Abstract
Introduction
We characterized the association of 3 metabolic conditions – obesity, metabolic syndrome, and nonalcoholic fatty liver disease (NAFLD) – with increased inflammation and subclinical atherosclerosis.
Methods
We conducted cross-sectional analysis of 3,976 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) with adequate CT imaging to diagnose NAFLD. Obesity was defined as BMI ≥30 kg/m2, metabolic syndrome by AHA/NHLBI criteria, and NAFLD using non-contrast cardiac CT and a liver/spleen attenuation ratio (L/S) <1. Increased inflammation was defined as high sensitivity C-reactive protein (hsCRP) ≥2 mg/L and subclinical atherosclerosis as coronary artery calcium (CAC) >0. We studied the association of a stepwise increase in number of these metabolic conditions (0–3) with increased inflammation and CAC, stratifying results by gender and ethnicity.
Results
Mean age of participants was 63 (±10) years, 45% were male, 37% white, 10% Chinese, 30% African American, and 23% Hispanic. Adjusting for obesity, metabolic syndrome and traditional risk factors, NAFLD was associated with a prevalence odds ratio for hsCRP ≥2 mg/L and CAC >0 of 1.47 (1.20–1.79) and 1.37 (1.11–1.68) respectively. There was a positive interaction between female gender and NAFLD in the association with hsCRP ≥2 mg/L (p= 0.006), with no interaction by race. With increasing number of metabolic conditions, there was a graded increase in prevalence odds ratios of hsCRP ≥2 mg/L and CAC >0.
Conclusion
NAFLD is associated with increased inflammation and CAC independent of traditional risk factors, obesity and metabolic syndrome. There is a graded association between obesity, metabolic syndrome, and NAFLD with inflammation and CAC.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is an important condition with an estimated worldwide prevalence of 20%1. There is increasing recognition that NAFLD is associated with cardiovascular disease (CVD). Cross-sectional epidemiologic studies have shown NAFLD to be linked to a higher prevalence of CVD independent of traditional risk factors2. NAFLD has also been associated with an increased risk of CVD events in patients with type 2 diabetes independent of obesity, other metabolic syndrome components, and traditional risk factors3. Indeed, the most common cause of death among individuals with NAFLD is CVD1.
In addition to manifest CVD, NAFLD has been associated with increased inflammation4–7 and subclinical atherosclerosis8–12 both of which are well known predictors of CVD in asymptomatic patients13–19. NAFLD has also been closely associated with obesity and metabolic syndrome - two conditions known to be associated with CVD, subclinical atherosclerosis, and systemic inflammation20–24. The associations of these metabolic conditions with inflammation and subclinical atherosclerosis have not been well characterized. As a result, some investigators have contended that NAFLD is an epiphenomenon as opposed to a mediator in the development of CVD25. The relationship between NAFLD, obesity, metabolic syndrome, and CVD is further complicated by the known ethnic differences in the prevalence of both NAFLD and metabolic risk factors26.
We hypothesized that NAFLD would be associated with high sensitivity C-reactive protein (hsCRP) as well as coronary artery calcium (CAC) independent of obesity and metabolic syndrome, and that this relationship would be similar across ethnic groups. Furthermore, we hypothesized that there would be a graded association between the number of these metabolic conditions present and the prevalence of high hsCRP and CAC.
Methods
Study Design
MESA is an observational cohort of 6,814 men and women aged 45–84 years without known CVD at the time of enrollment. White, Black, Chinese, and Hispanic individuals were enrolled at six different US field centers (Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York City, New York; and St. Paul, Minnesota) from July 2000 through September 2002. The MESA study design has been described in detail previously27. The study was approved by the institutional review board of each site, and all participants gave written informed consent.
A total of 4,384 individuals had an adequate non-contrast cardiac CT imaging to diagnose fatty liver. Compared to those who were excluded for inadequate CT imaging, our population was in general older, more likely to be female and had a higher prevalence of obesity, diabetes and hypertension. Those with adequate imaging were also more likely to be black and Hispanic but less likely to be Chinese. We excluded 285 individuals for a history of heavy alcohol use (> 14 drinks/week for men, > 7 drinks/week for women), known cirrhosis, oral corticosteroid or amiodarone use. An additional 123 individuals were excluded for missing covariates. The final population consisted of 3,976 participants.
