Abstract
Objective
To test the effectiveness of a telehealth service delivery model for the treatment of children with attention-deficit/hyperactivity disorder (ADHD) that provided pharmacological treatment and caregiver behavior training.
Method
The Children’s ADHD Telemental Health Treatment Study (CATTS) was a randomized controlled trial with 223 children referred by 88 primary care providers (PCPs) in 7 communities. Children randomized to the experimental telehealth service model received 6 sessions over 22 weeks of combined pharmacotherapy, delivered by child psychiatrists through videoconferencing, and caregiver behavior training, provided in person by community therapists who were supervised remotely. Children randomized to the control service delivery model received treatment with their PCPs augmented with a telepsychiatry consultation. Outcomes were diagnostic criteria for ADHD and oppositional defiant disorder (ODD) and role performance on the Vanderbilt ADHD Rating Scale (VADRS) completed by caregivers (VADRS-Caregivers) and teachers (VADRS-Teachers) and impairment on the Columbia Impairment Scale-Parent Version (CIS-P). Measures were completed at 5 assessments over 25 weeks.
Results
Children in both service models improved. Children assigned to the telehealth service model improved significantly more than children in the augmented primary care arm for VADRS-Caregiver criteria for inattention (χ2[4]=19.47, p<.001), hyperactivity (χ2[4]=11.91, p=0.02), combined ADHD (χ2[4]=14.90, p=0.005), ODD (χ2[4]=10.05, p=0.04), and VADRS-Caregiver role performance (χ2 [4]=12.40, p=0.01) and CIS-P impairment (χ2[4]=20.52, p<.001). For the VADRS-Teacher diagnostic criteria, children in the telehealth service model had significantly more improvement in hyperactivity (χ2[4]=11.28, p=0.02) and combined ADHD (χ2[4]=9.72, p=0.045).
Conclusion
The CATTS trial demonstrated the effectiveness of a telehealth service model to treat ADHD in communities with limited access to specialty mental health services.
Clinical trial registration information
Children’s Attention Deficit Disorder With Hyperactivity (ADHD) Telemental Health Treatment Study; http://clinicaltrials.gov; NCT00830700.
Keywords: Telepsychiatry, telehealth with children, telemental health with children, telehealth for ADHD, mental health treatment for children in rural communities
Introduction
Children who live outside of metropolitan areas experience disproportionately poor access to the expert mental health workforce and to evidence-based mental health treatment.1–5 Federal mandates have promoted the use of technology to address such disparities. The American Recovery and Reinvestment Act (ARRA, February 17, 2009; http://www.recovery.gov/arra/About/Pages/The_Act.aspx) emphasized the use of information technologies to improve healthcare delivery, and the Patient Protection and Affordable Care Act (ACA; Public Law 111-148; March 23, 2010; http://www.hhs.gov/strategic-plan/goal1.html) specifically proposed the meaningful use of telehealth technologies to improve health care and population health for all citizens. The Health Resources and Services Administration (HRSA) defines telehealth as “The use of electronic information and telecommunications technologies to support and promote long-distance clinical health care, patient and professional health-related education, public health, and health administration (http://www.hrsa.gov/ruralhealth/about/telehealth/glossary.html). When telehealth relies on synchronous (interactive) technologies, such as videoconferencing or telephony to deliver medical care to patients, the Center for Medicare and Medicaid (CMS; (http://www.cms.gov/Telemedicine/) uses the term “telemedicine”; and when that care specifically involves mental health or psychiatric services, the terms “telemental health” (TMH) and “telepsychiatry,” respectively, are generally used.6 Asynchronous, or delayed, telehealth technologies promote the dissemination of evidence-based care by viewing recordings of clinical care or sharing information through the use of patient portals, websites, and social media.
In response to federal mandates,7 telehealth programs have developed rapidly across the country, but the evidence base supporting their effectiveness is emerging gradually. Several studies have shown that synchronous TMH that delivers services directly to children is feasible and acceptable to primary care providers (PCPs),8–9 parents,10 and youth,11 and that TMH can be used reliably to establish diagnoses.12–13 A few preliminary studies have suggested that care provided through synchronous telehealth is effective in improving outcomes for children with mental health conditions, but these studies are limited by pre- to post-intervention study designs and/or very small samples.14–17 Asynchronous telehealth technologies show promise for training clinicians in the delivery of evidence-based mental health care. One study examined the impact of technology on improving pediatricians’ adherence to guideline-based care for the assessment and treatment of children with attention-deficit/hyperactivity disorder (ADHD).18 Pediatric practices were randomized to a quality improvement model, including a Web-based ADHD registry to track patients’ care and to manage data collected from standardized rating scales versus practice as usual. The intervention group significantly improved their adherence to guideline-based care, sustained these gains over six months, and showed further continuous quality improvements at two years.19
To bring telehealth service delivery models for children into mainstream mental healthcare, additional well-designed effectiveness studies are needed. ADHD is a disorder that is well suited to test the effectiveness of telehealth service models, as it is a prevalent, impairing, and treatable condition with well-established treatment guidelines. 20–22 Most children with ADHD living in non-metropolitan areas are treated by their PCPs,23 for whom the delivery of empirically supported mental health care continues to be challenging,24–26 particularly as most children with ADHD described in community studies have comorbid mental health conditions.27- 29 Telehealth can provide access to the expert mental health workforce to assist PCPs and patients with low mental health service capacity.6–7; 30–33 Within this context, we present findings from a community-based effectiveness trial designed to determine whether children who received treatment through a telehealth service model demonstrated better outcomes than children who received treatment in primary care augmented with a single telepsychiatry consultation.
METHODS
Study Design
The Children’s ADHD Telehealth Treatment Study (CATTS) was a community-based randomized controlled trial (RCT) that compared the effects of two service delivery models on outcomes for children with ADHD. The RCT was approved by the institutional review board of Seattle Children’s Research Institute and monitored by a data safety and monitoring board. The methodology for the trial has been described in detail elsewhere 34–35 and is summarized below.
Study Sample and Setting
Between November 2009 and August 2012, we recruited children (5.5 to 12 years old) from the practices of PCPs in seven underserved communities distributed over a 40,000 square mile geographic area that spanned from western to central Washington and north central Washington to north central Oregon. Any PCP practicing in proximity to the study sites could refer children with possible ADHD to the trial regardless of whether they had participated in our telepsychiatry service.36 All study services were provided at community clinics that had high bandwidth videoconferencing connections (384 kbits/sec to 1.0 MB/sec). Sites included outreach clinics of Seattle Children’s Hospital (SCH) (n=4), an SCH-affiliated specialty clinic (n=1), a large pediatric practice (n=1), and a frontier community mental health center (n=1). Children were eligible if they met diagnostic criteria for ADHD, were between ages 5.5 and 12.9 years, resided with English-speaking caregivers, and were enrolled in school Exclusionary criteria included being in state custody, unavailability of the legal guardian, or having medical, developmental, or psychiatric disorders that required interventions beyond the scope of the study. Based on experience in our telepsychiatry clinic.36 we anticipated that PCPs would refer mainly children with ADHD and comorbid mental health conditions to the trial.
Procedures
Clinical eligibility was determined in a three-step process. We reviewed records submitted by referring PCPs to determine exclusionary conditions. Caregivers then completed the Child Behavior Checklist (CBCL)37 online. If the CBCL ADHD diagnostic subscale T-score was ≥ 65, the child was eligible for recruitment. Caregivers then met in person at the community clinic with a CATTS therapist who administered informed consent and three modules of the Computerized Diagnostic Interview Schedule for Children based on the DSM-IV 20 (CDISC-IV) 38 to confirm that the child had a diagnosis of ADHD during the past month and to establish the presence of comorbid generalized anxiety disorder (GAD) and/or oppositional defiant disorder (ODD). Consistent with prior approaches,29, 39 to establish baseline comorbid disorders, we considered an anxiety disorder (AD) present if caregivers endorsed criteria of the CDISC-IV module for GAD or if the child’s T-score on the CBCL DSM-IV-Oriented Anxiety Problem Subscale was ≥70. Similarly, we considered ODD present if the child met diagnostic criteria on the CDISC-IV ODD module or if the child’s T-score on the CBCL DSM-IV-Oriented Oppositional-Defiant Problems Subscale was ≥ 70.
Children were then administered assent; caregivers and teachers completed a baseline assessment.
