20118. doi:10.1002/hbm.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton RJ, Heller W, Banich MT, Palmieri PA, Miller GA. Responding to threat: Hemispheric asymmetries and interhemispheric division of input. Neuropsychology. 2000;14:254–264. doi: 10.1037//0894-4105.14.2.254. doi:10.1037/0894-4105.14.2.254. [DOI] [PubMed] [Google Scholar]
- Cook EW, Miller GA. Digital filtering: Background and tutorial for psychophysiologists. Psychophysiology. 1992;29:350–367. doi: 10.1111/j.1469-8986.1992.tb01709.x. doi:10.1111/j.1469-8986.1992.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. The Journal of Neuroscience. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. doi:10.1016/S0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Dunkin JJ, Leuchter AF, Cook IA, Kasl-Godley JE, Abrams M, Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. Journal of Affective Disorders. 2000;60:13–23. doi: 10.1016/s0165-0327(99)00157-3. doi:10.1016/S0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Stewart JL, Miller GA. Digital filtering in EEG/ERP research. In: Handy TC, editor. Event-related potentials: A handbook. MIT Press; Cambridge, MA: 2005. pp. 85–113. [Google Scholar]
- Eisenberger NI, Liberman MD, Satpute AJ. Personality from a controlled processing perspective: An fMRI study of neuroticism, extraversion, and self-consciousness. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:169–181. doi: 10.3758/cabn.5.2.169. doi:10.3758/CABN.5.2.169. [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Mohanty A, Herrington JD, Banich MT, Webb AG, et al. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology. 2007;44:352–363. doi: 10.1111/j.1469-8986.2007.00518.x. doi: 10.1111/j.1469-8986.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Spielberg JM, Warren SL, Sutton BP, Banich MT, Miller GA. Co-occurring anxiety influences patterns of brain activation in depression. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:141–156. doi: 10.3758/CABN.10.1.141. doi:10.3758/CABN.10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck, Derakshan, Santos, Calvo Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. doi:10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. NeuroImage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. doi:10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders – non-patient edition (SCID-I/NP, version 2.0 – 4/97 revision) Biometrics Research Department; New York: 1997. [Google Scholar]
- Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Research. 2006;148:33–45. doi: 10.1016/j.pscychresns.2006.04.006. doi:10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Fox E. Attentional bias in anxiety: selective or not? Behavior Research and Therapy. 1993;31:487–493. doi: 10.1016/0005-7967(93)90129-i. doi:10.1016/0005-7967(93)90129-I. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Himle J, Niesenson LG. Action monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11:1–6. doi: 10.1111/1467-9280.00206. doi:10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring HA, Pazzaglia PJ, Post RM. Blunted left cingulate activation in mood disorder subjects during a response interference task (the stroop) The Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9:55–63. doi: 10.1176/jnp.9.1.55. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E. Information processing as a vulnerability factor for depression. Behavior Therapy. 1998;29:603–617. doi:10.1016/S0005-7894(98)80020-8. [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joorman Y. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004;113:127–135. doi: 10.1037/0021-843X.113.1.121. doi:10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biological Psychiatry. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. doi:10.1016/S0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Fossati P, Pochon JB, Levy R, Lebastard G, Lehericy S, Dubois B. Cognitive control and brain resources in major depression: An fMRI study using the n-back task. NeuroImage. 2005;26:860–869. doi: 10.1016/j.neuroimage.2005.02.048. doi:10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Heller W. Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology. 1993;4:476–489. doi: 10.1037/0894-4105.7.4.476. [Google Scholar]
- Heller W, Nitschke JB. Regional brain activity in emotion: A framework for understanding cognition in depression. Cognition and Emotion. 1997;11:637–661. doi:10.1080/026999397379845a. [Google Scholar]
- Heller W, Etienne MA, Miller GA. Patterns of perceptual asymmetry in depression and anxiety: Implications for neuropsychological models of emotion and psychopathology. Journal of Abnormal Psychology. 1995;104:327–333. doi: 10.1037//0021-843x.104.2.327. doi:10.1037/0021-843X.104.2.327. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Heller W, Mohanty A, Engels A, Banich MT, Webb AW, Miller GA. Localization of asymmetric brain function in emotion and depression. Psychophysiology. doi: 10.1111/j.1469-8986.2009.00958.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel PT. Depression and memory: Are impairments remediable through attentional control? Current Direction in Psychological Science. 1994;3:190–194. doi:10.1111/1467-8721.ep10770707. [Google Scholar]
