Abstract
Purpose
Intravesical instillation of liposomes is a potentially new therapeutic option for subjects with interstitial cystitis/bladder pain syndrome (IC/BPS). The aim of this study was to explore the safety and clinical outcomes of 4 weekly instillations of sphingomyelin liposomes in an open-label cohort of subjects with IC/BPS.
Methods
A total of fourteen symptomatic IC/BPS subjects were treated with intravesical liposomes once a week for 4 weeks. Safety measurements included lab specimen collection, vital signs, post void residual (PVR), and assessment of adverse events (AEs). Efficacy measurements included pain visual analog scales (VAS), voiding diaries, global response assessments (GRAs), and O'Leary-Sant Interstitial Cystitis Symptom and Problem Indices (ICSI and ICPI).
Results
No treatment-related adverse events (AE) were reported at any time over the course of the study. Urgency VAS scores significantly decreased at 4 weeks (p=0.0029) and 8 weeks (p=0.0112) post-treatment. Pain VAS scores significantly decreased at 4 weeks post-treatment (p=0.0073). Combined ICSI and ICPI scores improved significantly at 4 weeks and 8 weeks (p=0.002 for both time points) post-treatment. Responses to GRA showed improvement at 4 weeks post- instillation. No significant decrease in urinary frequency was found.
Conclusion
Sphingomyelin liposome instillations were well tolerated in subjects with IC/BPS with no AEs attributed to the test article. Treatment was associated with improvements in pain, urinary urgency, and overall symptom scores. Placebo controlled clinical trials are needed to assess this potential therapy for IC/BPS.
Keywords: Cystitis: Interstitial, Administration, Intravesical, Liposome
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a multifactorial syndrome of pelvic pain, urinary frequency, and urgency.1 Currently, IC/BPS is a largely incurable, chronic illness that often negatively impacts sexual function, ability to work, and overall quality of life. Estimates of the prevalence of IC/BPS in the U.S. range from 10 to 500 cases per 100,000 people primarily affecting women.2-4
Intravesical instillation of liposomes is a potentially new therapeutic option for subjects with IC/BPS. Biophysical studies have suggested that liposomes can be adsorbed by, or will fuse or transfer lipids to the cell membrane, as well as be endocytosed to the cell interior.5 Liposomes currently serve as a component of certain topical formulations applicable to body cavities: such as the vagina and cul-de sac of the eye.6, 7 Certain liposomal formulations have been developed to be biochemically similar to the phospholipid bilayers comprising human cellular membranes.8 Certain liposomal phospholipid constituents contribute to epithelial membrane impermeability, as well as modulate inflammation.9, 10
Sphingomyelin is a common phospholipid found in most animal cell membranes. It is consequently a common component of our food supply, as well as a common component of parenteral nutrition products. The safety of oral and intravenous administration of sphingomyelin is thus well known. However, the safety of an intravesical administration has not been established.
The aim of this study was to explore the safety and clinical outcomes of 4 weekly instillations of sphingomyelin liposomes (LP08) in an open-label cohort of subjects with IC/BPS.
Methods
Fifteen subjects with IC/BPS were identified and recruited subsequent to investigators receiving FDA and institutional review board (IRB) approval. One subject withdrew prior to treatment; thus 14 subjects completed the study. The basic inclusion criteria were that subjects must have: 1) been at least 18 years of age, 2) had history of refractory IC/BPS for at least 6 months, 3) had urinary frequency exceeding eight times per day recorded on the three-day voiding diary, and 4) had a bladder pain score equal to or > 4 on the Pain VAS (0-10; none to severe) within the 24 h prior to the screening visit. The patients were diagnosed clinically with IC/BPS based on NIDDK criteria. They are refractory to at least one pharmacotherapy for their disease. IC/BPS women of childbearing age without history of bilateral tubal ligation or post-menopausal status for at least one year agreed to use an acceptable means of contraception during the entire 4-week course of therapy and 1 week thereafter.