We carried out block randomization with stratification by age group (5.5–9.9 vs. 10.0–12.9 years) and site (n=7). A statistician generated a set of random numbers with an allocation ratio of 1:1 for assignment to one of two service models: 1) the CATTS telehealth service delivery model and 2) the primary care service delivery model with teleconsultation. The statistician used the random numbers to prepare a logbook with assignments listed in consecutive order.34 Participants and clinicians were not blinded to intervention assignment, but teachers and research assistants who managed data collection were blinded.
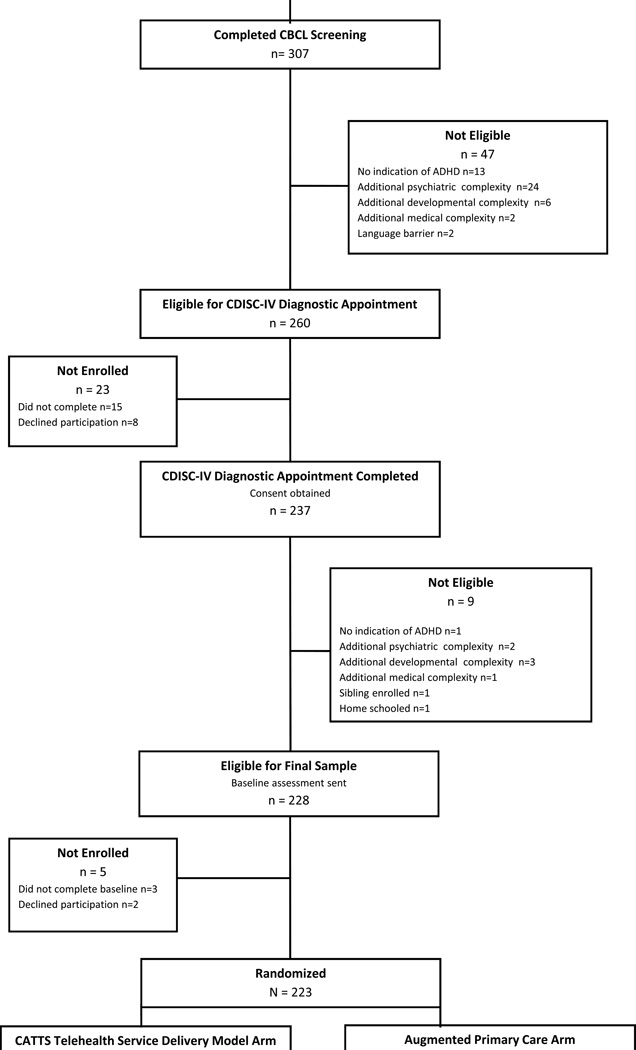
Caregivers and teachers completed follow-up assessments online at four time points: 4, 10, 19, and 25 weeks post-randomization.34 The 25-week assessment was timed to be administered three weeks after the final treatment session. The same caregiver completed each assessment and designated one teacher to complete all teacher assessments. However, because enrollment occurred throughout the year, some children advanced to the next grade or moved classrooms during the trial. Thus, for 14% of participants in each arm, two teachers were identified to complete assessments. Of the five research assessments, caregivers completed a mean of 4.8 ± 0.7 and teachers completed a mean of 3.3 ± 0.7 assessments; 91.0% and 94.7% of caregivers in the CATTS telehealth service delivery model and augmented primary care arms, respectively, completed at least four assessments. The Consolidated Standards of Reporting Trials (CONSORT) diagram is shown in Figure 1.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram. Note: ADHD = attention-deficit/hyperactivity disorder; CATTS = Children’s ADHD Telemental Health Treatment Study; CBCL = Child Behavior Checklist; CDISC-IV = Computerized Diagnostic Interview Schedule for Children, Based on the DSM-IV; PCP = primary care providers.
Interventions: CATTS Telehealth Service Delivery and Augmented Primary Care
The CATTS trial adopted a telehealth service delivery model appropriate for time-limited collaboration with PCPs, focusing on reduction of children’s ADHD-related symptoms and improved caregiver management of children’s behaviors. The telehealth service delivery model utilized both synchronous and asynchronous technologies. We used videoconferencing to provide children with the scarce resource of child psychiatrists who were located at Seattle Children’s Hospital. We used multiple asynchronous telehealth technologies to train master’s-level community therapists in an evidence-based caregiver behavior training intervention for children with ADHD.40, 41 Trained therapists would then serve as an enduring resource for communities. The two service-delivery arms are summarized below. 34, 40
CATTS Telehealth Service Delivery
The CATTS telehealth service delivery provided six sessions spaced three to four weeks apart over 22 weeks consisting of combined pharmacotherapy and caregiver behavior training. Our decision to provide combined treatment was based on findings of high rates of comorbid psychiatric conditions, particularly oppositional defiant behaviors and anxiety symptoms, among community samples of children with ADHD, 27–29 and on the increasing expectation for PCPs to manage routine cases of ADHD in their practices.18, 23–26 Empirical evidence 29, 42–43 and the practice guidelines for ADHD 21–22 indicate that a combined treatment model yields optimal outcomes for children with ADHD and comorbid oppositional defiant behaviors and anxiety symptoms.
Telepsychiatry
The principal investigator (K.M.) developed a manual to train the child psychiatrists to deliver evidence-based pharmacological care for ADHD through videoconferencing. Each of the six sessions included pharmacotherapy based on the five ADHD algorithms in the Texas Children’s Medication Algorithm Project (TCMAP) 44 and a session-specific module of psychoeducation based on the neurobiology of ADHD.45 The modules included: 1) Role of Medication in ADHD Treatment; 2) Central Nervous System (CNS) Involvement: ADHD Symptoms and Treatment Focus; 3) CNS Involvement: The Prefrontal Cortex and Executive Functioning; 4) Conditions Comorbid with ADHD; 5) Long Term Course and Potential Consequences of ADHD; 6) Review and Implications for the Individual Child.
Caregiver Behavior Training
The study’s research psychologist (C.M.) developed and recorded a series of behavior training sessions for caregivers.40 Master’s-trained community therapists hired for the study accessed the digitized recordings through a secure website, discussed the training cases with the psychologist, and practiced the intervention with two volunteer families. They then provided the caregiver behavior training in person.
The caregiver behavior training intervention and manual were developed based on key elements of evidence-based parent training programs for elementary school-aged children.46–48 The manual included a description of the sequentially delivered core elements for each session with sample scripts for teaching skills to caregivers. The six modules included: 1) Understanding ADHD and Your Child: Understanding Antecedents and Consequences of Behavior; 2) School Advocacy; 3) Praising and Ignoring Skills; 4) Giving Clear Instructions and Follow-Through; 5a) Time-Out and Other Consequences (5.5–9 years old) and 5b) Point System for Behavior (10–12 years old); and 6) Putting It All Together. Therapists gave caregivers handouts and assigned skills to practice between sessions. Just prior to the end of each telepsychiatry session, the community therapist joined the session at the patient site and transitioned to the caregiver behavior training. We conducted team meetings weekly with the therapists at their sites and staff at the research hub through videoconferencing. The psychologist carried out small group supervision via telephone. Between sessions, therapists exchanged documents with the research staff through an asynchronous Web portal; during sessions, they shared clinical data with the telepsychiatrists through a synchronous website. 34, 41
Primary Care Service Delivery Augmented With a Teleconsultation
Children randomly assigned to the control condition remained in the care of their PCPs and received a single consultation with a telepsychiatrist, who shared treatment recommendations with the referring PCP. The telepsychiatrists were instructed to provide a comprehensive consultation consistent with their outpatient practice, including medication alternatives and any relevant recommendations for other mental health specialists and school programs. PCPs were not restricted from referring to other resources, including other services at Seattle Children’s Hospital. We included an active control arm for several reasons. As telehealth programs develop, clinical, organizational, and financial stakeholders such as third party payers must decide whether to provide consultation to PCPs, a model that efficiently utilizes the expert mental health workforce, or to provide direct service to youth and families, a model that approximates parity with traditional mental health care. Comparing results of these two service delivery models would help stakeholders in their decision-making. As PCPs in non-metropolitan communities usually do not have the opportunity to participate in research,49 we also thought that an active control arm would incentivize them to refer to the trial. Finally, as many of the participating communities had telepsychiatry services, we deemed that ethically we could not proscribe access to an established community resource.
