Abstract
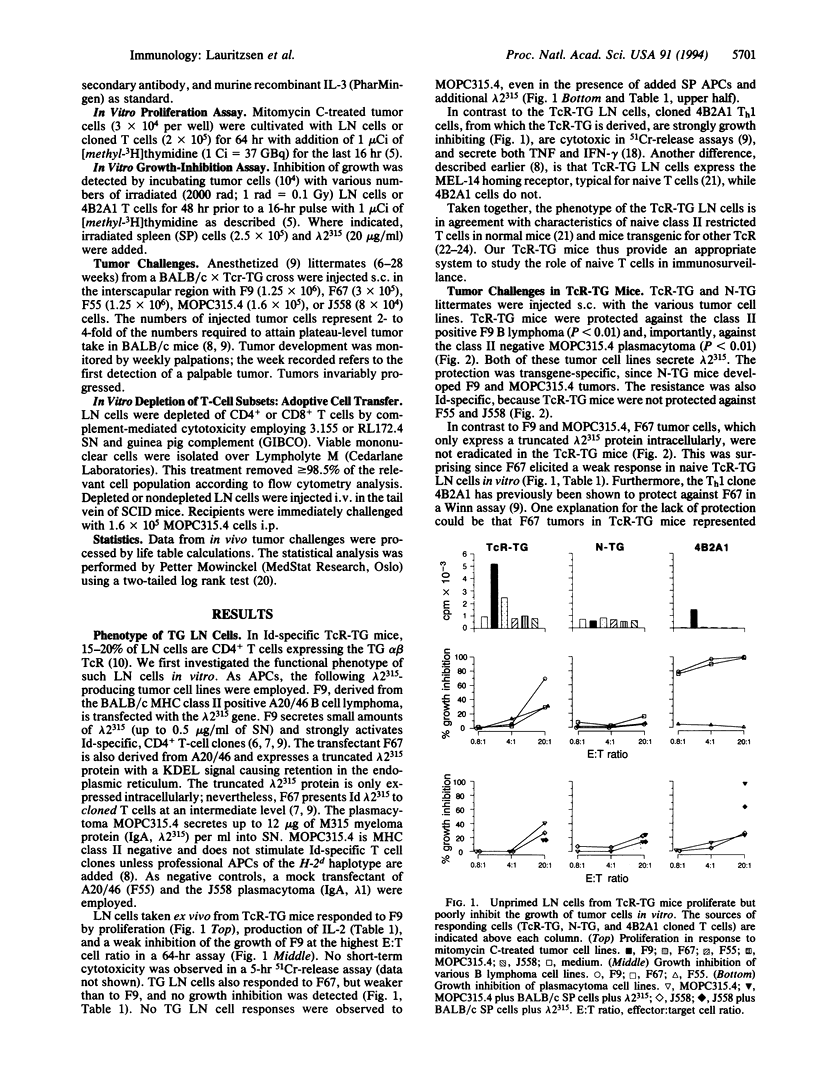
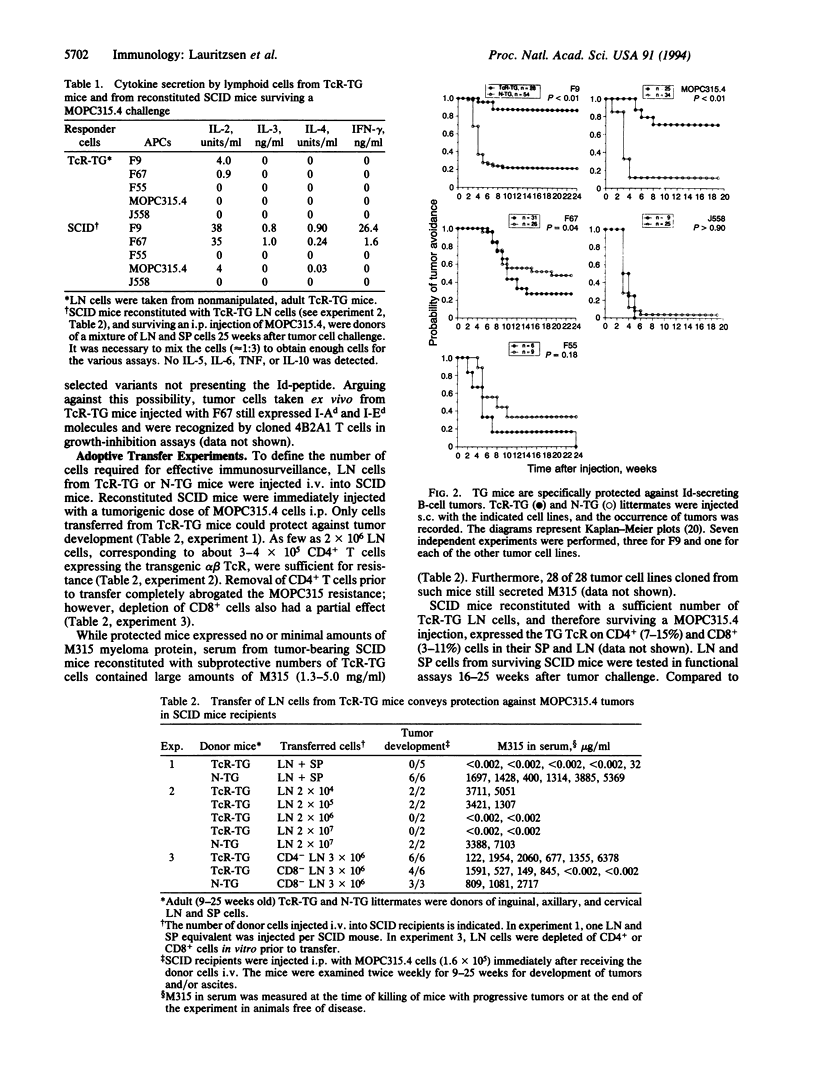
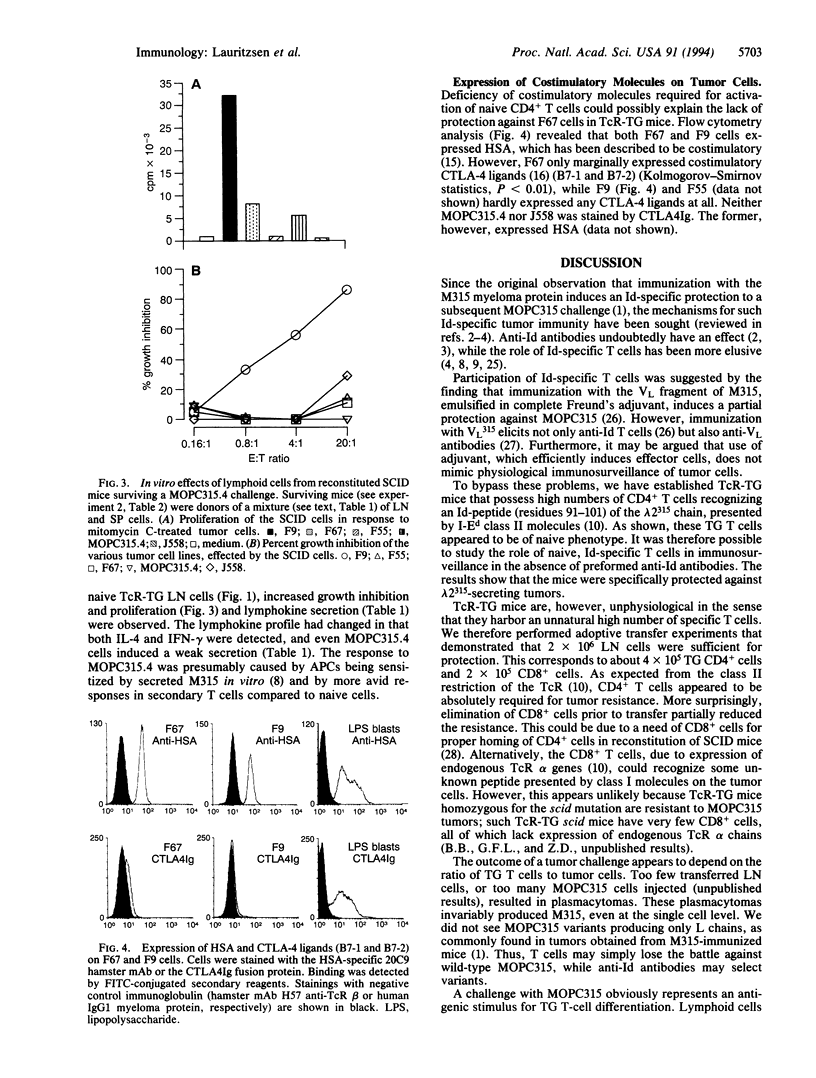
The immunosurveillance hypothesis suggests that lymphocytes can recognize tumor-specific antigens expressed by transformed cells and initiate their elimination. Immunosurveillance implies that lymphocytes of naive phenotype can home to a tumor site and become activated by tumor-specific antigens. In this study, we have employed T-cell receptor transgenic mice as a source of naive, tumor-specific T cells. The transgenic, CD4+ T cells recognize a 91- to 101-residue fragment of the lambda 2(315) immunoglobulin light chain presented by I-Ed class II molecules. Such naive, idiotype-specific, CD4+ T cells protected against tumor development of a class II negative plasmacytoma (MOPC315) and a class II positive B lymphoma (F9), which both secrete lambda 2(315) immunoglobulin. Adoptive transfer experiments demonstrated that 2 x 10(6) lymph node cells were sufficient for protection against MOPC315. Depletion of T-cell subsets indicated that transgenic CD4+ cells were indispensable for tumor resistance. However, an additional role of CD8+ T cells is not ruled out. In contrast to the resistance against the secreting MOPC315 and F9 cells, transgenic mice were not protected against B lymphoma cells (F67), which do not secrete lambda 2(315) but express a truncated lambda 2(315) chain intracellularly. The results suggest that lambda 2(315) is processed and presented by host antigen-presenting cells, which in turn activate naive, idiotype-specific T cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baskar S., Ostrand-Rosenberg S., Nabavi N., Nadler L. M., Freeman G. J., Glimcher L. H. Constitutive expression of B7 restores immunogenicity of tumor cells expressing truncated major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5687–5690. doi: 10.1073/pnas.90.12.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen B. Antibody responses to lambda 1J558 and lambda 2315 light chains. Specificity and genetic regulation. Scand J Immunol. 1984 Nov;20(5):413–424. doi: 10.1111/j.1365-3083.1984.