Abstract
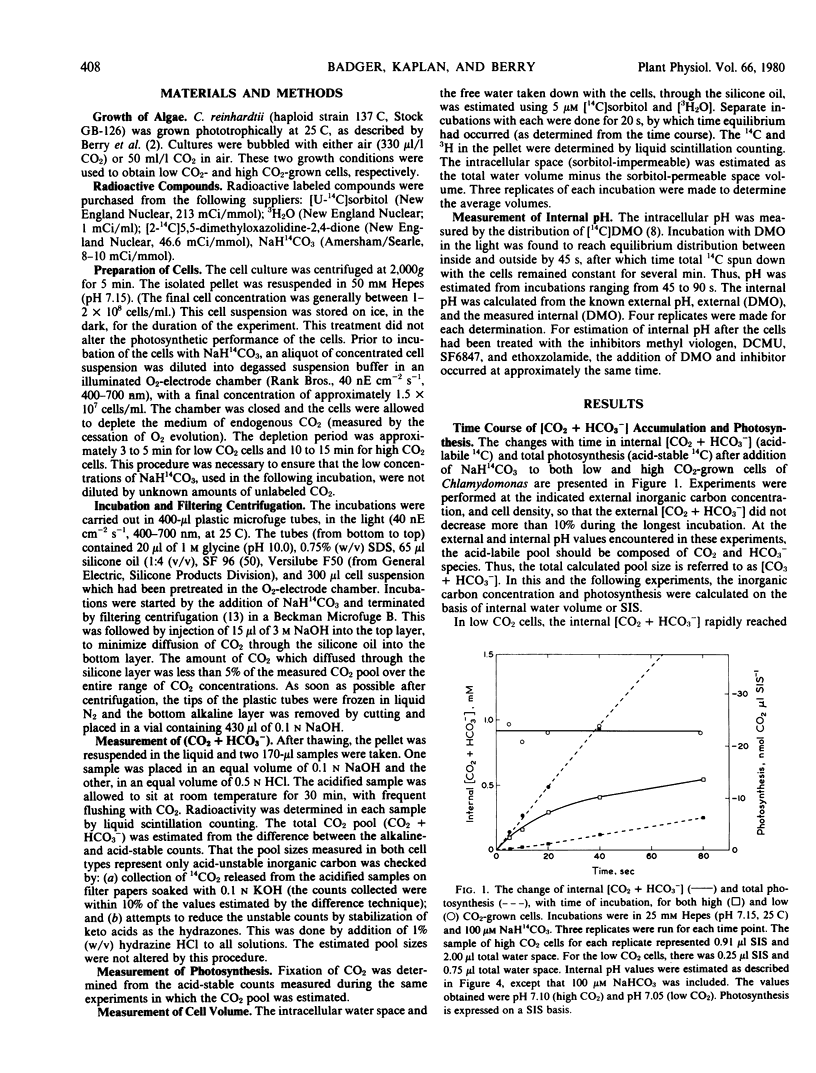
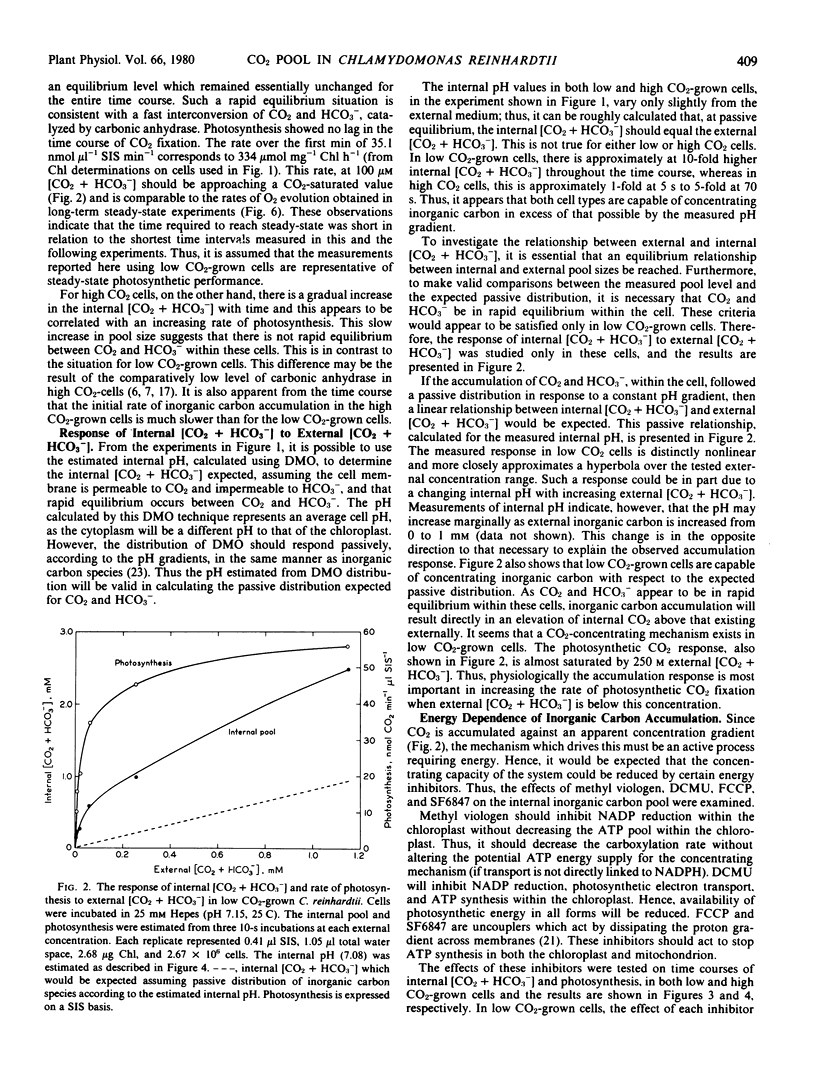
The external inorganic carbon pool (CO2 + HCO3−) was measured in both high and low CO2-grown cells of Chlamydomonas reinhardtii, using a silicone oil layer centrifugal filtering technique. The average internal pH values were measured for each cell type using [14C]dimethyloxazolidinedione, and the internal inorganic carbon pools were recalculated on a free CO2 basis. These measurements indicated that low CO2-grown cells were able to concentrate CO2 up to 40-fold in relation to the external medium. Low and high CO2-grown cells differed in their photosynthetic affinity for external CO2. These differences could be most readily explained as being due to the relative CO2-concentrating capacity of each cell type. This physiological adaptation appeared to be based on changes in the abilities of the cells actively to accumulate inorganic carbon using an energy-dependent transport system.
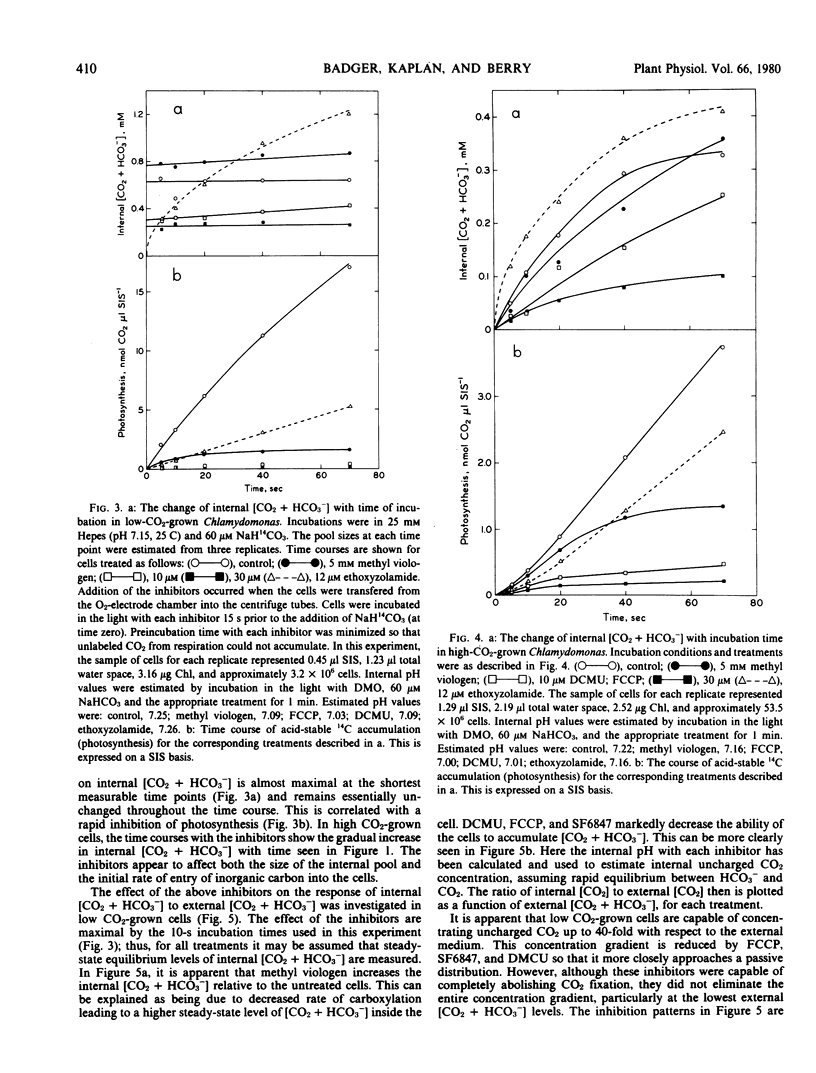
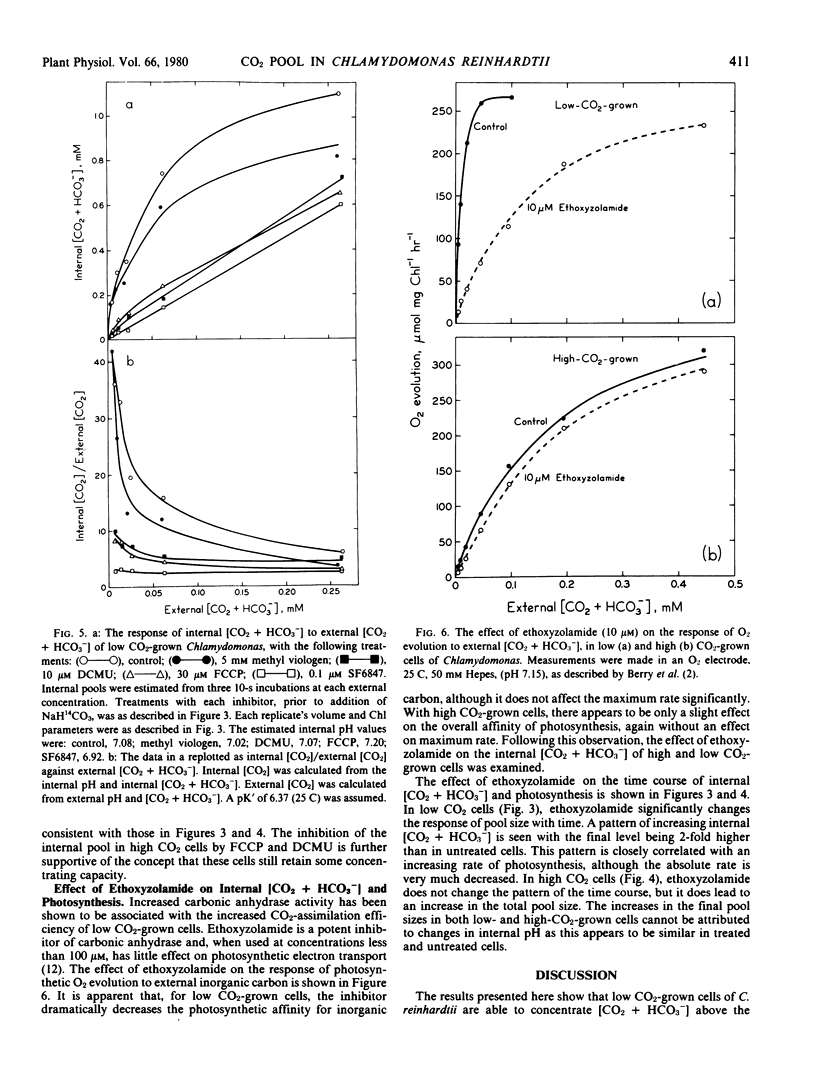
