Abstract
Aim
Retinal cell remodelling has been reported as a consistent feature of ageing. However, the degree to which this results in transretinal degeneration is unclear. To address this, the authors used multiphoton microscopy to quantify retinal degeneration in postmortem human eyes of two age groups.
Methods
Retinas from six young subjects (18–33 years old) and six older subjects (74–90 years old) were prepared as wholemount preparations. All retinas were stained with 4,6-diamidino-2-phenylindole and imaged by multiphoton confocal microscopy to quantify neuron densities in the retinal ganglion cell layer (RGCL), inner nuclear layer (INL) and outer nuclear layer (ONL). Neurons were counted using automated cell identification algorithms. All retinas were imaged hydrated to minimise tissue artefacts.
Results
In both groups, 56% of the area within the central 4 mm eccentricity and 27% of the area with eccentricity between 4 mm and 7 mm were imaged. Compared with young subjects, the peak RGCL neuron loss in the aged subjects (25.5%) was at 1 mm eccentricity. INL and ONL neuron densities significantly decreased at 1–2 mm eccentricity (8.7%) and 0.5–4 mm eccentricity (15.6%) respectively (P <0.05). The reduction in neuron density in the INL corresponded, spatially, to the region with the greatest neuron loss in the RGCL and ONL.
Conclusions
This is the first study to correlate neurodegeneration in different populations of cells in the ageing retinas. These data confirm that the greatest neuronal loss occurs in the RGCL and ONL in human ageing retinas, whereas the INL is relatively preserved.
INTRODUCTION
Most older people experience some loss of vision, even in the absence of any identifiable eye disease. Advancing age has brought a spectrum of degenerative or regressive events which manifest visually as a reduction in visual acuity and in peripheral and scotopic sensitivities.1, 2 Dark adaptation is also delayed in older people owing to changes in the kinetics of rod function.3 These age-related changes have functional effects that span the retina. Standard full-field electrophysiology studies indicate a significant increase in implicit times and a decrease in the amplitudes of a waves (photoreceptor responses) and b waves (bipolar and Müller cell responses) in older people.4, 5 The amplitude of multifocal oscillatory potentials, which are thought to reflect inner retinal function and rod–cone interactions, decreases linearly with age, whereas their latency increases with age.6 Histological studies have provided a neural basis for this loss in function, reporting reductions in retinal ganglion cell (RGC),7 rod bipolar cells, photoreceptors (especially rods)8–10 and pigmented epithelium (RPE).10
What is not clear from these observations is whether neural losses within the retinal layers occur independently or transneuronal degeneration contributes to the effects of ageing. Transneuronal degeneration refers to neuron loss in one area affecting linked neurons in neighbouring regions. Knowledge of these changes is important in the development of therapies for the restoration of vision in older people. Recently, neuronal plasticity was noted in senescent retinas in mouse11 and human12 where the neurons in the inner nuclear layer (INL) extended their dendrites to the outer nuclear layer (ONL). Our group reported a pattern of neuronal loss that was consistent with retinal transneuronal degeneration in glaucoma in which neuronal loss in the inner retina influences the viability of outer retina.13 Glaucoma has been reported to result in transneuronal degeneration in the retinogeniculate pathways by several investigators.14
Multiphoton confocal microscopy has a higher sensitivity than conventional imaging techniques–for example, phase-contrast Nomarski and single-photon confocal microscopy, for the quantification of neuronal population in intact tissues. It has the advantage of deeper tissue penetration as a result of a near-simultaneous absorption of multiple photons. With this imaging technique, we have been able to preserve the topographic relationship between retinal layers while quantifying different populations of neurons in the RGC layer (RGCL), INL and ONL. These data provide valuable information concerning the pattern and mechanism of neuronal loss in the ageing human retina.