In both arms, telepsychiatrists and therapists used checklists that outlined the essential treatment components to address during each session. All sessions were recorded, and sessions were randomly selected to rate the clinicians’ coverage of these components. Telepsychiatrists and therapists showed high fidelity to their protocols.34–35, 40
Outcome Measures
The outcomes were caregiver- and teacher-rated improvement in ADHD-related symptoms and behaviors, role performance, and functional impairment across the 25-week follow-up period (http://clinicaltrials.gov/show/NTC00830700). ADHD, ODD, and role performance were assessed with the Vanderbilt ADHD Rating Scales (VADRS) by caregivers (VADRS-Caregivers) and teachers (VADRS-Teachers).50 The VADRS scales include subscales of inattention (9 items), hyperactivity/impulsivity (9 items), oppositional defiant behaviors (8 items), and role performance related to academic, classroom, and interpersonal functioning (8 items). VADRS diagnosis-based items are based upon the DSM-IV 20 criteria for ADHD and are rated on a 4-point Likert scale. The VADRS shows a 2-factor model of inattention and hyperactivity/impulsivity consistent with a diagnosis of ADHD and has demonstrated solid psychometric properties.51–52 In the CATTS trial, the concurrent validity of the item total of the VADRS-Caregiver and the ADHD symptom scores on the CDISC-IV was high (r = .79). We scored the VADRS forms using their diagnosis-based algorithms for inattention, hyperactivity, combined ADHD, and ODD, thereby producing binary outcomes (diagnosis yes/no). The continuous role performance scale was scored by summing the item severity ratings.
The child’s level of functional impairment was assessed with the Columbia Impairment Scale, Parent-Report Version (CIS-P).53 The CIS-P has 13 items that tap impairment across domains of interpersonal relations, psychopathology, schoolwork or job, and use of leisure time and map onto a one-dimensional factor. Its reported internal consistency reliability and validity are very good.53 In the CATTS trial, internal consistency at baseline was high (Chronbach’s alpha = .84). The CIS-P is scored by summing the item severity ratings.
Statistical Analysis
We conducted intention-to-treat analyses. To evaluate the robustness of the randomization, we tested whether there were differences in demographic characteristics and baseline clinical status using chi-square test of proportions for diagnostic/binary variables and T-test for continuous variables. We found the number of comorbid conditions was unbalanced between the two arms and, therefore, controlled for comorbidity in subsequent regression analyses.
To test overall intervention effects, we applied Wald Chi-square tests to determine whether all four interaction terms were zero simultaneously or whether significantly more improvement in one arm occurred at least once during the four assessments. Then we evaluated individual interaction coefficients to determine when the significant differences occurred during the longitudinal assessments.54
In the longitudinal data analyses, we applied logistic mixed effects models to the binary outcomes and linear mixed effects models to the continuous outcomes. A patient-specific random intercept was included in all models to account for the within-subject correlations due to repeated assessments. In the mixed effects models, we included main effects for treatment arms, time (week of assessment), comorbid conditions, and interaction terms between treatment arms and time. We modeled time as a discrete predictor with five levels corresponding to baseline, week 4, 10, 19, and 25. Then we evaluated the coefficients on the interaction terms to capture the differences in rates of improvement from baseline to each follow-up assessment between the two study arms. The coefficient on the main group effect tested differences between study arms at baseline. The coefficients on the main time effect tested the differences between each follow-up assessment and baseline for the augmented primary care arm. The coefficients on the group-by-time interaction tested whether children in the CATTS telehealth service delivery achieved more improvement from baseline than children in the augmented primary care arm. For these binary outcomes, the odds ratio estimates on the interaction terms or their logarithmic transformations provide adjusted effect size estimates.55
For the continuous outcomes measuring functioning, we reported beta coefficients from linear mixed effects models. In addition, we reported Cohen’s d based on between-study arm comparisons of within-individual changes in scale scores from baseline to 25-week follow-up. In a study such as ours, where adjustments are warranted for uneven distribution of confounding variables across study arms and for features of the study design (longitudinal, nested structure), the Cohen’s d statistic provides a rough indication of effect size.
Analyses were conducted using Stata 12.1.56 We set the significance level at 0.05, a priori.
RESULTS
Participants, Engagement, and Characteristics of the Study Arms
Eighty-eight PCPs made successful referrals to the trial. The sample consisted of 223 children with mean age 9.25 (± 2.0) years, predominantly of European/white ancestry (91.5%), with a median annual family income of $35,000 to $75,000. Seventy-five percent of children (n=168) had at least one comorbid disorder. Forty-two percent (n=93) met criteria for ODD, and 6% (n=13) met criteria for AD; 28% (n=62) met criteria for ODD and AD.
Of the six possible sessions, participants randomized to the CATTS telehealth service delivery arm (n=111) attended an average of 5.2 (range 0 to 6) telepsychiatry sessions and an average of 5.1 (range 0 to 6) caregiver behavior training sessions. Of participants randomized to the augmented primary care arm (n=112), 94.6% attended their telepsychiatry consultation session.
As shown in Table 1, the demographic characteristics of children in the CATTS telehealth service delivery and augmented primary care arms were comparable with no statistically significant differences between the groups. Clinical characteristics were also well balanced with one exception. A higher proportion of children in the CATTS telehealth service delivery arm had ADHD with both comorbid disorders; therefore, we adjusted for comorbidity status (0,1, 2 comorbidities) in multivariate analyses.
Table 1.
Sample Demographic and Clinical Characteristics
| Demographic Variables | CATTS Telehealth Service Delivery (n=111) |
Augmented Primary Care (n=112) |
|
|---|---|---|---|
| Child Age, M (SD) | 9.2 (2.0) | 9.3 (2.0) | |
| Child Gender, n (%) | |||
| Male | 76 (68.5) | 87 (77.7) | |
| Female | 35 (31.5) | 23 (22.3) | |
| Child Race, n (%) | |||
| White | 104 (93.7) | 100 (89.3) | |
| Black | 1 (0.9) | 1 (0.9) | |
| Asian | 0 (0) | 2 (1.8) | |
| NH/PI | 0 (0) | 4 (3.6) | |
| AI/AN | 4 (3.6) | 2 (2.7) | |
| Other | 2 (1.8) | 2 (1.8) | |
| Child Ethnicity, n (%) | |||
| Hispanic | 10 (9.0) | 19 (17.0) | |
| Non-Hispanic | 101 (91.0) | 93 (83.0) | |
| Marital Status, n (%) | |||
| Married/Cohabitating | 76 (68.5) | 83 (74.8) | |
| Other | 35 (31.5) | 28 (25.2) | |
| Household Income, n (%) | |||
| <35k | 41 (36.9) | 36 (32.4) | |
| 35k-75k | 28 (25.2) | 42 (37.8) | |
| 75k-100k | 22 (19.8) | 13 (11.7) | |
| >100k | 20 (18.0) | 20 (18.0) | |
| Comorbid Disorders, n (%)* | |||
| ADHD alone | 20 (18.0) | 35 (31.3) | |
| ADHD+ODD | 44 (39.6) | 49 (43.8) | |
| ADHD+AD | 7 (6.3) | 6 (5.4) | |
| ADHD+ODD+AD | 40 (36.0) | 22 (19.6) | |
| Clinical Measures | |||
| Caregiver Ratings | |||
| Vanderbilt ADHD Rating Scale, Diagnostic Scoring | |||
| Inattention diagnosis, n (%) | 92 (83) | 92 (82) | |
| Hyperactivity/Impulsivity diagnosis, n (%) | 74 (67) | 65 (58) | |
| ADHD-combined diagnosis, n (%) | 67 (60) | 58 (52) | |
| ODD diagnosis, n (%) | 68 (61) | 57 (51) | |
| Measures of Functioning | |||
| VADRS-Role Performance Score, M (sd) | 3.51 (0.59) | 3.52 (0.55) | |
| CIS-P Functioning Score, M (sd) | 24.71 (9.18) | 22.89 (8.95) | |
| Teacher Ratings | |||
| Vanderbilt ADHD Rating Scale, Diagnostic Scoring | |||
| Diagnostic Criteria per Algorithmic Scoring | |||
| Inattention Diagnosis, n (%) | 56 (50) | 64 (57) | |
| Hyperactivity/Impulsivity diagnosis, n (%) | 42 (38) | 32 (29) | |
| ADHD-combined Diagnosis, n (%) | 26 (23) | 19(17) | |
| ODD Diagnosis, n (%) | 34 (31) | 35 (31) | |
| Measure of Functioning | |||
| VADRS-Role Performance Score, M (SD) | 3.75 (0.72) | 3.90 (0.66) | |
Note: AD = anxiety disorder; ADHD = attention-deficit/hyperactivity disorder; AI/AN = American Indian/Alaska Native; CATTS = Children’s ADHD Telemental Health Treatment Study; CIS-P = Columbia Impairment Scale-Parent Version; NH/PI = Native Hawaiian/Pacific Islander; ODD = oppositional defiant disorder; VADRS = Vanderbilt ADHD Rating Scales.