- Hertel PT. Impairments in inhibition or cognitive control in psychological disorders. Applied and Preventive Psychology. 2007;12:149–153. [Google Scholar]
- Hochman SH. The effects of stress on Stroop color-word performance. Psychonomic Science. 1967;9:475–476. [Google Scholar]
- Holmes AJ, Pizzagalli DA. Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia. 2008;46:2904–2913. doi: 10.1016/j.neuropsychologia.2008.05.028. doi:10.1016/j.neuropsychologia.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J, Berns S, Uzelac S, Davis-Conway S. Neurocognitive deficits and disability in major depressive disorder. Psychiatry Research. 2006;145:39–48. doi: 10.1016/j.psychres.2005.11.011. doi:10.1016/j.psychres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. doi:10.1016/S1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Keller J, Nitschke JB, Bhargava T, Deldin PJ, Gergen JA, Miller GA, Heller W. Neuropsychological differentiation of depression and anxiety. Journal of Abnormal Psychology. 2000;109:3–10. doi:10.1037/0021-843X.109.1.3. [PubMed] [Google Scholar]
- Kessler RC, Dupont RL, Berglund P, Wittchen H-U. Impairment in pure and comorbid generalized anxiety disorder and major depression at 12 months in two national surveys. American Journal of Psychiatry. 1999;156:1915–1923. doi: 10.1176/ajp.156.12.1915. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, Blazer DG. Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. British Journal of Psychiatry. 1996;168:17–30. [PubMed] [Google Scholar]
- Kessler RC, Zhao S, Blazer DG, Swartz MD. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. Journal of Affective Disorders. 1997;45(1):19–30. doi: 10.1016/s0165-0327(97)00056-6. doi:10.1016/S0165-0327(97)00056-6. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Gruber SA, Yurgelun-Todd DA. Depressed mood and lateralized prefrontal activity during a Stroop task in adolescent children. Neuroscience Letters. 2007;416:43–48. doi: 10.1016/j.neulet.2007.01.081. doi:10.1016/j.neulet.2007.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug MK, Carter CS. Adding fear to conflict: A general purpose cognitive control network is modulated by trait anxiety. Cognitive, Affective, and Behavioral Neuroscience. doi: 10.3758/CABN.10.3.357. in press. [DOI] [PubMed] [Google Scholar]
- Levin RL, Heller W, Mohanty A, Herrington JD, Miller GA. Cognitive deficits in depression and functional specificity of regional brain activity. Cognitive Therapy and Research. 2007;31:211–233. doi:10.1007/s10608-007-9128-z. [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Functional dissociation of attentional selection within PFC: Response and non-response related aspects of attentional selection as ascertained by fMRI. Cerebral Cortex. 2006;16:827–834. doi: 10.1093/cercor/bhj026. doi:10.1093/cercor/bhj026. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. The MIT Press; London: 2005. [Google Scholar]
- MacKinnon DP. Introduction to Statistical Mediation Analysis. Tayor & Francis Group; New York, NY: 2008. [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: A proposed model of depression. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biological Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. doi:10.1016/S0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn state worry questionnaire. Behavior Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. doi:10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. Clinical Neurophysiology. 2004;115:2195–2222. doi: 10.1016/j.clinph.2004.06.001. doi:10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: Effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative thinking. Neuroimage. 2000;11:735–739. doi: 10.1006/nimg.2000.0568. doi:10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: An fMRI analysis of conflict specificity and functional differentiation. Human Brain Mapping. 2005;25:328–335. doi: 10.1002/hbm.20110. doi:10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Barad V. Competition for priority in processing increases prefrontal cortex's involvement in top-down control: An event-related fMRI study of the stroop task. Cognitive Brain Research. 2003;17:212–222. doi: 10.1016/s0926-6410(03)00108-3. doi:10.1016/S0926-6410(03)00108-3. [DOI] [PubMed] [Google Scholar]
- Miller GA, Gratton G, Yee CM. Generalized implementation of an eye movement correction procedure. Psychophysiology. 1988;25:241–243. doi:10.1111/j.1469-8986.1988.tb00999.x. [Google Scholar]
- Miller GA, Lutzenberger W, Elbert T. The linked-reference issue in EEG and ERP recording. Journal of Psychophysiology. 1991;5:273–276. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. doi:10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ho MR, Banich MT, Miller GA. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–351. doi: 10.1111/j.1469-8986.2007.00515.x. doi:10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Molina S, Borkovec TD. The Penn state worry questionnaire: Psychometric properties and associated characteristics. In: Davey GCL, Tallis F, editors. Worrying: Perspectives on Theory, Assessment and Treatment. Wiley; Chichester: 1994. pp. 265–283. [Google Scholar]
