Abstract
An ethylene binding component(s) has been partially purified from mung bean sprouts. Tissue was homogenized in 0.3 molar sucrose and 0.2 molar potassium phosphate buffer (pH 7.0). The homogenate was centrifuged, and resuspended fractions were assayed by incorporating them onto cellulose fibers (0.7 grams per milliliter). These were exposed to [14C]ethylene (3.7 × 10−2 microliters per liter of 120 millicurie per millimole) in the presence or absence of 1000 microliters per liter unlabeled ethylene. The cellulose was transferred to separate containers and the [14C]ethylene was absorbed in mercury perchlorate and counted. Distribution of ethylene binding to various fractions was: 0 to 3,000g, 3%; 3,000 to 12,000g; 4%; 12,000 to 100,000g, 69%; cellular debris, 24%; 100,000g supernatant, 0%. Adjustment of the pH to 4.0 precipitates the ethylene-binding component. Neutralization, addition of Triton X-100, and readjustment of the pH to 4.0 “solubilized” most of the binding component. Further purification was obtained by chromatography on CM-Sephadex in 10 millimolar potassium acetate buffer, (pH 5.0) containing 1% Triton X-100. Elution was with 200 millimolar potassium phosphate (pH 6.0) containing 1% Triton X-100. Upon treatment of the Triton “solubilized” component with cold acetone, over 90% of the binding capacity was lost. Extraction of the acetone-precipitated residue with 2% Triton X-100 restored some of the binding capacity which was found in the soluble fraction. The pH optimum for binding is 6.0. Passing the Triton X-100 extract of the acetone powder through Sepharose 6B provides considerable purification. The binding component moved ahead of most of the protein.