p < 0.05
Caregiver-Rated Outcomes by Intervention Status
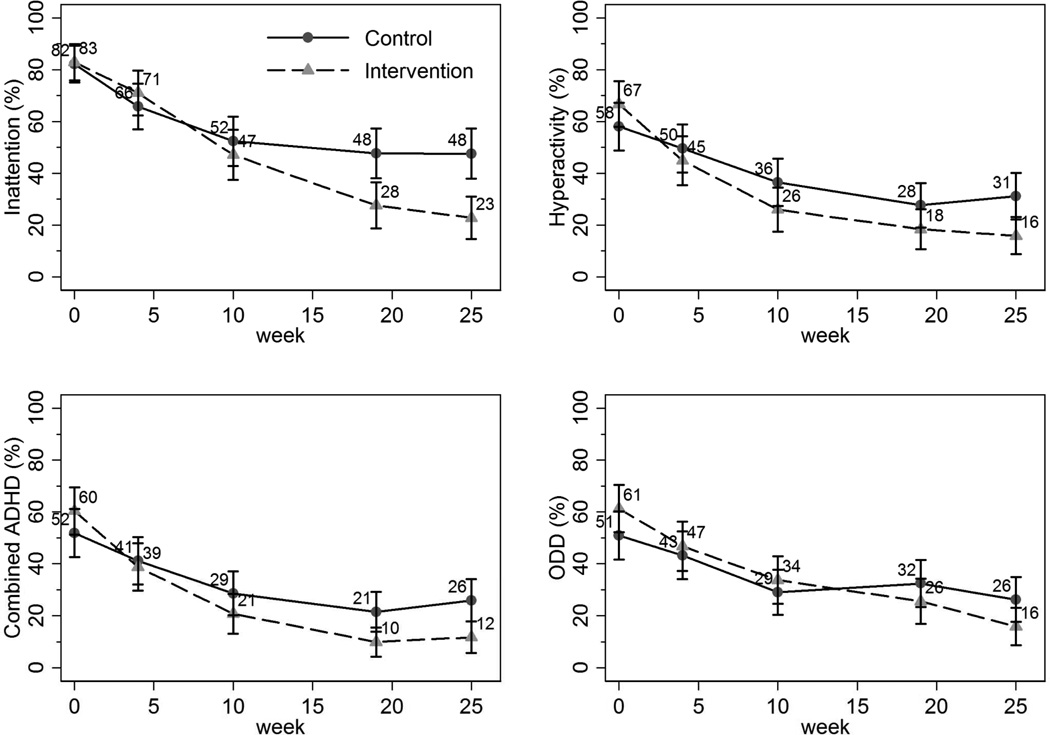
Figure 2 graphically shows the unadjusted proportions of participants in the CATTS telehealth service delivery and augmented primary care arms who met VADRS-Caregiver-based diagnostic criteria at baseline and at 4, 10, 19, and 25-week follow-up. At baseline, participants in the two arms had similar occurrence of meeting VADRS-Caregiver diagnostic criteria for inattention (intervention vs. control: 83% vs. 82%), hyperactivity (67% vs. 58%), combined ADHD (60% vs. 52%), and ODD (61% vs. 51%). Children in both study arms improved over time. At 25 weeks, compared with children in the augmented primary care arm, lower proportions of children in the CATTS intervention arm met diagnostic criteria on the VADRS-Caregiver: inattention (23% vs. 48%), hyperactivity (16% vs. 31%), combined ADHD (12% vs. 26), and ODD (16% vs. 26%).
Figure 2.
Proportions (unadjusted) of children at each assessment meeting caregiver-reported diagnostic criteria on subscales of the Vanderbilt Attention-Deficit/Hyperactivity Disorder (ADHD) Rating Scale. Note: ODD = oppositional defiant disorder.
The steeper decline in the unadjusted proportions of children in the CATTS telehealth service delivery arm meeting diagnostic criteria shown in Figure 2 were formally tested. According to the tests of overall intervention effects, the CATTS telehealth service delivery arm demonstrated greater improvements at least once during follow-up assessment in all four VADRS-Caregiver diagnostic outcomes: inattention (Wald χ2=19.47, df=4, p<.001), hyperactivity/impulsivity (Wald χ2=11.91, df=4, p=0.02), combined ADHD (Wald χ2=14.90, df=4, p=.005), and ODD (Wald χ2=10.05, df=4, p=0.04).
Table 2 shows the course of the VADRS-caregiver ratings. The coefficients on the main time effect indicate the significant improvement for the augmented primary care arm, and the coefficients on the group-by-time interaction indicate the greater improvement for the CATTS telehealth service delivery arm, which were evident by week 10 for hyperactivity and combined ADHD, and by week 19 for inattention and ODD.
Table 2.
Treatment Outcomes: Meeting Diagnostic Criteria Based on Vanderbilt Attention-Deficit/Hyperactivity Disorder (ADHD) Rating Scales
| Caregiver Ratings (N = 223) |
Inattention OR (95% CI) |
Hyperactivity OR (95% CI) |
ADHD-Combined OR (95% CI) |
ODD OR (95% CI) |
|
|---|---|---|---|---|---|
| Group | |||||
| Intervention vs. Control | .822 (0.276–2.446) | 1.354 (0.516–3.556) | 1.338 (0.552–3.244) | 1.220 (0.431–3.452) | |
| Time (in weeks) | |||||
| 4 | .228 (0.100–0.522)*** | .524 (0.252–1.092) | .471 (0.233–0.951)* | .512 (0.232–1.129) | |
| 10 | .085 (0.036–0.200)*** | .190 (0.087–0.412)*** | .185 (0.088–0.390)*** | .137 (0.058–0.324)*** | |
| 19 | .059 (0.025–0.141)*** | .090 (0.039–0.206)*** | .100 (0.045–0.222)*** | .183 (0.079–0.427)*** | |
| 25 | .058 (0.024–0.139)*** | .120 (0.053–0.272)*** | .149 (0.070–0.318)*** | .103 (0.042–0.251)*** | |
| Group × Time | |||||
| 1 × 4 | 1.410 (0.439–4.528) | .368 (0.128–1.058) | .486 (0.179–1.316) | .535 (0.172–1.658) | |
| 1 × 10 | .604 (0.184–1.984) | .213 (0.069–0.664)** | .296 (0.101–0.874)* | .618 (0.186–2.051) | |
| 1 × 19 | .211 (0.060–0.745)* | .236 (0.070–0.794)* | .149 (0.044–0.512)** | .247 (0.072–0.853)* | |
| 1 × 25 | .140 (0.038–0.508)** | .137 (0.040–0.466)** | .132 (0.040–0.432)** | .148 (0.039–0.562)** | |
|
Teacher Ratings (n=149) |
Inattention OR (95% CI) |
Hyperactivity OR (95% CI) |
ADHD–Combined OR (95% CI) |
ODD OR (95% CI) |
|
| Group | |||||
| .518 (0.174–1.542) | 2.135 (0.646–7.052) | 1.746 (.511–5.967) | .834 (0.239–2.908) | ||
| Intervention vs. Control | |||||
| Time (in weeks) | |||||
| 4 | .234 (0.096–0.568)*** | .614 (0.232–1.629) | .337 (0.117–0.968)* | .841 (0.320–2.216) | |
| 10 | .272 (0.114–0.649)** | .785 (0.304–2.028) | .910 (0.315–2.628) | .354 (0.128–0.983)* | |
| 19 | .227 (0.095–0.541)*** | .164 (0.056–0.480)*** | .191 (0.060–0.608)** | .179 (0.062–0.520)** | |
| 25 | .254 (0.101–0.637)** | .171 (0.054–0.546)** | .153 (0.046–0.510)** | .332 (0.117–0.945)* | |
| Group × Time | |||||
| 1 × 4 | 2.046 (0.568–7.373) | .699 (0.169–2.884) | .731 (0.173–3.084) | .490 (0.113–2.132) | |
| 1 × 10 | .493 (0.133–1.822) | .073 (0.014–0.371)** | .077 (0.013–0.451)** | .673 (0.141–3.212) | |
| 1 × 19 | .504 (0.136–1.863) | .749 (0.164–3.417) | .443 (0.087–2.267) | .441 (0.084–2.312) | |
| 1 × 25 | .320 (0.079–1.300) | .311 (0.056–1.732) | .178 (0.027–1.174) | .108 (0.017–0.704)* | |
Note: Analyses per logistic mixed effects regression models; all models adjusted for baseline comorbid conditions; coefficients on the group × time interaction terms are the logarithmic ORs. Note: ODD = oppositional defiant disorder; VADRS = Vanderbilt ADHD Rating Scale.
p< 0.05;
p < 0.01;
p < 0.001
Functional improvements also differed by intervention status. At baseline, the average VADRS-Caregiver role performance scores were comparable between the CATTS telehealth service delivery and augmented primary care arms.