Treatment consisted of a single course of four intravesical instillations of 80 milligrams of pre-liposomal sphingomyelin lyophilate (LP08; Lipella Pharmaceuticals, Pittsburgh, PA) reconstituted with 40 mL of sterile water. Treatments occurred within 5- 14 days of one another. Prior to the initial instillation, vital signs, urinalysis, urine pregnancy test (if appropriate), PVR assessment via bladder scan, cystoscopy, and symptom assessments were conducted. Liposomes were instilled into the empty bladder via an 8 Fr catheter with 2% lidocaine jelly applied to the catheter tip prior to insertion. The instillate was retained in the bladder for 30 minutes and then removed via independent voiding. Subjects were monitored closely for adverse events (AEs) and urinary function symptoms including gross hematuria, urinary retention, urgency, frequency, and/or signs/symptoms of infection.
Clinical outcomes were assessed at 1, 4, and 8 weeks after the last instillation for a total of 12 weeks since treatment began. Efficacy measures included a pain VAS where subjects rated their current pain on a continuum of 0-10 (none to severe), and a three-day voiding diary for urinary frequency, urgency VAS (0-10; none to severe). Subjects also reported overall changes in symptoms since treatment on the seven-point Likert scale (“markedly worse” to “markedly better”) Global Response Assessment (GRA). Symptom and bother were also evaluated with validated O'Leary-Sant Interstitial Cystitis Symptom and Problem Indices (ICSI and ICPI), which when combined have an aggregate score ranging from 0 to 36 (higher scores indicated worse symptoms/bother). Other study tests included urine pregnancy test (if applicable), urine dipstick, measurement of PVR, and cystoscopy.
Clinical measures represented by a continuous scale were subjected to validity tests on a pairwise basis using either or both of the Student's t-test or Wilcoxon signed rank test depending on the validity of the respective normality assumptions. Hypothesis testing was also conducted on a cohort basis (i.e. not pairwise) via the Wilcoxon rank sums test. Population estimates of continuous measures (probability density functions) were conducted via a Gaussian kernel having a 20% data-range standard deviation. Clinical data acquired prior to the first instillation was considered baseline for evaluations depicted as a change from baseline. Differences in ordinal measures were evaluated via the Chi-squared likelihood ratio test, as well as pairwise via the Wilcoxon signed rank test.
Results
The average age of the cohort was 58 years (range 37-75) and there were a total of eleven women and three men. No treatment-related AEs or side effects were reported at any time over course of the study. A total of nine AEs occurred in four subjects during the study. Five AEs were mild in intensity; four AEs were moderate in intensity. One serious AE (pneumonia) occurred but this was unrelated to the test material and the patient fully recovered (Table 1). None of these AEs were determined to have any relationship to the test article, all were resolved, and no subject terminated the study prematurely because of an AE.
Table 1.
Summary of adverse events (AEs) during the study
| Number or AEs | AE Description | Intensity | Outcome | Relationship |
|---|---|---|---|---|
| Local adverse events | ||||
| 1 | Gross hematuria | Mild | Resolved | Not related |
| 1 | Irritable bowel flare | Mild | Resolved | Not related |
| 1 | Groin pull | Mild | Resolved | Not related |
| 1 | Bladder infection* | Mild | Resolved | Not related |
| Other Adverse Events | ||||
| 1 | Nausea | Mild | Resolved | Not related |
| 1 | Elbow pinched nerve | Moderate | Resolved | Not related |
| 1 | Surgery Right arm | Moderate | Resolved | Not related |
| 1 | Yeast Infection | Moderate | Resolved | Not related |
| 1 | Pneumonia | Serious | Resolved | Not related |
AE occurred 4 weeks after final LP-08 instillation
Cystoscopy at baseline and at 8 weeks post-treatment was performed to assess for bladder changes. Bladder inflammation/ulcers (Hunner's lesions) were identified and recorded. Cystoscopic images were taken to increase the accuracy of comparisons. Between baseline and eight weeks post-treatment there were no changes in bladder appearance for 10 subjects, 3 had improved, and one worsened. The subject with the worsening bladder appearance had 2 ulcers at baseline and 3 ulcers at the end of the study period.