HsCRP Measurement
HsCRP was measured at the baseline examination using a particle-enhanced immunonepholometric assay on the BNII nephelometer (Dade-Behring, Inc., Deerfield, IL)28 at the University of Vermont, Burlington, Vermont. We defined increased inflammation as hsCRP ≥2 mg/L29.
CAC Score Measurement
Details of the MESA scanning protocol have been reported previously30. All participants underwent CAC scoring using computed tomography (CT) as part of the baseline examination. CAC was measured with either a cardiac-gated electron-beam CT scanner (Chicago, Los Angeles, New York), or a multidetector CT (Baltimore, Forsyth County, St. Paul). Individuals were scanned twice, and mean CAC (Agaston) score was calculated and used for all analyses31. All images were interpreted at the MESA CT reading center (Los Angeles Biomedical Research Institute, Torrance CA). We defined subclinical atherosclerosis as CAC >0.
Liver Fat Assessment
Details of the liver fat measurement within MESA have been previously reported26,32. Briefly, baseline cardiac CT scans were utilized to measure hepatic and splenic attenuation values (Hounsfield Units) using a region of interest of ≥ 100 mm2 in area. Two regions in the right hepatic lobe and one in the spleen were measured. The liver/spleen (L/S) attenuation ratio was calculated using the mean of the hepatic measurements divided by the splenic attenuation value. NAFLD was defined as L/S attenuation ratio < 1.
Risk Factor Measurement
Information pertaining to demographics, medical history, cigarette smoking, and alcohol use was collected at the baseline visit using standardized questionnaires, as previously described27. Waist circumference was measured at the umbilicus. Body mass index (BMI) was calculated as weight in kilograms divided by height divided in meters squared. Using an automated sphygmomanometer (Critikon, Tampa, FL), systolic and diastolic blood pressure (SBP & DBP) were measured 3 times and the mean of the last two measurements was used. A central laboratory (Fairview-University Medical Center, Minneapolis, MN) measured levels of total and high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) after a 12-hour fast. Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation. Obesity was defined as BMI ≥ 30 kg/m2. Metabolic syndrome was defined by the American Heart Association/National Heart, Lung, and Blood Institute criteria as at least 3 of the following: waist circumference ≥102 cm for men and ≥88 cm for women; TG ≥ 150mg/dL or drug treatment for elevated TG; HDL-C < 40mg/dl for men and <50mg/dL for women or drug treatment for reduced HDL-C; SBP ≥130 mmHg or DBP ≥85 mmHg or drug treatment for hypertension; and fasting blood glucose level ≥ 100 mg/dL or drug treatment for elevated glucose33.
Statistical Analysis
We described baseline characteristics by presence or absence of NAFLD. We used logistic regression models to study the cross-sectional association between NAFLD and the outcomes of hsCRP ≥2 mg/L and CAC >0. The first model was adjusted for age, gender, and ethnicity as well as other CVD risk factors not included in the metabolic syndrome: smoking status, LDL-cholesterol, use of lipid-lowering medication, and level of education. In order to assess the strength of association for NAFLD independent of obesity and metabolic syndrome, a second logistic regression model was performed adjusting for obesity and components of the metabolic syndrome (each individual component coded as binary) in addition to the covariates used in model 1. These two logistic regression models were stratified by ethnicity and gender and estimates were calculated for each ethnicity or gender category. Formal multiplicative interaction testing was also performed for the ethnicity and gender variables with NAFLD. As a sensitivity analysis we adjusted for overweight rather than obesity and obtained unchanged results.
In order to assess a possible gradient-response, logistic regression (Model 1) was used to examine the association of a stepwise increase in the number (0–3) of metabolic conditions (NAFLD, obesity, metabolic syndrome) with increased inflammation and subclinical atherosclerosis. We also calculated the p-value for linear trend of the prevalence odds ratios.
All analyses were performed using STATA version 13.1 (Stata Corp, College Station, Texas).