MATERIALS AND METHODS
Storage and research using human tissue samples were carried out according to Human Tissue Authority regulations under the UK Human Tissue Act 2004. Six young (18–33 years old) and six ageing retinas (74–90 years old) were analysed. In all cases, age, gender, ethnic origin and cause of death were known. The retinas were fixed within 24 h of death in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Clinical examination of the optic nerve and retina did not indicate the presence of glaucoma or macular degeneration in any of the eyes. Following the removal of anterior segment and vitreous humour, the retina was dissected as a whole-mount preparation and freed from the retinal pigment epithelium. Four linear cuts were made in the retina to provide four approximately equal segments, which were then stained by 4,6-diamidino-2-phenylindole (DAPI, Sigma, UK) solution (3 μg/ml in PBS) overnight at 4°C. The segments were whole-mounted RGC side uppermost in a hard set mounting medium for fluorescence imaging (VECTASHIELD Mounting Medium, Vector Laboratories, Peterborough, UK).
Details of the multiphoton imaging strategy have been described in detail elsewhere.13 Briefly, in order to relate retinal pathology to clinical data, a sampling grid was used which retinotopically corresponded to the spacing stimuli in a Humphrey 30–2 visual field. DAPI molecules were excited by the 843 nm line of the Ti-Sapphire Tsunami multiphoton laser (SpectraPhysics) and images acquired using a Leica TCS SP2 confocal system with a Leica DMRE microscope (HCX PL Apo 40/1.25 NA oil CS objective lens; Leica, Milton Keynes, UK). Images were analysed using ImageJ (version 1.34, US National Institutes of Health) to derive counts for neuronal nuclei in the RGCL, INL and ONL. These DAPI-labelled nucleus layers were separated by regions of unlabelled neurite. The whole depth of the RGCL was imaged, but since INL and ONL were several cells deep, the automated identification of all cells in these layers within a region of interest was not possible. For the INL and ONL, we therefore restricted counting to a 10 μm thick segment within these layers and then multiplied the count by a depth correction factor that corrected for the thickness of the respective nuclear layer. The scanning interval for all layers was 1 μm, and the image stacks were analysed as a projected image (ie, collapsed in the Z dimension). Comparison of manual and automated counting methods in two eyes (eight sample areas each from the INL, ONL and 25 sample areas for the RGC layer) indicated excellent agreement for the RGCL(r2=0.84, p<0.01) and INL (r2=0.96, p<0.01) and good agreement for the ONL (r2=0.66, p<0.01). Any truncated nuclei that were smaller than the threshold size were excluded. Endothelial cells, astrocytes15 and glial cells16 were distinguished from neurons by their laminar location, small size and nuclear morphology. Endothelial cells were identified by their small and elongated nuclei. Astrocyte nuclei were mainly in the nerve fibre layer (NFL) which were not counted. Müller cell nuclei were angular and were located in the internal nuclear layer (INL). In addition, ‘forbidden lines’ were used in which cell nuclei that touched the top and right edges of the square region of interest were excluded from the analysis17 to avoid overestimating cell counts. Our counting method could not distinguish between RGC and amacrine cells, so we used literature values to offset the count for easy comparison–that is, 0.3% to 24% amacrine cells between 0.5 and 4 mm eccentricity in the human retina.7, 18
Statistics
Statistical analysis was performed using SPSS 14.0 for Windows. A general linear model was used to compare neuron densities and ratios at different eccentricities of the retina in young and aged groups. In each retinal layer, given that the same measurements were carried out on the same individuals at six different eccentricities, a repeated-measures analysis was performed with a Bonferroni correction. Differences were considered significant at p<0.05 or lower.
RESULTS
At each sampling point, neurons at the RGCL, INL and ONL were imaged sequentially to preserve the topographical correlation. Typical image stacks for young and old eyes are reconstructed as 3D models in figure 1. We found a close agreement between our RGC densities and those reported by Curcio7, 8, 18, 19 for similar age groups (figure 2).