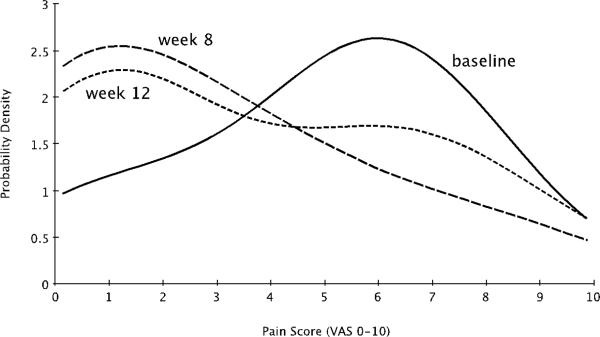
Efficacy evaluations were performed on all subjects that received a full course of liposome instillations. Pain scores showed significant improvement at 4 weeks post-treatment as shown in Table 2. The pain score reduction at 8 weeks post-treatment was not statistically significant. Figure 1 shows the distribution of pain VAS scores for the cohort at baseline, as well as four and 8 weeks post-treatment. At both time points, the average pain score for the population shifted from 5.9 to 1.2 (an 80% reduction). The shift at four weeks post therapy was more pronounced then at 8 weeks post therapy. The distribution at 8 weeks post-therapy shows evidence of the population eventually returning to baseline.
Table 2.
Summary of efficacy evaluations (change from baseline)
| Weeks post-treatment | Mean | SEM | Median | IQR | p value |
|---|---|---|---|---|---|
| Pain VAS | |||||
| 4 | −2 | 0.62 | −1.45 | (−3.82, 0.00) | 0.0073 |
| 8 | −1.14 | 0.87 | −0.55 | [−3.90, −0.90] | 0.2885 |
| Urgency VAS | |||||
| 4 | −1.55 | 0.44 | −1.1 | (−3.02, 0.00) | 0.0029 |
| 8 | −1.58 | 0.57 | −1.25 | (−3.38, −0.40) | 0.0112 |
| ICSS + ICPS | |||||
| 4 | −4 | (−7.00, −1.00) | 0.002 | ||
| 8 | −4 | (−8.00, −1.00) | 0.002 |
Fig 1.
Probability density functions for pain scores of the sample population at baseline and at four and eight weeks after the conclusion of each subject's course of 4 weekly LP-08 instillations. The most likely pain score for the population shifted from 5.9 to 1.2 (an 80% reduction). The shift at 4 weeks post therapy was more pronounced than at eight weeks post therapy. The Wilcoxon rank sums test yielded a p-value of 0.01 at 4 weeks post therapy, however a p-value of 0.29 8 weeks post therapy.
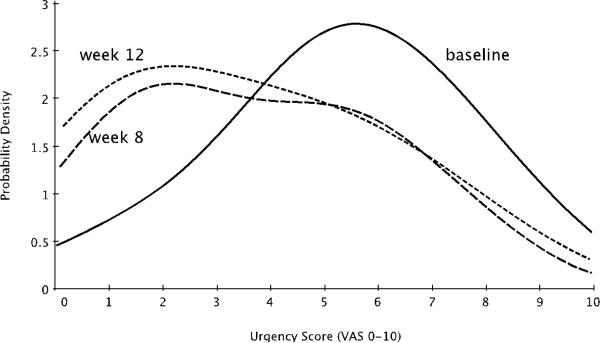
Urgency scores showed significant improvement at both 4 and 8 weeks post-treatment as shown in Table 2. In both cases, the mean reduction was approximately 1.5 units on the VAS scale. Figure 2 shows the distribution of urgency VAS scores for the cohort at baseline, as well as 4 and 8 weeks post-treatment. The distribution was approximately Gaussian at baseline, and significantly skewed post-treatment. The average urgency severity score for the population shifted from 5.4 to 2.3 (a 57% reduction).
Fig 2.
Probability density functions for urgency severity scores of the sample population at baseline and at four and eight weeks after the conclusion of each subject's course of four weekly LP-08 instillations. The most likely urgency severity score for the population at both time points shifted from 5.4 to 2.3 (a 57% reduction). Wilcoxon rank sums tests for the two time points vs. baseline yield p-values of p=0.076 and p=0.084 respectively.