Results
There were 670 (16.9%) individuals with NAFLD. Baseline characteristics according to presence/absence of NAFLD are shown in Table 1. Mean age differed slightly, 61 (± 9.6) years for those with NAFLD and 63 (± 10.5) years for those without. Gender distribution was similar in each group (46% male). The ethnic makeup of the two groups differed significantly, with a predominance of Hispanics in the NAFLD group. Individuals with NAFLD were more likely to be obese and meet criteria for metabolic syndrome. The crude prevalence of hsCRP ≥2 mg/L and CAC >0 was greater in those with NAFLD compared to those without (63% vs. 46%) and (54% vs. 50%) respectively.
Table 1.
Baseline characteristics of the population by presence or absence of NAFLD
| Overall (N=3,976) |
No NAFLD/liver spleen ratio ≥1 (n=3306) |
NAFLD/ liver spleen ratio <1 (n=670) |
p-value | |
|---|---|---|---|---|
| Age (years), mean (SD) | 62.9 (10.4) | 63.3 (10.5) | 61.2 (9.6) | <0.001 |
| Gender | 0.82 | |||
| Male (%) | 1,794 (45.1) | 1,489 (45.9) | 305 (45.5) | |
| Female (%) | 2,182 (54.9) | 1,817 (55.0) | 365 (54.5) | |
| Race/Ethnicity | <0.001 | |||
| Whites (%) | 1,466 (36.9) | 1,250 (37.8) | 216 (32.2) | |
| Chinese (%) | 388 (9.8) | 314 (9.5) | 74 (11.0) | |
| African American (%) | 1,192 (30.0) | 1,060 (32.1) | 132 (19.7) | |
| Hispanics (%) | 930 (23.4) | 682 (20.6) | 248 (37.0) | |
| Current smokers (%) | 447 (11.2) | 380 (11.5) | 67 (10) | 0.09 |
| Obese1 (%) | 1,328 (33.4) | 972 (29.4) | 356 (53.1) | <0.001 |
| Metabolic syndrome2 (%) | 1,482 (37.3) | 1,056 (31.9) | 426 (63.6) | <0.001 |
| LDL-C (mg/dL), mean (SD) | 117.5 (31.2) | 117.9 (31.2) | 115.7 (31.1) | 0.10 |
| HDL-C (mg/dL), mean (SD) | 50.5 (14.5) | 51.7 (14.7) | 45 (11.8) | <0.001 |
| Triglycerides (mg/dL), median (IQR) | 110 (82) | 104 (75) | 149 (98) | 0.0001 |
| Waist circumference (cm), mean (SD) | 98.8 (13.9) | 97.4 (13.7) | 105.7 (13.2) | <0.001 |
| SBP, mean (SD) | 127.2 (21.6) | 126.7 (21.7) | 129.9 (20.9) | 0.0005 |
| DBP, mean (SD) | 71.7 (10.2) | 71.4 (10.1) | 73.2 (10.3) | <0.001 |
| Hypertension (%)3 | 1,862 (46.8) | 1,506 (45.6) | 356 (53.1) | <0.001 |
| Diabetes mellitus4 (%) | 517 (13.0) | 370 (11.2) | 147 (21.9) | <0.001 |
. Obesity was defined as BMI≥ 30 kg/m2
. Metabolic syndrome was defined by the National Cholesterol Education Program Adult Treatment Panel III criteria as 3 or more of the following: waist circumference greater than 102 cm for men and greater than 88 cm for women, triglyceride level 150 mg/dL or more, HDL-C less than 40 mg/dL for men and less than 50 mg/dL for women, blood pressure 130/85 mm Hg or more, and a fasting blood glucose level 110 mg/dL or more
. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg and diastolic blood pressure (DBP) ≥90 mmHg
. Diabetes mellitus was defined as a fasting blood glucose level of ≥126 mg/dL or the use of hypoglycemic medications
NAFLD and inflammation
Multivariable adjusted prevalence odds ratios (OR) in demonstrate a 2.2 fold greater odds of hsCRP ≥2mg/L in individuals with NAFLD [OR= 2.22, 95% CI (1.85–2.67)] (Table 2, Model 1). In analysis stratified by ethnicity, the association between NAFLD and increased inflammation remained significant across all ethnic groups. Additionally, when the population was stratified by gender, the association remained significant for men and women. Notably, the association between NAFLD and increased inflammation was stronger in women and was confirmed by formal interaction testing (p = 0.006). There was no significant interaction according to ethnicity (p=0.14).