According to the tests of overall differences, children in the CATTS telehealth service delivery demonstrated significantly greater improvement from baseline to follow-up assessments in VADRS-Caregiver role performance (Wald χ2=12.40, df=4, p=0.01) and CIS-P impairment scores (Wald χ2=20.52, df=4, p<.001). As noted in Table 3, these significant intervention differences were noted at 19 weeks for CIS-P impairment (12.80 ± 6.95 vs. 15.11 ± 9.58, p=0.05) and at 25 weeks for VADRS-Caregiver role performance (2.96 ± 0.67 vs. 3.21 ± 0.77, p=0.01). Looking at within-participant changes between baseline and 25-week follow-up, we estimated that the CATTS telehealth service delivery achieved an effect size of 0.38 (Cohen’s d) on the VADRS-Caregiver rated role performance, and 0.44 on the CIS-P.
TABLE 3.
Treatment Outcomes: Role Performance and Impairment Scale Scores
| Caregiver VADRS-Role Performance Scale β (95% CI) (N=223) |
Caregiver-Report CIS-P Scale β (95% CI) (N=223) |
Teacher VADRS-Role Performance Scale β (95% CI) (n=149) |
||
|---|---|---|---|---|
| Group | ||||
| Intervention vs. Control | −.052 (−.195 – .091) | .447 (−1.617 – 2.512) | −.159 (−.352 – .034) | |
| Time (in weeks) | ||||
| 4 | −.180 (−.274 – −.085) | −4.555 (−5.797 – −3.313) | −.176 (−.278 – −.074) | |
| 10 | −.245 (−.357 – −.132) | −5.973 (−7.419 – −4.529) | −.253 (−.381 – −.126) | |
| 19 | −.339 (−.447 – −.232) | −7.040 (−8.666 – −5.413) | −.405 (−.543 – −.267) | |
| 25 | −.317 (−.434 – −.200) | −8.111 (−9.720 – −6.502) | −.245 (−.395 – −.096) | |
| Group × Time | ||||
| 1 × 4 | .055 (−.064 – .175) | 1.047 (−.642 – 2.737) | .045 (−.100 – .191) | |
| 1 × 10 | .023 (−.135 – .182) | −1.386 (−3.546 – .774) | −.149 (−.349 – .050) | |
| 1 × 19 | −.140 (−.306 – .025) | −3.593 (−5.885 – −1.301)** | −.113 (−.318 – .092) | |
| 1 × 25 | −.220 (−.389 – −.051)* | −3.678 (−5.870 – −1.485)** | −.223 (−.462 – .016) |
Note: Analyses per linear mixed effects models; all models adjusted for baseline comorbid conditions. CIS-P = Columbia Impairment Scale, parent-report version; VADRS = Vanderbilt Attention-Deficit/Hyperactivity Disorder (ADHD) Rating Scale.
p< 0.05;
p < 0.01
Teacher-Rated Outcomes by Intervention Status
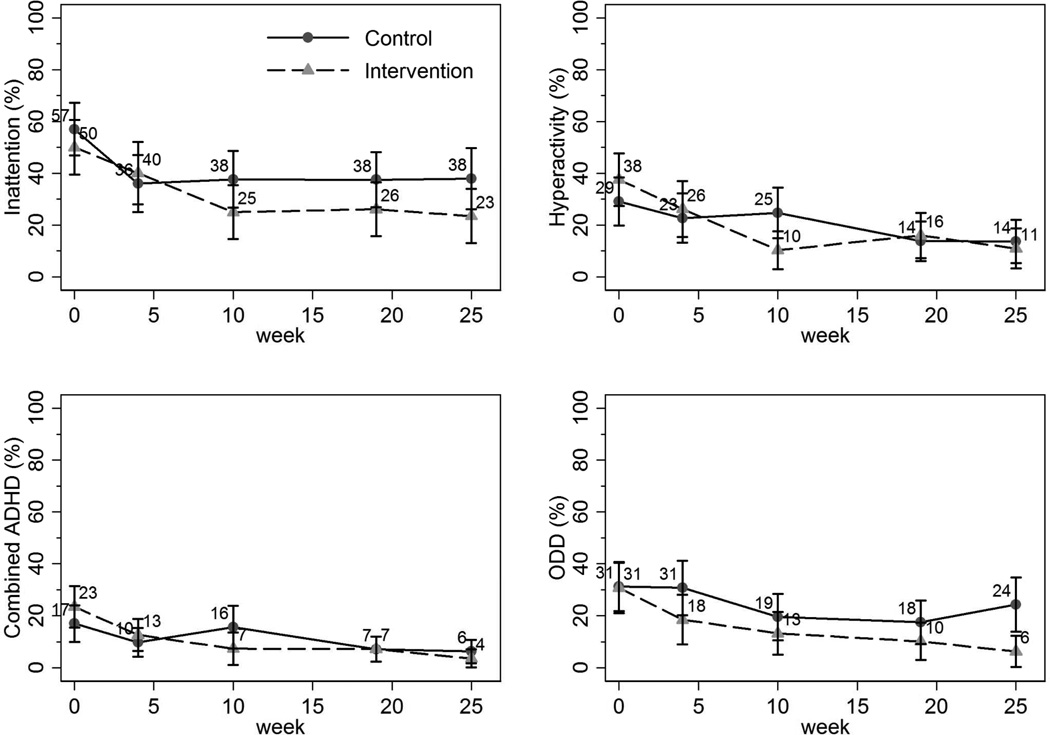
Figure 3 graphically depicts the unadjusted proportions of participants in the CATTS telehealth service delivery and augmented primary care arms who met VADRS-Teacher-based diagnostic criteria at baseline and at 4-, 10-, 19-, and 25-week follow-up. At baseline, participants in the two arms had a similar occurrence of meeting VADRS-Teacher diagnostic criteria for inattention, hyperactivity, combined ADHD, and ODD, and both groups improved over time.
Figure 3.
Proportions (unadjusted) of children at each assessment meeting teacher-reported diagnostic criteria on subscales of the Vanderbilt Attention-Deficit/Hyperactivity Disorder (ADHD) Rating Scale. Note: ODD = oppositional defiant disorder.
According to the tests of overall intervention effects, the VADRS-Teacher scores demonstrated significantly greater improvements at least once for the CATTS telehealth service delivery arm for hyperactivity (Wald χ2=11.28, df=4, p=0.02) and total ADHD (Wald χ2=9.72, df=4, p=0.045). As indicated in Table 2, results of logistic mixed effects regression models show that these intervention effects reflected significant differences only at week 10. There was no difference in outcomes between the intervention arms for inattention and ODD.
As shown in Table 3, results of linear mixed effects models indicate that improvements in VADRS-Teacher-reported role performance scores did not differ significantly between the two study arms. Evaluation of the within-subject changes between baseline and 25-week follow-up yields an estimated effect size of 0.37 (Cohen’s d) for the CATTS telehealth service delivery arm, similar to caregiver-reported role performance.
We conducted sensitivity analyses with additional demographics (age, gender, and parental education) and site indicators in the models. There were no significant changes in the intervention effects estimates, consistent with the balance among these factors achieved in our randomization.
DISCUSSION
The CATTS trial is the first community-based investigation testing the effectiveness of a telehealth service delivery model to improve mental health outcomes for children, and the only trial comparing a telehealth service delivery model with a primary care service model. The trial demonstrated that both the short-term CATTS telehealth service delivery and the augmented primary care treatment arms were associated with reductions in caregiver-reported ADHD and ODD diagnoses and improvement in role performance and impairment over 25 weeks, and that the telehealth service model performed better than the augmented primary care model. These results are broadly consistent with prior findings, that children with ADHD and comorbid disorders who were treated in person over a longer duration with a combined intervention improved more than those treated in primary care.57 They support the “added value” of providing short-term expert care through telehealth over treatment in primary care.