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Bogen B., Dembic Z., Weiss S. Clonal deletion of specific thymocytes by an immunoglobulin idiotype. EMBO J. 1993 Jan;12(1):357–363. doi: 10.1002/j.1460-2075.1993.tb05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen B., Gleditsch L., Weiss S., Dembic Z. Weak positive selection of transgenic T cell receptor-bearing thymocytes: importance of major histocompatibility complex class II, T cell receptor and CD4 surface molecule densities. Eur J Immunol. 1992 Mar;22(3):703–709. doi: 10.1002/eji.1830220313. [DOI] [PubMed] [Google Scholar]
- Bogen B., Malissen B., Haas W. Idiotope-specific T cell clones that recognize syngeneic immunoglobulin fragments in the context of class II molecules. Eur J Immunol. 1986 Nov;16(11):1373–1378. doi: 10.1002/eji.1830161110. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Chen L., Ashe S., Brady W. A., Hellström I., Hellström K. E., Ledbetter J. A., McGowan P., Linsley P. S. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992 Dec 24;71(7):1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- Croft M., Duncan D. D., Swain S. L. Response of naive antigen-specific CD4+ T cells in vitro: characteristics and antigen-presenting cell requirements. J Exp Med. 1992 Nov 1;176(5):1431–1437. doi: 10.1084/jem.176.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen H. N., Simms E. S., Potter M. Mouse myeloma proteins with antihapten antibody acitivity. The protein produced by plasma cell tumor MOPC-315. Biochemistry. 1968 Nov;7(11):4126–4134. doi: 10.1021/bi00851a048. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Borriello F., Hodes R. J., Reiser H., Hathcock K. S., Laszlo G., McKnight A. J., Kim J., Du L., Lombard D. B. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science. 1993 Nov 5;262(5135):907–909. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]
- Greenberg P. D. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- Hathcock K. S., Laszlo G., Dickler H. B., Bradshaw J., Linsley P., Hodes R. J. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993 Nov 5;262(5135):905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- Hsieh C. S., Heimberger A. B., Gold J. S., O'Garra A., Murphy K. M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen T., Gaudernack G., Hannestad K. Immunization with the light chain and the VL domain of the isologous myeloma protein 315 inhibits growth of mouse plasmacytoma MOPC315. Scand J Immunol. 