Table 2.
Prevalence odds ratios (95% CI) for the association between NAFLD and hsCRP ≥2 mg/L
| N hsCRP≥2 mg/L / N Overall |
Model 1 | Model 2 | |
|---|---|---|---|
| Overall | 1,956/3,976 | 2.22 (1.85–2.67) | 1.47 (1.20–1.79) |
| Race/Ethnicity | |||
| Whites | 667/1,466 | 2.76 (2.02–3.78) | 1.68 (1.20–2.36) |
| Chinese | 87/388 | 2.28 (1.27–4.11) | 1.64 (0.87–3.10) |
| African American | 681/1,192 | 1.64 (1.10–2.45) | 1.22 (0.80–1.88) |
| Hispanic | 521/930 | 2.12 (1.54–2.91) | 1.40 (0.98–1.99) |
| Gender | |||
| Female | 1,255/2,182 | 2.90 (2.21–3.80) | 1.78 (1.32–2.40) |
| Male | 701/1,794 | 1.70 (1.31–2.21) | 1.29 (0.98–1.70) |
Model 1: adjusted for age, gender, ethnicity, smoking status, LDL cholesterol, use of lipid-lowering medication, highest level of education completed
Model 2: adjusted for Model 1 + obesity and components of metabolic syndrome
Bolded items are significant (p<0.05)
Additional analyses were conducted further adjusting for obesity and components of the metabolic syndrome to test for a true independent effect of NAFLD on increased inflammation (Table 2, Model 2). The overall association remained significant with a 1.5 fold greater odds of hsCRP ≥2 mg/L [OR= 1.47, 95% CI (1.20–1.79)]. When this fully adjusted model was stratified by ethnicity, the association remained significant for whites only. Formal interaction testing for ethnicity was nonsignificant (p= 0.48). In gender-specific analysis, the association was only significant for women. Formal interaction testing for gender was again significant (p= 0.006).
NAFLD and subclinical atherosclerosis
The presence of NAFLD was also associated with an increased prevalence of CAC. Multivariable adjusted prevalence odds ratios show a 1.6-fold greater odds of CAC >0 [OR= 1.63, 95% CI (1.34–1.98)] (Table 3, Model 1). In ethnicity-stratified analysis this association remained significant among white and Hispanic individuals. In gender-specific analysis, both men and women with NAFLD had an increased prevalence of CAC. Formal interaction testing for both ethnicity and gender were non-significant (p=0.21 and p=0.83 respectively).
Table 3.
Prevalence odds ratios (95% CI) for the association between NAFLD and CAC >0
| N CAC >0/ N Overall |
Model 1 | Model 2 | |
|---|---|---|---|
| Overall | 2,002/3,976 | 1.63 (1.34–1.98) | 1.37 (1.11–1.68) |
| Race/Ethnicity | |||
| Whites | 861/1,466 | 1.60 (1.11–2.29) | 1.22 (0.83–1.80) |
| Chinese | 198/388 | 1.14 (0.64–2.04) | 0.97 (0.52–1.80) |
| African American | 531/1,192 | 1.48 (0.98–2.22) | 1.32 (0.87–2.00) |
| Hispanic | 412/930 | 2.00 (1.41–2.83) | 1.72 (1.19–2.50) |
| Gender | |||
| Female | 887/2,182 | 1.71 (1.30–2.23) | 1.47 (1.10–1.95) |
| Male | 1,115/1,794 | 1.49 (1.11–2.00) | 1.25 (0.92–1.71) |
Model 1: adjusted for age, gender, ethnicity, smoking status, LDL cholesterol, use of lipid-lowering medication, highest level of education completed
Model 2: adjusted for Model 1 + obesity and components of metabolic syndrome
Bolded items are significant (p<0.05)
When the model was further adjusted for obesity and components of metabolic syndrome, the overall association remained significant with a 1.4-fold greater odds of CAC >0 [OR= 1.37, 95% CI (1.11–1.68)] (Table 3, Model 2). In analysis stratified by ethnicity, the association remained significant only for Hispanic individuals. In gender-specific analysis, the association was only significant for women. Formal interaction testing for ethnicity and gender were non-significant (p=0.18 and p=0.85 respectively).