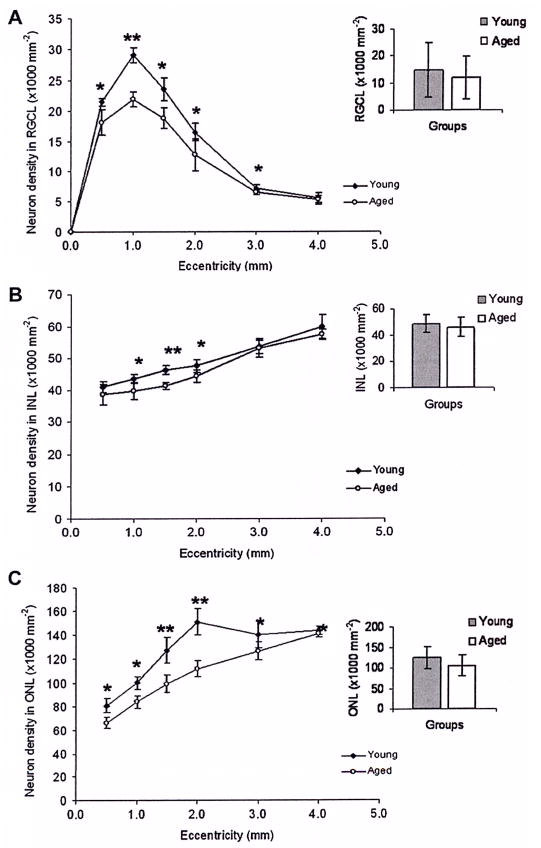
Figure 1.

Multiphoton images of 4,6-diamidino-2-phenylindole stained cell nuclei in the retinal ganglion cell layer (RGCL), inner nucleus layer (INL) and outer nucleus layer (ONL) of the young (n=6) and older (n=6) human retinas. 3D models of the retinal layers for young and older retinas were generated by Imaris software. An example from one retina of each group is shown. The images were sequentially taken with the RGCL uppermost, focusing from the RGCL to the ONL. The whole depth of RGCL was imaged. For INL and ONL, the z stack is 10 μm thick. The sampling interval between planes is 1 μm. The projection image for any given layer was generated from the sum of the component z planes.
Figure 2.
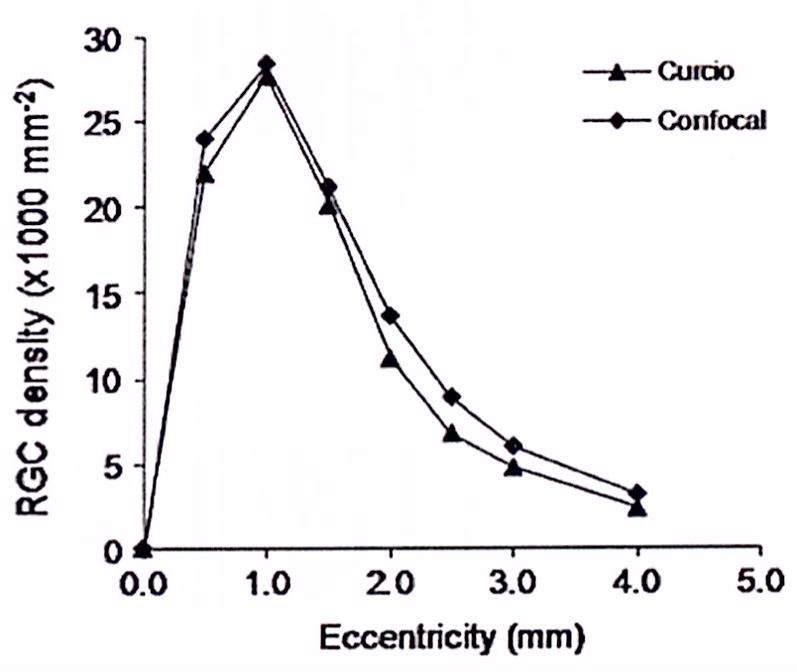
Comparisons of retinal ganglion cell (RGC) quantities measured by the multiphoton-4,6 diamidino-2-phenylindole method in young adults and literature values.18
In both groups of the retinas, 56% of the total area within central 4 mm eccentricity and 27% between 4 mm and 7 mm eccentricity were imaged. In total, 262 532 neurons were counted in young (n=6) and 228 140 in older human retinas (n=6). The mean neuron densities were compared for young and older eyes in figure 3. Neuron density in the RGCL in the older eyes decreased significantly between 0.5 and 3 mm eccentricity (figure 3A); within the central 4 mm eccentricity, the mean density decreased by approximately 15.4% (from 14 405 to 12 186/mm2); the greatest RGC loss was at 1 mm eccentricity of 24.7%. In the INL and ONL, significant reductions in neuron densities were seen at 1–2 mm eccentricity of 8.7% (figure 3B) and 0.5–4 mm eccentricity of 15.6% (figure 3C), respectively (p<0.05). The greatest reductions in cell densities were seen in the RGC and ONL layers with comparative preservation of the INL.