For most measures, significant differences between the CATTS telehealth service delivery and the augmented primary care arms did not emerge until 10- to 19-weeks post-randomization, possibly reflecting the CATTS short-term intervention model with fewer and less frequent caregiver training sessions than delivered in prior trials conducted in person, or the lack of an initial washout and titration of medication to an individual optimal dose.27, 28, 42, 48, 57–58 Alternatively, the single teleconsultation may have led PCPs to initiate successful treatment, consistent with other telehealth studies, 59–61 thereby delaying the emergence of differences between the treatment arms, but a teleconsultation may have been insufficient to sustain benefits, consistent with adult studies that have shown inadequate management of chronic conditions in primary care. 62–63 By the 10-week assessment, families had participated in three telepsychiatry and three caregiver behavior training sessions, and by the 19-week assessment, they had participated in 5 combined sessions. There may be a critical minimal number of sessions needed to detect group differences, and/or the caregiver training sessions may need to be conducted more closely together to detect differences over a shorter timeframe.
The more limited benefits for the CATTS intervention for teacher-rated outcomes reflect several factors. Behavioral treatments yield the largest effects in the setting in which they are implemented,48, 58 and interventions provided directly to teachers have shown improved ADHDrelated behaviors per both teacher ratings and observations.57, 64–65 The CATTS service delivery model may have benefitted with the addition of a school component in which teachers provided more frequent feedback during medication adjustment through an asynchronous website and received consultation regarding classroom behavior management through videoconferencing.65 Methodological issues may have contributed to the teacher-reported outcomes. Eligibility was determined on the basis of caregiver ratings, but caregivers and teachers only correlate moderately in their descriptions of youth characteristics. Consistent with prior studies,65, 67 teachers rated lower levels of ADHD symptoms, ODD, and role performance than caregivers on the same scales leaving less room for improvement over the course of the trial and attenuating observed effect sizes. Future work should consider both caregiver and teacher reports in determining study eligibility and use telehealth technologies to include teachers in intervention trials. Outcomes should include objective school outcomes measures, such as behavioral observations or homework completion.
There are several implications of the CATTS trial for future work. Investigators may be interested in determining the relative contributions of the pharmacologic and behavioral treatment components of the CATTS telehealth service delivery, particularly for subgroups of children with ADHD, for example those with and without comorbid disorders or learning disabilities, or for families that have access to one but not both of the treatment components. Of note in the current study, children in the CATTS telehealth service delivery showed improvements in both ADHD symptoms, which may preferentially respond to pharmacologic interventions,68–69 as well as ODD, role performance, and impairment outcomes, which may preferentially respond to behavioral interventions. 70–71
Sustainability of the two models is important to further development of telehealth programs. Increasingly, states are mandating coverage for telehealth,72–73 and third party payers are reimbursing synchronous telehealth services 74–76 using specific Current Procedural Terminology (CPT) codes.77–78 Telepsychiatry appears to be reimbursed more predictably than teletherapies, particularly by federal and state programs.74–76, 78 Further work to establish the effectiveness of delivering caregiver behavior training through videoconferencing will promote the sustainability of teletherapies that can help to reduce the burden of mental illness for families who are not well served by usual models of care.79
Telehealth is well poised to contribute to efforts by Accountable Care Organizations (ACOs) to provide mental health care to children living in underserved areas,78 and future work should examine the cost to benefit of a range of telehealth models of care for ADHD implemented at the population level80 and determine the optimal mix of intervention components and number of sessions needed to effectively and efficiently improve children’s mental health.
The CATTS trial had several limitations. The augmentation of primary care treatment with a teleconsultation may have yielded smaller group differences than would have been evident if we had used it as a treatment-as-usual control. Methodological issues may have contributed to the teacher-reported outcomes. Compared to caregivers, teachers completed fewer assessments with inconsistency in teacher reporters for some children, which introduced greater variance for teacher-reported outcomes. Overall, the trial may have been underpowered to detect group differences for teacher-rated outcomes. The sample may have been biased by referral from PCPs who were willing to use telehealth services and/or who referred children with more complicated symptomatology, and by the exclusion of children with complex medical, developmental, and psychiatric disorders.
CLINICAL GUIDANCE.
The CATTS trial supports the effectiveness of treating children with ADHD using telehealth.
A direct telehealth service model offers undeserved communities potential parity with metropolitan communities in accessing guideline-based interventions.
An augmented primary care model may optimally redistribute the child psychiatry workforce and support PCPs’ efforts to treat children’s mental health disorders..
Asynchronous telehealth technologies offer therapists an opportunity to upgrade their skills in treating children and to strengthen the child mental health workforce in underserved communities.
Stakeholders who are considering telehealth should determine whether a short-term direct service model or an augmented primary care model best meets their community’s needs and resources.81–82
Child and adolescent psychiatrists interested in a telehealth practice should consider the evidence base for providing care through telehealth technologies, collaborative models for working with clinicians at the patient site, supports needed at both the provider and the patient sites, and financial models to sustain a successful practice.
Acknowledgments
The design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript was supported by funding from the National Institute of Mental Health (NIMH; 1R01MH081997 and R01MH081997-04S1). Funding for pilot data was provided by the University of Washington Institute of Translational Health Sciences Small Pilot Project Grant program (566821); the University of Washington Royalty Research Fund program (65-4020); and the American Academy of Child and Adolescent Psychiatry Abramson Fund (506200020101).
Dr. Zhou served as the statistical expert for this research.
The authors wish to thank all of the families who participated in the Children’s ADHD Telemental Health Treatment Study. They are grateful to the staff and therapists from the Seattle Children’s Research Institute who worked with the families and helped to bring the study to completion: Heather Violette, PhD; Gina Kim, BA; Sarah Grover, BS; Jane Koltracht, BA; Yuet Juhn Tse, BA; Jessica Garcia, BA; Laura Aviles, MSW; Aloma Burrows, Med; Lila Waldron, MS; Corrie Piper, MMFT; and Joan Arrasmith, MEd. A special thanks to Kelly Thompson, MSW, from the University of Washington.
Disclosure: Dr. Myers has received research funding from the Seattle Children’s Research Institute (Intramural). She is an editor of Telemental Health: Clinical, Technical, and Administrative Foundations for Evidence-Based Practice, published by Elsevier, and Child and Adolescent Psychiatry: The Essentials, published by Lippincott, Williams, and Wilkins. Dr. Vander Stoep has received research funding from the National Institutes of Health, Fogarty International Center; the US Department of Education, National Center for Education Research, Institute of Education Sciences; the Don Loeb Family Foundation; the Developmental Pathways Project: Young Adult Study; and NIMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Drs. Zhou, McCarty, and Katon report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Holzer CE, Goldsmith HF, Ciarlo JA. Mental Health, United States. DHHS Publication No, (SMA) 99-3285. Washington, DC: Superintendent of Documents, U.S. Government Printing Office; 1998. Effects of rural-urban county type on the availability of health and mental health care providers. Chapter 16. [Google Scholar]
- 2.Thomas CR, Holzer CE., 3rd The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1023–1031. doi: 10.1097/01.chi.0000225353.16831.5d. [DOI] [PubMed] [Google Scholar]