Collective association of obesity and metabolic syndrome and NAFLD
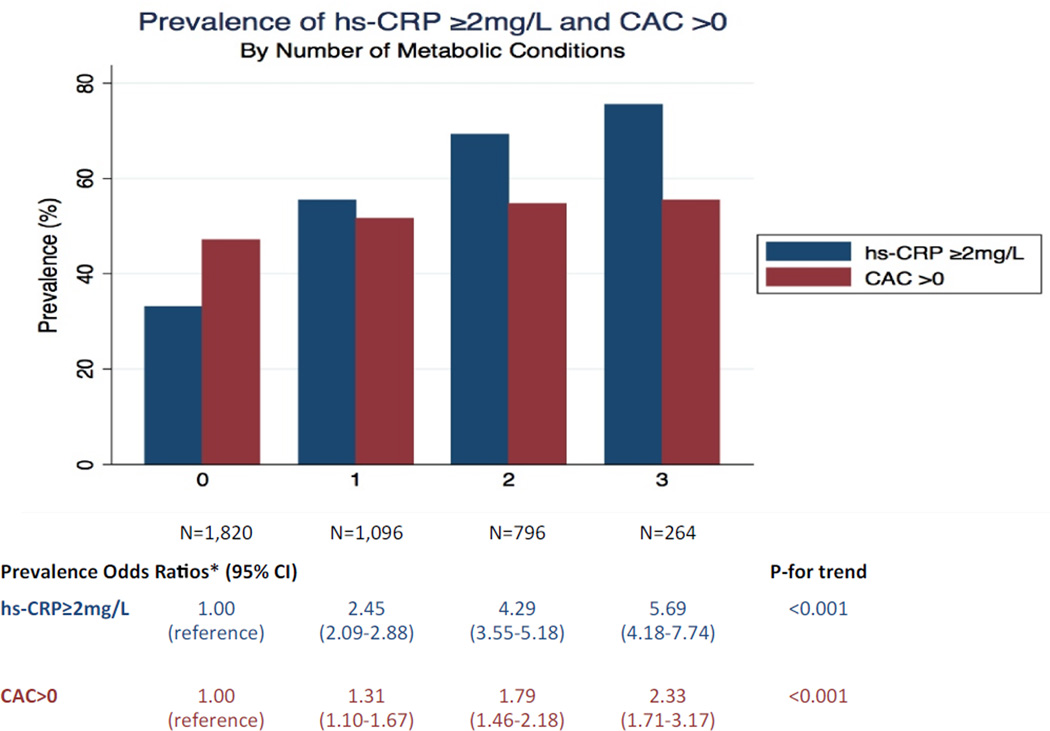
Figure 1 demonstrates the gradient response relationship between the number of metabolic conditions (obesity, metabolic syndrome, and NAFLD) with both inflammation and subclinical atherosclerosis. There was a nearly constant absolute increase in prevalence odds ratios, suggesting a possible graded association. Compared to absence of these metabolic conditions, the presence of 1, 2 and all 3 of these conditions was associated with a prevalence odds ratios of [2.45 (95% CI 2.09–2.88), 4.29 (95% 3.55–5.18) and 5.69 (4.18–7.74) respectively) for increased inflammation and [1.31 (95% 1.10–1.57), 1.79 (95% 1.46–2.18) and 2.33 (95% CI 1.71–3.18) respectively) for presence of CAC; p-value for trend <0.001 for both outcomes
Figure 1. Prevalence of hsCRP ≥2 mg/L and CAC >0 by Number of Metabolic Conditions.
*Adjusted for age, gender, ethnicity, smoking status, LDL cholesterol, use of lipid-lowering medica on, highest level of educa on completed
Discussion
The results of our cross-sectional analysis from this multi-ethnic cohort demonstrate that the presence of NAFLD is associated with systemic inflammation and subclinical atherosclerosis. This relationship remained significant after adjusting for traditional risk factors, obesity, and metabolic syndrome. We have also shown that there is a gradient response relationship with increasing number of these metabolic conditions suggesting a possible graded relationship of obesity, metabolic syndrome, and NAFLD on increased systemic inflammation and subclinical atherosclerosis.