Figure 3.
Comparison of neuron densities in the retinal ganglion cell layer (RGCL), inner nucleus layer (INL) and outer nucleus layer (ONL) of young (n=6) and ageing retinas (n=6). Mean neuron densities are shown for each eccentricity (±SD). Ageing retinas show a significant reduction in neuron densities of 30% between 0.5 and 2 mm eccentricities. In the INL and ONL, there are significant reductions of 8.7% (1–2 mm eccentricity) for the INL 15.6% for the ONL (0.5–4 mm eccentricity). The column graphs compare the mean neuron densities at all the eccentricities for each retinal layer, which are not significantly different. *p<0.05, **p<0.01.
DISCUSSION
Our results are consistent with previous reports of age-related neurodegeneration in both human and animal models.7, 9, 10, 20–23 The RGC density in four quadrants of the retina is similar to literature values18 (figure 3), but the degree of RGC degeneration (19%) in the central 11° of vision is lower than that in Curcio’s report (‘one-fourth’) in older eyes compared with retinas from young adults,7 which might reflect the interindividual variability. An important limitation of the method used in the present study is that we could not reliably discriminate between RGC and amacrine cells, and therefore used literature values to offset the retinal ganglion cell layer count. In the same region, the densities of photoreceptors in the older eyes were 16% lower than in young eyes, This is comparable with a previous report of a 15% reduction in photoreceptors.9 The neuronal loss in the central 11° of visual field may be responsible for the visual function deficits in older individuals.24 We could not distinguish between rods and cones using the muliphoton DAPI method.
The contribution of the present study is that we have matched changes in the inner and outer retina for any given retinal region of interest. Among the three layers of the retina examined, the greatest neuronal loss was seen in the ONL between 0.5 and 4 mm eccentricity in older retinas (figure 3). Although INL degeneration occurs in a smaller region (1–2 mm eccentricity), the reduction in neuron densities in the INL corresponds to the region with the greatest reduction in RGCL and ONL cell counts (figure 3). The resilience of the INL suggests that loss in the retinal ganglion cell and ONL layers is relatively independent. This contrasts with our findings in glaucomatous eyes in which we found a gradient of cell loss from inner to outer retina which is consistent with a transneuronal degeneration initiated in the retinal ganglion cell layer.13
It is not clear why, in a closely linked visual network, both inner and outer retinas undergo significant cell loss, whereas the middle layer is relatively preserved. Most electrophysiological studies also indicate outer retina degeneration in older people. These data do not rule out the possibility of INL cell loss reflecting damage to the neighbouring layers as it is in the region where RGCL and ONL damage was greatest. The comparable loss of neurons in the RGCL and INL has implications for strategies directed at the repair of the ageing retina. While the present study did not include patients with age-related macular degeneration, given the age of patients affected by this condition, it is likely that treatments directed at the replacement of neurons lost in the ONL25 may be limited by inner retina degeneration. Our findings suggest that strategies should be devised to address both inner and outer retinal degeneration in age-related retinal diseases.
Acknowledgments
The technical support from C Thrasivoulou and D Ciantar and statistical support from P McGeoghan are very much appreciated. We are also very grateful for the comments of J Singgers, Imperial College London, on the manuscript.
Funding Research into Ageing Grant 271. Grants: Research Into Ageing (Grant No 271, YL, JEM), NIH EY 15736 (MPF), NIH EY 07065 (MPF, DHJ).