Full text
PDF
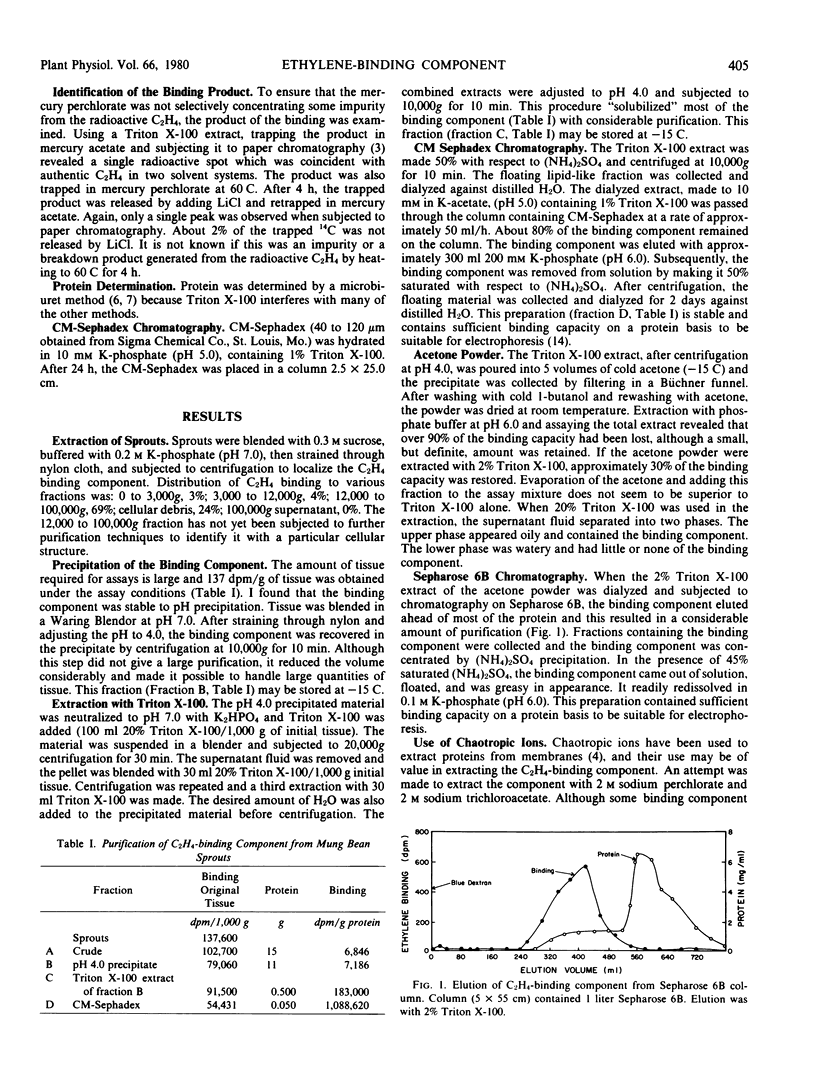
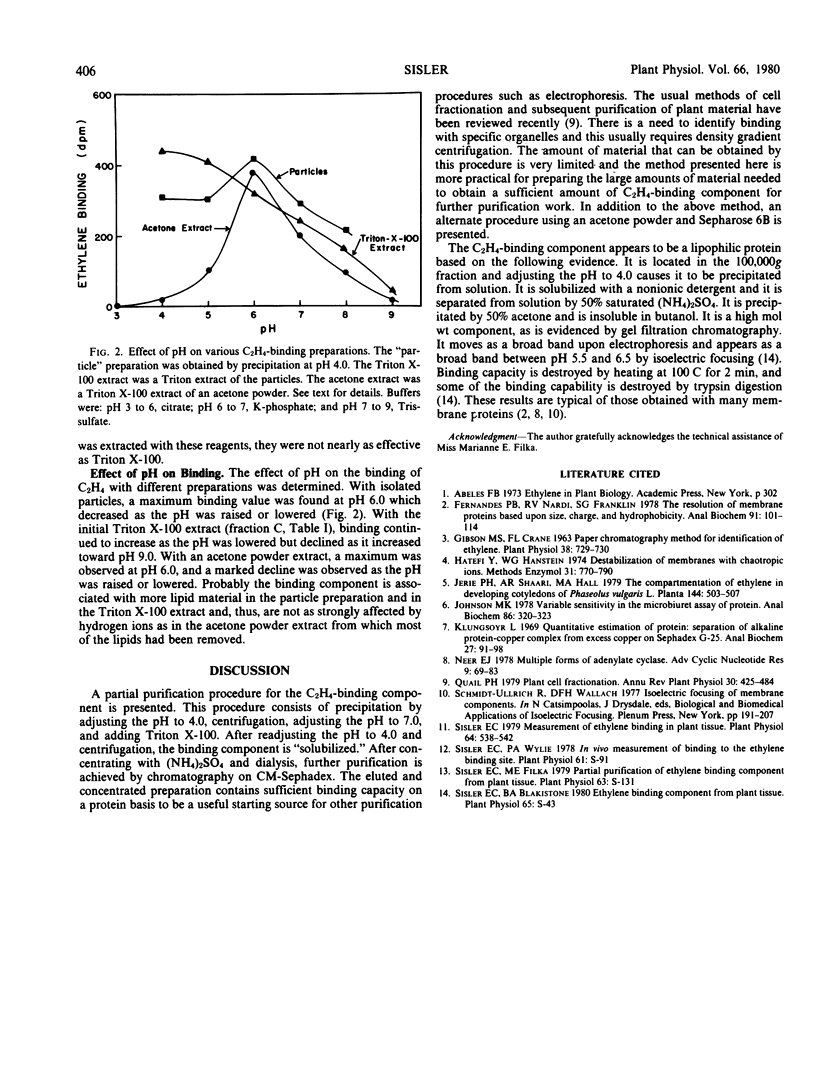
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fernandes P. B., Nardi R. V., Franklin S. G. The resolution of membrane proteins based upon size, charge, and hydrophobicity. Anal Biochem. 1978 Nov;91(1):101–114. doi: 10.1016/0003-2697(78)90820-5. [DOI] [PubMed] [Google Scholar]
- Gibson M. S., Crane F. L. Paper Chromatography Method for Identification of Ethylene. Plant Physiol. 1963 Nov;38(6):729–730. doi: 10.1104/pp.38.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Destabilization of membranes with chaotropic ions. Methods Enzymol. 1974;31:770–790. doi: 10.1016/0076-6879(74)31080-4. [DOI] [PubMed] [Google Scholar]
- Johnson M. K. Variable sensitivity in the microbiuret assay of protein. Anal Biochem. 1978 May;86(1):320–323. doi: 10.1016/0003-2697(78)90349-4. [DOI] [PubMed] [Google Scholar]
- Klungsöyr L. Quantitative estimation of protein. Separation of alkaline protein-copper complex from excess copper on Sephadex G-25. Anal Biochem. 1969 Jan;27(1):91–98. doi: 10.1016/0003-2697(69)90222-x. [DOI] [PubMed] [Google Scholar]
- Neer E. J. Multiple forms of adenylate cyclase. Adv Cyclic Nucleotide Res. 1978;9:69–83. [PubMed] [Google Scholar]
- Sisler E. C. Measurement of ethylene binding in plant tissue. Plant Physiol. 1979 Oct;64(4):538–542. doi: 10.1104/pp.64.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]