- 3.Muskie School of Public Service, Research and Policy Brief. [Accessed December 29, 2014];Rural Children Don’t Receive the Mental Health Care They Need. Maine Rural Health Research Center. http://muskie.usm.maine.edu/Publications/rural/pb39/Rural-Children-Mental-Health-Services.pdf. Published 2009. [Google Scholar]
- 4.Ziller EC, Coburn AF, Loux SL, Hoffman C, McBride TD. Health insurance coverage in rural America. (No. 4093) Washington, DC: The Kaiser Commission on Medicaid and the Uninsured, University of Southern Maine; 2003. [Google Scholar]
- 5.Burns BJ, Costello EJ, Angold A, et al. Children’s mental health service use across service sectors. Health Affairs. 1995;14(3):147–159. doi: 10.1377/hlthaff.14.3.147. [DOI] [PubMed] [Google Scholar]
- 6.Yellowlees PM, Shore J, Roberts L. Practice Guidelines for Videoconferencing-Based Telemental Health – October 2009. Telemedicine and e-Health. 2010;16(10):1074–1089. doi: 10.1089/tmj.2010.0148. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary. The National Academies Press; [Accessed December 30, 2014]. website. http://www.nap.edu/catalog.php?record_id=13466, Published 2012. [PubMed] [Google Scholar]
- 8.Elford DR, White H, St John K, Maddigan B, Ghandi M, Bowering R. A prospective satisfaction study and cost analysis of a pilot child telepsychiatry service in Newfoundland. J Telemed Telecare. 2001;7(2):73–81. doi: 10.1258/1357633011936192. [DOI] [PubMed] [Google Scholar]
- 9.Myers K, Valentine JM, Melzer SM. Feasibility, acceptability, and sustainability of telepsychiatry for children and adolescents. Psychiatr Serv. 2007;58(11):1493–1496. doi: 10.1176/ps.2007.58.11.1493. [DOI] [PubMed] [Google Scholar]
- 10.Myers KM, Valentine JM, Melzer SM. Child and adolescent telepsychiatry: utilization and satisfaction. Telemed J E Health. 2008;14:131–137. doi: 10.1089/tmj.2007.0035. [DOI] [PubMed] [Google Scholar]
- 11.Boydell KM, Volpe T, Pignatiello A. A qualitative study of young people's perspectives on receiving psychiatric services via televideo. J Can Acad Child Adolesc Psychiatry. 2010;19:5–11. [PMC free article] [PubMed] [Google Scholar]
- 12.Elford R, White H, Bowering R, et al. A randomized, controlled trial of child psychiatric assessments conducted using videoconferencing. J Telemed Telecare. 2000;6(2):73–82. doi: 10.1258/1357633001935086. [DOI] [PubMed] [Google Scholar]
- 13.Reese RM, Jamison R, Wendland M, Fleming K, Braun MJ, Turek J. Evaluating interactive videoconferencing for assessing symptoms of autism. Telemed J E Health. 2013;19(9):671–677. doi: 10.1089/tmj.2012.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yellowlees PM, Hilty DM, Marks SL, Neufeld J, Bourgeois JA. A retrospective analysis of a child and adolescent eMental Health program. J Am Acad Child Adolesc Psychiatry. 2008;47(1):103–107. doi: 10.1097/chi.0b013e31815a56a7. [DOI] [PubMed] [Google Scholar]
- 15.Reese RJ, Slone NC, Soares N, Sprang R. Telehealth for underserved families: an evidence-based parenting program. Psychol Serv. 2012;9(3):320–322. doi: 10.1037/a0026193. [DOI] [PubMed] [Google Scholar]
- 16.Nelson E, Barnard M, Cain S. Treating childhood depression over teleconferencing. Telemed J E Health. 2003;9:49–55. doi: 10.1089/153056203763317648. [DOI] [PubMed] [Google Scholar]
- 17.Xie Y, Dixon JF, Yee OM, et al. A study on the effectiveness of videoconferencing on teaching parent training skills to parents of children with ADHD. Telemed J E Health. 2013;19(3):1–8. doi: 10.1089/tmj.2012.0108. [DOI] [PubMed] [Google Scholar]
- 18.Epstein JN, Langberg JM, Lichtenstein PK, et al. Attention-deficit/hyperactivity disorder outcomes for children treated in community-based pediatric settings. Arch Pediatr Adolesc Med. 2010;164(2):160–165. doi: 10.1001/archpediatrics.2009.263. [DOI] [PubMed] [Google Scholar]
- 19.Epstein JN, Langberg JM, Lichtenstein PK, Kolb RC, Stark LJ. Sustained improvement in pediatrician’s ADHD practice behaviors in the context of a community-based quality improvement initiative. Children’s Health Care. 2010;39:296–311. [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 21.Subcommittee on Attention-Deficit/Hyperactivity Disorder. Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–1022. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- 23.American Academy of Pediatrics. Committee on Psychosocial Aspects of Child and Family Health. The new morbidity revisited: a renewed commitment to the psychosocial aspects of pediatric care. Pediatrics. 2001;108(5):1227–1230. doi: 10.1542/peds.108.5.1227. [DOI] [PubMed] [Google Scholar]
- 24.Power TJ, Mautone JA, Manz PH, Frye L, Blum NJ. Managing attention-deficit hyperactivity disorder in primary care:a systematic analysis of roles and challenges. Pediatrics. 2008;121(1):e65–e72. doi: 10.1542/peds.2007-0383. [DOI] [PubMed] [Google Scholar]
- 25.Knapp CA, Hinojosa M, Baron-Lee J, Fernandez-Baca D, Hinojosa R, Thompson L. Factors associated with a medical home among children with attention-deficit hyperactivity disorder. Maternal Child Health J. 2012;16(9):1771–1778. doi: 10.1007/s10995-011-0922-6. [DOI] [PubMed] [Google Scholar]
- 26.Leslie LK, Weckerly J, Plemmons D, Landsverk J, Eastman S. Implementing the American Academy of Pediatrics attention-deficit/hyperactivity disorder diagnostic guidelines in primary care settings. Pediatrics. 2004;114:129–140. doi: 10.1542/peds.114.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolko DJ, Campo J, Kilbourne AM, Hart J, Sakolsky D, Wisniewski S. Collaboration care outcomes for pediatric behavior health problems: A cluster randomized trial. Pediatrics. 2014;133:e981–e992. doi: 10.1542/peds.2013-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorell L. The Community Parent Education program (COPE). Treatment effects in a clinical and a community sample. Clin Child Psychol Psychiatry. 2009;14(3):373–387. doi: 10.1177/1359104509104047. [DOI] [PubMed] [Google Scholar]
- 29.Jensen PS, Hinshaw SP, Kraemer HC, et al. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. J Am Acad Child Adolesc Psychiatry. 2001;40(2):147–158. doi: 10.1097/00004583-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 30.United States Public Health Service Office of the Surgeon General. Mental Health: A Report of the Surgeon General. Rockville, MD: Department of Health and Human Services, US Public Health Service; 1999. [Google Scholar]
- 31.Baum RA, Epstein JN, Kelleher K. Healthcare reform, quality, and technology: ADHD as a case study. Curr Psychiatry Rep. 2013;15(7):369–375. doi: 10.1007/s11920-013-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Health Resources and Services Administration. [Accessed December 30, 2014];Health Information Technology: TeleHealth. US Department of Health and Human Services website. http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Telehealth.
- 33.Centers for Medicare and Medicaid. [Accessed December 30, 2014];Telemedicine. Medicaid website. http://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Delivery-Systems/Telemedicine.html.
- 34.Vander Stoep A, Myers K. Methodology for conducting the Children's Attention-deficit Hyperactivity Disorder Telemental Health Treatment Study in multiple underserved communities. Clin Trials. 2013;10(6):949–958. doi: 10.1177/1740774513494880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers K, Vander Stoep A, Lobdell C. Feasibility of conducting a randomized controlled trial of telemental health with children diagnosed with attention-deficit/hyperactivity disorder in underserved communities. J Child Adolesc Psychopharmacol. 2013;23(6):372–378. doi: 10.1089/cap.2013.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers KM, Vander Stoep A, McCarty CA, et al. Child and adolescent telepsychiatry: variations in utilization, referral patterns and practice trends. J Telemed Telecare. 2010;16(3):128–133. doi: 10.1258/jtt.2009.090712. [DOI] [PubMed] [Google Scholar]
- 37.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- 38.Shaffer D, Fisher P, Dulcan MK, et al. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): description, acceptability, prevalence rates, and performance in the MECA Study. Methods for the Epidemiology of Child and Adolescent Mental Disorders Study. J Am Acad Child Adolesc Psychiatry. 1996;35(7):865–877. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Lewczyk CM, Garland AF, Hurlburt MS, Gearity J, Hough RL. Comparing DISC-IV and clinician diagnoses among youths receiving public mental health services. J Am Acad Child Adolesc Psychiatry. 2003;42(3):349–356. doi: 10.1097/00004583-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 40.McCarty CA, Vander Stoep A, Violette H, Myers K. Interventions developed for psychiatric and behavioral treatment in the children's ADHD Telemental Health Treatment Study [epub online ahead of print] J Child Fam Studies. 2014 May [Google Scholar]
- 41.Geyer J, Myers K, Vander Stoep A, McCarty C, Palmer N, DeSalvo A. Implementing a low-cost web-based clinical trial management system for community studies: a case study. Clin Trials. 2011;8(5):634–644. doi: 10.1177/1740774511416384. [DOI] [PubMed] [Google Scholar]
- 42.Majewicz-Hefley A, Carlson JS. A meta-analysis of combined treatments for children diagnosed with ADHD. J Attention Dis. 2007;10(3):239–250. doi: 10.1177/1087054706289934. [DOI] [PubMed] [Google Scholar]