- Nitschke JB, Heller W. The neuropsychology of anxiety disorders: Affect, cognition, and neural circuitry. In: D'haenen H, den Boer JA, Westenberg H, Willner P, editors. Textbook of biological psychiatry. Wiley; Chichester, UK: 2002. pp. 975–988. [Google Scholar]
- Nitschke JB, Heller W, Imig JC, McDonald RP, Miller GA. Distinguishing dimensions of anxiety and depression. Cognitive Therapy and Research. 2001;25:1–22. doi:10.1023/A:1026485530405. [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628–637. doi:10.1017/S0048577299972013. [PubMed] [Google Scholar]
- Nitschke JB, Miller GA, Cook EW., III Digital filtering in EEG/ERP analysis: Some technical and empirical comparisons. Behavior Research Methods, Instruments & Computers. 1998;30:54–67. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. doi:10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error related negativity (ERN) and psychopathology: Toward an endophenoype. Clinical Psychology Review. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. doi:10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso S, Lamberty GJ, Garvey MJ, Robinson RG. Cognitive impairment in the euthymic phase of chronic unipolar depression. The Journal of Nervous and Mental Disease. 1997;185:748–754. doi: 10.1097/00005053-199712000-00005. doi:10.1097/00005053-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Simmons A, Stein MB. Anterior cingulate activation in high trait anxious subjects is related to altered error processing during decision making. Biological Psychiatry. 2004;55(12):1179–1187. doi: 10.1016/j.biopsych.2004.02.023. doi:10.1016/j.biopsych.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF. Assessing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. doi:10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Ravnkilde B, Videbech P, Clemmensen K, Egander A, Rasmussen NA, Rosenberg R. Cognitive deficits in major depression. Scandinavian Journal of Psychology. 2002;43:239–251. doi: 10.1111/1467-9450.00292. doi:10.1111/1467-9450.00292. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Bradshaw JL, Pantelis C, Phillips JG. Frontostriatal deficits in unipolar major depression. Brain Research Bulletin. 1998;47:297–310. doi: 10.1016/s0361-9230(98)00126-9. doi:10.1016/S0361-9230(98)00126-9. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale J. Mindfulness-based cognitive therapy for depression: A new approach to preventing relapse. Guilford Press; New York: 2002. [Google Scholar]
- Siegle GJ, Ghinassi F, Thase ME. Neurobehavioral therapies in the 21rst century: summary of an emerging field and an extended example of cognitive control therapy for depression. Cognitive Research and Therapy. 2007;31:235–262. doi:10.1007/s10608-006-9118-6. [Google Scholar]
- Silton RL, Miller GA, Towers DN, Engels AS, Edgar JC, Spielberg JM, Sass SM, Stewart JL, Sutton BP, Banich MT, Heller W. The time course of anterior cingulate and prefrontal cortical activity during top-down attentional control. NeuroImage. 2010;50:1292–1302. doi: 10.1016/j.neuroimage.2009.12.061. doi:10.1016/j.neuroimage.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stordal KI, Lundervold AJ, Egeland J, Mykletun A, Asbjornsen A, Landro NI, Lund A. Impairment across executive functions in recurrent major depression. Nordic Journal of Psychiatry. 2004;58:41–47. doi: 10.1080/08039480310000789. doi:10.1080/08039480310000789. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Factors affecting speed in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. doi:10.1037/h0054651. [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: Evidence from functional magnetic resonance imaging. Psychological Science. 2003;14:347–353. doi: 10.1111/1467-9280.24411. doi:10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Gammelgaard L, Egander A, Clemmensen K, Rasmussen NA, Rosenberg R. The Danish PET/depression project: Performance on stroop's test linked to white matter lesions in the brain. Psychiatry Research. 2004;130:117–130. doi: 10.1016/j.pscychresns.2003.10.002. doi:10.1016/j.pscychresns.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, Schlosser RGM. Cortical inefficiency in patients with unipolar depression: An event-related FMRI study with the stroop task. Biological Psychiatry. 2006;59:958–965. doi: 10.1016/j.biopsych.2005.10.025. doi:10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Ward DB. Simultaneous inference for FMRI data. 2000. http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf, accessed July 27, 2006.
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995a;104:15–25. doi: 10.1037//0021-843x.104.1.15. doi:10.1037/0021-843X.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995b;104:3–14. doi: 10.1037//0021-843x.104.1.3. doi:10.1037/0021-843X.104.1.3. [DOI] [PubMed] [Google Scholar]
- Weiland-Fiedler P, Erickson K, Waldeck T, Luckenbaugh DA, Pike D, Bonne O, Neumeister A. Evidence for continuing neuropsychological impairments in depression. Journal of Affective Disorders. 2004;82:253–258. doi: 10.1016/j.jad.2003.10.009. doi:10.1016/j.jad.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith S. Temporal autocorrelation in univariate linear modeling of fMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. doi:10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]