1980;11(1):29–35. doi: 10.1111/j.1365-3083.1980.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Lauritzsen G. F., Bogen B. Idiotype-specific, major histocompatibility complex restricted T cells are of both Th1 and Th2 type. Scand J Immunol. 1991 Jun;33(6):647–656. doi: 10.1111/j.1365-3083.1991.tb02537.x. [DOI] [PubMed] [Google Scholar]
- Lauritzsen G. F., Bogen B. The role of idiotype-specific, CD4+ T cells in tumor resistance against major histocompatibility complex class II molecule negative plasmacytoma cells. Cell Immunol. 1993 Apr 15;148(1):177–188. doi: 10.1006/cimm.1993.1100. [DOI] [PubMed] [Google Scholar]
- Lauritzsen G. F., Weiss S., Bogen B. Anti-tumour activity of idiotype-specific, MHC-restricted Th1 and Th2 clones in vitro and in vivo. Scand J Immunol. 1993 Jan;37(1):77–85. doi: 10.1111/j.1365-3083.1993.tb01668.x. [DOI] [PubMed] [Google Scholar]
- Levy R., Miller R. A. Therapy of lymphoma directed at idiotypes. J Natl Cancer Inst Monogr. 1990;(10):61–68. [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991 Sep 1;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jones B., Aruffo A., Sullivan K. M., Linsley P. S., Janeway C. A., Jr Heat-stable antigen is a costimulatory molecule for CD4 T cell growth. J Exp Med. 1992 Feb 1;175(2):437–445. doi: 10.1084/jem.175.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jones B., Brady W., Janeway C. A., Jr, Linsley P. S., Linley P. S. Co-stimulation of murine CD4 T cell growth: cooperation between B7 and heat-stable antigen. Eur J Immunol. 1992 Nov;22(11):2855–2859. doi: 10.1002/eji.1830221115. [DOI] [PubMed] [Google Scholar]
- Luqman M., Bottomly K. Activation requirements for CD4+ T cells differing in CD45R expression. J Immunol. 1992 Oct 1;149(7):2300–2306. [PubMed] [Google Scholar]
- Lynch R. G., Graff R. J., Sirisinha S., Simms E. S., Eisen H. N. Myeloma proteins as tumor-specific transplantation antigens. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1540–1544. doi: 10.1073/pnas.69.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch R. G. Immunoglobulin-specific suppressor T cells. Adv Immunol. 1987;40:135–151. doi: 10.1016/s0065-2776(08)60239-4. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Fiorentino D. F., Leverah J., Moore K. W., Bond M. W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990 Nov 1;145(9):2938–2945. [PubMed] [Google Scholar]