- 43.Strand MT, Hawk LW, Bubnik M, Shiels K, Pelham WE, Waxmonsky JG. Improving working memory in children with attention-deficit/hyperactivity disorder: the separate and combined effects of incentives and stimulant medication. J Abnorm Child Psychol. 2012;40(7):1193–1207. doi: 10.1007/s10802-012-9627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pliszka SR, Crismon ML, Hughes CW, et al. Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. The Texas Children's Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/ hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(6):642–657. doi: 10.1097/01.chi.0000215326.51175.eb. [DOI] [PubMed] [Google Scholar]
- 45.Stahl SM. Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. 3rd ed. New York: Cambridge University Press; 2008. [Google Scholar]
- 46.McMahon RJ, Forehand R. Helping the Noncompliant Child, Second Edition: Family-Based Treatment for Oppositional Behavior. New York: The Guilford Press; 2003. [Google Scholar]
- 47.Barkely RA. Attention-Deficit Hyperactivity Disorder, Third Edition: A Handbook for Diagnosis and Treatment. 3rd Edition. New York: The Guilford Press; 2006. [Google Scholar]
- 48.Fabiano G, Pelham W, Coles E, Gnagy E, Chronis-Tuscano A, O'Connor B. A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder. Clin Psychol Rev. 2009;29(2):129–140. doi: 10.1016/j.cpr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Baquet CR, Commiskey P, Mullins CD, Mishra SI. Recruitment and participation in clinical trials: Socio-demograpic rural/urban, and health care access predictors. Cancer Detect Prev. 2006;30(1):24–33. doi: 10.1016/j.cdp.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jellinek M, Patel B, Froehle M, editors. Bright Futures in Practice: Mental Health—Volume II. Tool Kit. Arlington, VA: National Center for Education in Maternal and Child Health; 2002. No. II. [Google Scholar]
- 51.Wolraich M, Lambert W, Doffing M, Bickman L, Simmons T, Worley K. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. J Pediatr Psychol. 2003;28(8):559–567. doi: 10.1093/jpepsy/jsg046. [DOI] [PubMed] [Google Scholar]
- 52.Wolraich M, Bard D, Neas B, Doffing M, Beck L. The psychometric properties of the Vanderbilt Attention-Deficit Hyperactivity Disorder Diagnostic Teacher Rating Scale in a community population. J Dev Behav Pediatr. 2013;34(2):83–93. doi: 10.1097/DBP.0b013e31827d55c3. [DOI] [PubMed] [Google Scholar]
- 53.Bird HR, Shaffer D, Fisher P, Gould MS. The Columbia Impairment Scale: pilot findings on a measure of global impairment for children and adolescents. Int J Methods Psychiatr Res. 1993;3(3):167–176. [Google Scholar]
- 54.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis (Wiley Series in Probability and Statistics) Hoboken, NJ: John Wiley and Sons, Wiley-Interscience; 2004. Chapters 11 and 12. [Google Scholar]
- 55.Fleiss JL. Measures of effect size for categorical data. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. New York: Russell Sage Foundation; 1994. pp. 245–260. [Google Scholar]
- 56.Stata Statistical Software: Release 12. [computer program] College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 57.The MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56(12):1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 58.Chronis AM, Chacko A, Fabiano GA, Wymbs BT, Pelham WE., Jr. Enhancements to the behavioral parent training paradigm for families of children with ADHD: review and future directions. Clin Child Fam Psychol Rev. 2004;7(1):1–27. doi: 10.1023/b:ccfp.0000020190.60808.a4. [DOI] [PubMed] [Google Scholar]
- 59.Greenberg N, Boydell K, Volpe T. Pediatric telepsychiatry in Ontario: Caregiver and service provider perspectives. J Behav Health Sci Res. 2006;33(1):105–111. doi: 10.1007/s11414-005-9001-3. [DOI] [PubMed] [Google Scholar]
- 60.Lau ME, Way BB, Freemont WP. Assessment of SUNY Upstate Medical University’s child telepsychiatry consultation program. Int J Psychiatry Med. 2011;42:93–104. doi: 10.2190/PM.42.1.g. [DOI] [PubMed] [Google Scholar]
- 61.Hilty DM, Yellowlees PM, Nesbitt TS. Evolution of telepsychiatry to rural sites: changes over time in types of referral and in primary care providers' knowledge, skills and satisfaction. General Hospital Psychiatry. 2006;28(5):367–373. doi: 10.1016/j.genhosppsych.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 62.Katon WJ, Russo J, Lin EH, et al. Diabetes and poor disease control: is comorbid depression associated with poor medication adherence or lack of treatment intensification? Psychosom Med. 2009;71:965–972. doi: 10.1097/PSY.0b013e3181bd8f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solomon DH, Bitton A, Katz JN, Radner H, Brown E, Fraenkel L. Review: Treat to target in rheumatoid arthritis: Fact, fiction or hypothesis? Arthritis Rheumatol. 2014;4(66):775–782. doi: 10.1002/art.38323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fabiano GA, Vujnovic RK, Pelham WE, et al. Enhancing the effectiveness of special education programming for children with attention deficit hyperactivity disorder using a daily report card. Shool Psychol Rev. 2010;39(2):219–239. [Google Scholar]
- 65.Mikami AY, Griggs MS, Lerner MD, et al. A randomized trial of a classroom intervention to increase peers’ social inclusion of children with attention-deficit/hyperactivity disorder. J Conslt Clin Psychol. 2013;81(1):100–112. doi: 10.1037/a0029654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grady B, Lever N, Cunningham D, Stephan S. Telepsychiatry and school mental health. Child Adolesc Psychiatr Clin N Am. 2011;20(1):81–94. doi: 10.1016/j.chc.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 67.Lavigne JV, Dulcan MK, LeBailly SA, Binns HJ. Can parent reports serve as proxyfor teacher ratings in medication management of attention-deficit hyperactivity disorder? J Devel Behav Pediatrics. 2012;33(4):336–342. doi: 10.1097/DBP.0b013e31824afea1. [DOI] [PubMed] [Google Scholar]
- 68.Czerniak SM, Sikoglu EM, King JA, et al. Areas of the brain modulated by single-dose methylphenidate treatment in youth with ADHD during task-based fMRI: A systematic review. Harvard Rev Psychiatry. 2013;21(3):151–162. doi: 10.1097/HRP.0b013e318293749e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schulz KP, Fan J, Bedard A, et al. Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2012;69(9):952–961. doi: 10.1001/archgenpsychiatry.2011.2053. [DOI] [PubMed] [Google Scholar]
- 70.Evans SW, Owens JS, Bunford N. Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2014;43(4):527–551. doi: 10.1080/15374416.2013.850700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Daley D, van der Oord S, Ferrin M, et al. Behavioral interventions in attentiondeficit/ hyperactivity disorder: A meta-analyses of randomized controlled trials across multiple outcome domains. J Am Acad Child Adolesc Psychiatry. 2014;53(8):835–847. doi: 10.1016/j.jaac.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 72.State Telemedicine Policy Center. [Accessed December 30, 2014];State Telemedicine Gaps Analysis. American Telemedicine Association website. http://www.americantelemed.org/policy/state-telemedicine-policy Published 2012. [Google Scholar]
- 73. [Accessed December 30, 2014];American Psychological Association: Telehealth 50-State Review. 2013b Retrieved from http://www.apapracticecentral.org/advocacy/state/telehealth-slides.pdf Published October 2013. [Google Scholar]
- 74.American Telemedicine Association. [Accessed December 29, 2014];Telemedicine and Telehealth Services: Reimbursement. http://www.americantelemed.org/docs/default-source/policy/medicare-payment-of-telemedicine-and-telehealth-services.pdf. Published January 2013. [Google Scholar]
- 75.Center for Medicare and Medicaid Services. [Accessed December 29, 2014];Telehealth. CMS website. http://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/index.html. Modified January 2014.
- 76. [Accessed December 29, 2014];Keeping America Healthy. Medicaid website. http://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Delivery-Systems/Telemedicine.html.
- 77.American Medical Association. CPT 2015 Standard Codebook. Chicago: American Medical Association; 2014. [Google Scholar]
- 78.Center for Medicare and Medicaid Services (CMS) [Accessed December 30, 2014];Accountable Care Organizations. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ACO/index.html. Modified January 2015.
- 79.Kazdin AE, Blasé S. Rebooting psychotherapy research and practice to reduce the burden of mental illness. Perspectives Psychol Sci. 2011;6(1):21–37. doi: 10.1177/1745691610393527. [DOI] [PubMed] [Google Scholar]
- 80.Koepsell TD, Zatzick DF, Rivara FP. Estimating the population impact of preventive interventions from randomized trials. Am J Prev Med. 2011;40(2):191–198. doi: 10.1016/j.amepre.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Georgetown University Health Policy Institute. [Accessed December 27, 2014];Center for Children and Families. website. http://ccf.georgetown.edu/ [Google Scholar]
- 82. [Accessed December 29 2014];Health Reform: Children’s Health Coverage: Medicaid, CHIP and the ACA. The Henry J. Kaiser Family Foundation. website. http://kff.org/health-reform/issue-brief/childrens-health-coverage-medicaid-chip-and-the-aca/. Published March 2014. [Google Scholar]