Several prior studies have evaluated the independent association between NAFLD and inflammation as well as the relationship between NAFLD and subclinical atherosclerosis. The data demonstrating the positive association between NAFLD and increased inflammation has been fairly consistent4–7. In contrast, the data evaluating the association between NAFLD and CAC has been mixed. Several prior studies have demonstrated an increased burden of CAC among individuals with NAFLD independent of traditional risk factors and measures of abdominal adiposity8–12, whereas others have not34,35. A previous subgroup analysis from the MESA cohort evaluated the association between NAFLD and CAC in 398 black and white individuals from a single field center in North Carolina34. In this group, NAFLD was not associated with CAC. The small population size from a single center likely accounts for the difference in results from our current analysis. Furthermore, the ethnic makeup of the smaller cohort consisted of black and white individuals as opposed to the more diverse MESA cohort. The recently published analysis from the Coronary Artery Risk Development in Young Adults Study (CARDIA) evaluated the association between NAFLD and CAC in 2,424 white and black individuals with a mean age of 50 years. In this cohort, there was no significant association between NAFLD and CAC after adjustment for measures of adiposity35. The younger population may explain the difference in results compared with our analysis as well as other studies in which the mean age of the populations was generally higher.
A key strength of this analysis is the ethnic diversity of the MESA cohort. The majority of prior studies evaluating the impact of NAFLD on inflammation as well as subclinical atherosclerosis have been performed in ethnically homogeneous populations. Given the known ethnic variations in prevalence of NAFLD, it is important to evaluate the impact of NAFLD in a multi-ethnic cohort. In the current analysis, when the population was stratified by ethnicity, the multivariable-adjusted association between NAFLD and increased inflammation only remained significant among whites, whereas the relationship between NAFLD and CAC only remained significant among Hispanic individuals. Importantly however, formal multiplicative interaction testing between NAFLD and ethnicity was non-significant for increased inflammation or subclinical atherosclerosis, suggesting that the ethnic variation in strength of association may have been due to chance, possibly in the setting of the limited sample size of the ethnic subgroups.
This study has limitations that warrant acknowledgement. The use of CT is not as specific for the diagnosis of fatty liver as histology or magnetic resonance spectroscopy36. However, CT has been used in several prior epidemiologic cohorts for the diagnosis of fatty liver and has compared reasonably well to other imaging modalities37. Additionally, the cross-sectional nature of this analysis is an important limitation that precludes conclusions regarding temporality. Furthermore, we used categorical risk factor data rather than continuous variables because of the binary method in which metabolic syndrome is defined.
In conclusion, our results suggest that NAFLD is associated with both increased inflammation and subclinical atherosclerosis, independent of traditional risk factors, obesity and metabolic syndrome. Additionally, with an increasing burden of the three closely-linked metabolic conditions (NAFLD, obesity, and metabolic syndrome) there is a stepwise increase in the prevalence of increased inflammation and subclinical atherosclerosis, suggesting a graded association. Further research looking at the longitudinal effects of NAFLD may help discern whether this is a marker or mediator of disease.
NAFLD was associated with inflammation and subclinical atherosclerosis independent of obesity and metabolic syndrome
Different results were seen when stratifying by gender and race
With increasing burden of NAFLD, obesity and metabolic syndrome, there is a stepwise increase in the prevalence of inflammation and subclinical atherosclerosis
Acknowledgements
This research was supported by R01 HL071739 and contracts N01-HC-95159 through N01-HC-95165 and N01 HC 95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There is no conflict of interest to be declared