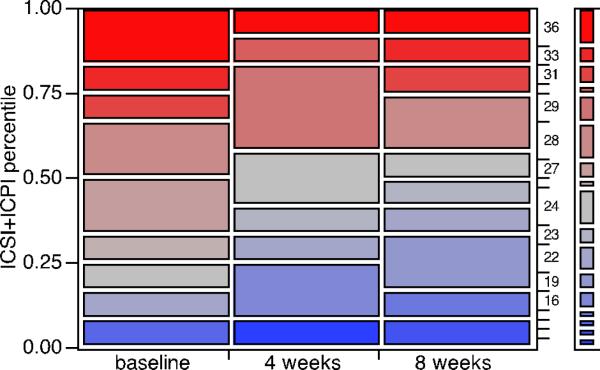
Interstitial cystitis symptom/pain index scores (ICSI+ICPI) significantly improved for the cohort at both four and eight weeks post-treatment (Table 2). The median reduction in total score from baseline was four units for both time points. The comparative distributions of this index total for both time points as well as baseline are shown via a mosaic plot in Figure 3. When pairing is not considered, the reduction at 8 weeks post-treatment is no longer statistically significant. However, the reduction at 4 weeks post-instillation remained statistically significant.
Fig 3.
The distributions of interstitial cystitis symptom/problem index total (ICSI+ICPI, scores from 0-36) for the cohort at baseline as well as four and eight weeks post-treatment are presented as a mosaic plot. The corresponding chi-squared likelihood ratio test p-values for the two time points vs. baseline were p=0.0434 and p=0.3858 respectively.
The GRA measure distribution for the cohort for both time points (4 and 8 weeks post-treatment) had a median value of “slightly improved ”, which is the first of three possible levels of positive improvement ranging from “slightly improved” to “markedly improved.” Wilcoxon signed rank test results at the two time points were p=0.002 and p=0.141 for 4 and 8 weeks post-treatment respectively.
Although a small decrease (10%) in urinary frequency was seen after the third instillation, no statistically significant differences were seen between baseline and any follow up period.
Discussion
Four weekly liposome instillations were successfully completed in a total of 14 subjects with chronic IC/BPS. Pain and urgency VAS showed a significant improvement in aggregate scores at 1 and 4 weeks post-treatment. GRA also showed significant improvement in aggregate scores at 4 weeks. There was a significant decrease in ICSI and ICPI total scores at 4 and 8 weeks post-treatment compared to baseline. No treatment-related AE were reported at any time over course of the study. Limitations of this preliminary study include lack of placebo control, small sample size and limited duration of follow-up.
Preclinical study data (obtained by using an IC/BPS model in female rats) have demonstrated normalization of urinary frequency indicating that liposomes may be a potent protectant of the bladder mucosa against inflammation and irritation.11 Liposomes were compared with DMSO and pentosan polysulfate sodium (Elmiron). Liposomes were able to significantly reverse the decrease in intercontractile interval (ICI) (the surrogate measurement of efficacy) induced by potassium chloride (KCl) following bladder injury with protamine sulfate while pentosan polysulfate sodium showed modest efficacy. On average, LP-08 increased ICI in the model rats by 132% with strong statistical significance. Control subjects demonstrated no statistically significant effect. Histology obtained from the animal studies demonstrated that liposomes act as a mechanical barrier to the passage of irritants through the mucosal cell lining. Once liposome are instilled into the bladder, they do not penetrate into the interior surface of the bladder, and thus are not absorbed systemically.12 There has not been any direct comparison between hyaluronic acid and liposome, and it would be something interesting to investigate in future studies.
The FDA previously approved a compassionate use IND single-subject trial to evaluate the safety and clinical outcomes of intravesical instillation of liposomes in a woman with ulcerative IC/BPS.13 A 48-year old woman, diagnosed with ulcerative IC/BPS, received four weekly instillations of intravesical liposomes. Subsequently she was evaluated for 8 weeks post bladder instillation. No side effects or adverse events were reported during the 12 week study period. The subject denied experiencing any problems related to urinary functioning including gross hematuria, urinary retention, urgency, frequency, and/or signs/symptoms of infection. Bladder instillations were well tolerated. At each treatment visit, the subject denied bladder irritation after liposome instillation and drainage. She was able to void without difficulty after the liposomes were drained from her bladder. Improvements across multiple domains were demonstrated. Voids per day decreased from baseline of 18 voids per 24 hours to 11.3 voids per 24 hours at week 3, and 12.6 voids per 24 hours at 8 weeks after final instillation. Cystoscopy revealed a marked improvement in bladder appearance. Three bladder ulcers were noted at baseline, but were absent at the 8 week post-treatment visit. Additionally there was no evidence of bladder inflammation.