- Reimann J., Rudolphi A., Claesson M. H. Selective reconstitution of T lymphocyte subsets in scid mice. Immunol Rev. 1991 Dec;124:75–95. doi: 10.1111/j.1600-065x.1991.tb00617.x. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Dialynas D. P., Lancki D. W., Wall K. A., Lorber M. I., Loken M. R., Fitch F. W. Cloned T lymphocytes and monoclonal antibodies as probes for cell surface molecules active in T cell-mediated cytolysis. Immunol Rev. 1982;68:135–169. doi: 10.1111/j.1600-065x.1982.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson F. K., George A. J., Glennie M. J. Anti-idiotypic therapy of leukemias and lymphomas. Chem Immunol. 1990;48:126–166. [PubMed] [Google Scholar]
- Sugai S., Palmer D. W., Talal N., Witz I. P. Protective and cellular immune responses to idiotypic determinants on cells from a spontaneous lymphoma of NZB-NZW F1 mice. J Exp Med. 1974 Dec 1;140(6):1547–1558. doi: 10.1084/jem.140.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Bradley L. M., Croft M., Tonkonogy S., Atkins G., Weinberg A. D., Duncan D. D., Hedrick S. M., Dutton R. W., Huston G. Helper T-cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol Rev. 1991 Oct;123:115–144. doi: 10.1111/j.1600-065x.1991.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Townsend S. E., Allison J. P. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993 Jan 15;259(5093):368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Alaverdi N., Wade W. F., Linsley P. S. Induction of costimulatory molecule B7 in M12 B lymphomas by cAMP or MHC-restricted T cell interaction. J Immunol. 1993 Mar 15;150(6):2192–2202. [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Weiss S., Bogen B. B-lymphoma cells process and present their endogenous immunoglobulin to major histocompatibility complex-restricted T cells. Proc Natl Acad Sci U S A. 1989 Jan;86(1):282–286. doi: 10.1073/pnas.86.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S., Bogen B. MHC class II-restricted presentation of intracellular antigen. Cell. 1991 Feb 22;64(4):767–776. doi: 10.1016/0092-8674(91)90506-t. [DOI] [PubMed] [Google Scholar]