Footnotes
DH Johnson and MR Hernandez are deceased
Competing interests None.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Bonnel S, Mohand-Said S, Sahel JA. The aging of the retina. Exp Gerontol. 2003;38:825–31. doi: 10.1016/s0531-5565(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 2.Owsley C, Jackson GR, White M, et al. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001;108:1196–202. doi: 10.1016/s0161-6420(01)00580-2. [DOI] [PubMed] [Google Scholar]
- 3.Owsley CS. Vision and aging. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Vol. 4. Amsterdam: Elsevier; 1990. pp. 229–49. [Google Scholar]
- 4.Birch DG, Anderson JL. Standardized full-field electroretinography. Normal values and their variation with age. Arch Ophthalmol. 1992;110:1571–6. doi: 10.1001/archopht.1992.01080230071024. [DOI] [PubMed] [Google Scholar]
- 5.Weleber RG. The effect of age on human cone and rod ganzfeld electroretinograms. Invest Ophthalmol Vis Sci. 1981;20:392–9. [PubMed] [Google Scholar]
- 6.Kurtenbach A, Weiss M. Effect of aging on multifocal oscillatory potentials. J Opt Soc Am A Opt Image Sci Vis. 2002;19:190–6. doi: 10.1364/josaa.19.000190. [DOI] [PubMed] [Google Scholar]
- 7.Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer’s disease and aging. Ann Neurol. 1993;33:248–57. doi: 10.1002/ana.410330305. [DOI] [PubMed] [Google Scholar]
- 8.Curcio CA. Photoreceptor topography in ageing and age-related maculopathy. Eye. 2001;15:376–83. doi: 10.1038/eye.2001.140. [DOI] [PubMed] [Google Scholar]
- 9.Curcio CA, Millican CL, Allen KA, et al. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34:3278–96. [PubMed] [Google Scholar]
- 10.Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1–17. [PubMed] [Google Scholar]
- 11.Liets LC, Eliasieh K, van der List DA, et al. Dendrites of rod bipolar cells sprout in normal aging retina. Proc Natl Acad Sci U S A. 2006;103:12156–60. doi: 10.1073/pnas.0605211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eliasieh K, Liets LC, Chalupa LM. Cellular reorganization in the human retina during normal aging. Invest Ophthalmol Vis Sci. 2007;48:2824–30. doi: 10.1167/iovs.06-1228. [DOI] [PubMed] [Google Scholar]
- 13.Lei Y, Garrahan N, Hermann B, et al. Quantification of retinal transneuronal degeneration in human glaucoma: a novel multiphoton-DAPI approach. Invest Ophthalmol Vis Sci. 2008;49:1940–5. doi: 10.1167/iovs.07-0735. [DOI] [PubMed] [Google Scholar]
- 14.Gupta N, Yucel YH. Glaucoma and the brain. J Glaucoma. 2001;10:S28–9. doi: 10.1097/00061198-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 15.Ogden TE. Nerve fiber layer astrocytes of the primate retina: morphology, distribution, and density. Invest Ophthalmol Vis Sci. 1978;17:499–510. [PubMed] [Google Scholar]
- 16.Ramirez JM, Trivino A, Ramirez Al, et al. Structural specializations of human retinal glial cells. Vision Res. 1996;36:2029–36. doi: 10.1016/0042-6989(95)00322-3. [DOI] [PubMed] [Google Scholar]
- 17.Gundersen H. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J Microsc. 1977;111:219–23. [Google Scholar]
- 18.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- 19.Curcio CA, Sloan KR, Kalina RE, et al. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 20.Harman A, Abrahams B, Moore S, et al. Neuronal density in the human retinal ganglion cell layer from 16–77 years. Anat Rec. 2000;260:124–31. doi: 10.1002/1097-0185(20001001)260:2<124::AID-AR20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Kim CB, Tom BW, Spear PD. Effects of aging on the densities, numbers, and sizes of retinal ganglion cells in rhesus monkey. Neurobiol Aging. 1996;17:431–8. doi: 10.1016/0197-4580(96)00038-3. [DOI] [PubMed] [Google Scholar]
- 22.Danias J, Lee KC, Zamora MF, et al. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: comparison with RGC loss in aging C57/BL6 mice. Invest Ophthalmol Vis Sci. 2003;44:5151–62. doi: 10.1167/iovs.02-1101. [DOI] [PubMed] [Google Scholar]
- 23.Neufeld AH, Gachie EN. The inherent, age-dependent loss of retinal ganglion cells is related to the lifespan of the species. Neurobiol Aging. 2003;24:167–72. doi: 10.1016/s0197-4580(02)00059-3. [DOI] [PubMed] [Google Scholar]
- 24.Owsley CSM. Vision and aging. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. 1990. pp. 229–49. [Google Scholar]
- 25.MacLaren RE, Pearson RA. Stem cell therapy and the retina. Eye (London, England) 2007;21:1352–9. doi: 10.1038/sj.eye.6702842. [DOI] [PubMed] [Google Scholar]