Conclusion
Intravesical liposome (LP08) instillations were well tolerated in subjects with IC/BPS with no AEs attributed to the test article. Treatment was associated with improvement in pain, urinary urgency, and overall symptom scores. Multicenter placebo controlled clinical trials are needed to assess this potential therapy for IC/BPS.
Acknowledgments
Authors received funding from the National Institutes of Health DK085733.
Footnotes
ClinicalTrial.gov Identifier: NCT01731470
References
- 1.Nordling J. Interstitial cystitis: How should we diagnose it and treat it in 2004? Curr Opin Urol. 2004;14:323–327. doi: 10.1097/00042307-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Berry SH, Elliott MN, Suttorp M, et al. Prevalence and incidence of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemens JQ, Meenan RT, Rosetti MC, et al. Prevalence and incidence of interstitial cystitis in a managed care population. J Urol. 2005;173:98–102. doi: 10.1097/01.ju.0000146114.53828.82. [DOI] [PubMed] [Google Scholar]
- 4.Gardella B, Porru D, Ferdeghini F, et al. Insight into urogynecologic features of women with interstitial cystitis/painful bladder syndrome. Eur Urol. 2008;54:1145–1153. doi: 10.1016/j.eururo.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 5.Negrete HO, Lavelle JP, Berg J, et al. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol. 1996;271:F886–F894. doi: 10.1152/ajprenal.1996.271.4.F886. [DOI] [PubMed] [Google Scholar]
- 6.Gregoriadis G. The carrier potential of liposomes in biology and medicine (first of two parts). N Engl J Med. 1976;295:704–710. doi: 10.1056/NEJM197609232951305. [DOI] [PubMed] [Google Scholar]
- 7.Pavelic Z, Skalko-Basnet N, Jalsenjak I. Characterization and in vitro evaluation of bioadhesive liposome gels for local therapy of vaginitis. Int J Pharm. 2005;301:140–148. doi: 10.1016/j.ijpharm.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Tyagi P, Chancellor M, Yoshimura N, et al. Activity of different phospholipids in attenuating hyperactivity in bladder irritation. BJU Int. 2008;101:627–632. doi: 10.1111/j.1464-410X.2007.07334.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman J, Tyagi V, Anthony M, et al. State of the art in intravesical therapy for lower urinary tract symptoms. Rev Urol. 2010;12:e181–e189. [PMC free article] [PubMed] [Google Scholar]
- 10.Hill WG, Zeidel ML. Reconstituting the barrier properties of a water-tight epithelial membrane by design of leaflet-specific liposomes. J Biol Chem. 2000;275:30176–30185. doi: 10.1074/jbc.M003494200. [DOI] [PubMed] [Google Scholar]
- 11.Tyagi P, Hsieh VC, Yoshimura N, et al. Instillation of liposomes vs dimethyl sulphoxide or pentosan polysulphate for reducing bladder hyperactivity. BJU Int. 2009;104:1689–1692. doi: 10.1111/j.1464-410X.2009.08673.x. [DOI] [PubMed] [Google Scholar]
- 12.Chuang YC, Lee WC, Lee WC, et al. Intravesical liposome versus oral pentosan polysulfate for interstitial cystitis/painful bladder syndrome. J Urol. 2009;182:1393–1400. doi: 10.1016/j.juro.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Peters KM, Hasenau DL, Anthony M, et al. Novel therapy with intravesical liposomes for ulcerative interstitial cystitis/painful bladder syndrome. LUTS: Lower Urinary Tract Symptoms. 2012;4:51–53. doi: 10.1111/j.1757-5672.2011.00108.x. [DOI] [PubMed] [Google Scholar]