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 3.Targher G, Bertolini L, Poli F, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 4.Targher G. Relationship between high-sensitivity C-reactive protein levels and liver histology in subjects with non-alcoholic fatty liver disease. J Hepatol. 2006;45:879–881. doi: 10.1016/j.jhep.2006.09.005. author reply 881–2. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Bertolini L, Rodella S, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity (Silver Spring) 2008;16:1394–1399. doi: 10.1038/oby.2008.64. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Bertolini L, Scala L, Zoppini G, Zenari L, Falezza G. Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in nondiabetic men. Role of visceral adipose tissue. Diabet Med. 2005;22:1354–1358. doi: 10.1111/j.1464-5491.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- 7.Ndumele CE, Nasir K, Conceiçao RD, Carvalho JAM, Blumenthal RS, Santos RD. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1927–1932. doi: 10.1161/ATVBAHA.111.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos RD, Nasir K, Conceição RD, Sarwar A, Carvalho JAM, Blumenthal RS. Hepatic steatosis is associated with a greater prevalence of coronary artery calcification in asymptomatic men. Atherosclerosis. 2007;194:517–519. doi: 10.1016/j.atherosclerosis.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Kim D, Choi S-Y, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–613. doi: 10.1002/hep.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C-H, Nien C-K, Yang C-C, Yeh Y-H. Association between nonalcoholic fatty liver disease and coronary artery calcification. Dig Dis Sci. 2010;55:1752–1760. doi: 10.1007/s10620-009-0935-9. [DOI] [PubMed] [Google Scholar]
- 11.Sung K-C, Wild SH, Kwag HJ, Byrne CD. Fatty liver, insulin resistance, and features of metabolic syndrome: relationships with coronary artery calcium in 10,153 people. Diabetes Care. 2012;35:2359–2364. doi: 10.2337/dc12-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Musani SK, Bidulescu A, et al. Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African Americans: the Jackson Heart Study. Atherosclerosis. 2012;224:521–525. doi: 10.1016/j.atherosclerosis.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 15.Arad Y, Spadaro LA, Goodman K, Newstein D, Guerci AD. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol. 2000;36:1253–1260. doi: 10.1016/s0735-1097(00)00872-x. [DOI] [PubMed] [Google Scholar]
- 16.LaMonte MJ, FitzGerald SJ, Church TS, et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol. 2005;162:421–429. doi: 10.1093/aje/kwi228. [DOI] [PubMed] [Google Scholar]
- 17.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46:807–814. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 18.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 19.Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 20.Angulo P. Nonalcoholic Fatty Liver Disease — NEJM. [accessed May 28, 2014]; http://www.nejm.org/doi/full/10.1056/NEJMra011775. [Google Scholar]
- 21.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 22.Burke GL, Bertoni AG, Shea S, et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:928–935. doi: 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 24.Santos RD, Nasir K, Tufail K, Meneghelo RS, Carvalho JAM, Blumenthal RS. Metabolic syndrome is associated with coronary artery calcium in asymptomatic white Brazilian men considered low-risk by Framingham risk score. Prev Cardiol. 2007;10:141–146. doi: 10.1111/j.1520-037x.2007.888128.x. [DOI] [PubMed] [Google Scholar]
- 25.McKimmie RL, Daniel KR, Carr JJ, et al. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol. 2008;103:3029–3035. doi: 10.1111/j.1572-0241.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tota-Maharaj R, Blaha MJ, Zeb I, et al. Ethnic and sex differences in fatty liver on cardiac computed tomography: the multi-ethnic study of atherosclerosis. Mayo Clin Proc. 2014;89:493–503. doi: 10.1016/j.mayocp.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 28.DeGoma EM, French B, Dunbar RL, Allison MA, Mohler ER, Budoff MJ. Intraindividual variability of C-reactive protein: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2012;224:274–279. doi: 10.1016/j.atherosclerosis.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mora S, Ridker PM. Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)--can C-reactive protein be used to target statin therapy in primary prevention? Am J Cardiol. 2006;97:33A–41A. doi: 10.1016/j.amjcard.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 31.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 32.Kodama Y, Ng CS, Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 33.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol. 2005;4:198–203. doi: 10.1097/00132577-200512000-00018. Diagnosis and management of the metabolic s. [DOI] [PubMed] [Google Scholar]
- 34.Ding J, Kritchevsky SB, Hsu F-C, et al. Association between non-subcutaneous adiposity and calcified coronary plaque: a substudy of the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2008;88:645–650. doi: 10.1093/ajcn/88.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanWagner LB, Ning H, Lewis CE, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis. 2014;235:599–605. doi: 10.1016/j.atherosclerosis.2014.05.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–749. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong L, Chen JJ, Chen J, et al. Nonalcoholic fatty liver disease: quantitative assessment of liver fat content by computed tomography, magnetic resonance imaging and proton magnetic resonance spectroscopy. J Dig Dis. 2009;10:315–320. doi: 10.1111/j.1751-2980.2009.00402.x. [DOI] [PubMed] [Google Scholar]