The energy dependence of CO2 accumulation was investigated, using the inhibitors methyl viologen, 3-(3,4-dichlorophenyl)-1,1 dimethylurea, carbonyl cyanide trifluoromethoxyphenylhydrazone, and 3,5-di-tert-butyl-4-hydroxybenzylide nemalononitrile. It appears that the concentrating mechanism in both cell types may be dependent upon an energy supply linked to both phosphorylation in general and photophosphorylation. The treatment of low CO2-grown cells with the carbonic anhydrase inhibitor ethoxyzolamide decreased the apparent photosynthetic affinity for CO2. This was correlated with a decrease in the transport of inorganic carbon into the cells.
The nature of the CO2-concentrating mechanism, particularly with respect to a bicarbonate transport system, is discussed, and its possible occurrence in other algae is assessed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Andrews T. J. Effects of CO2, O2 and temperature on a high-affinity form of ribulose diphosphate carboxylase-oxygenase from spinach. Biochem Biophys Res Commun. 1974 Sep 9;60(1):204–210. doi: 10.1016/0006-291x(74)90192-2. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Filmer D. The active species of "CO2" utilized by ribulose diphosphate carboxylase. J Biol Chem. 1969 Feb 10;244(3):1081–1083. [PubMed] [Google Scholar]
- Graham D., Reed M. L. Carbonic anhydrase and the regulation of photosynthesis. Nat New Biol. 1971 May 19;231(20):81–83. doi: 10.1038/newbio231081a0. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Hess J. L., Tolbert N. E. Glycolate pathway in algae. Plant Physiol. 1967 Mar;42(3):371–379. doi: 10.1104/pp.42.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson B. S., Fong F., Heath R. L. Carbonic anhydrase of spinach: studies on its location, inhibition, and physiological function. Plant Physiol. 1975 Mar;55(3):468–474. doi: 10.1104/pp.55.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd N. D., Canvin D. T., Culver D. A. Photosynthesis and photorespiration in algae. Plant Physiol. 1977 May;59(5):936–940. doi: 10.1104/pp.59.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLBERT N. E., ZILL L. P. Excretion of glycolic acid by algae during photosynthesis. J Biol Chem. 1956 Oct;222(2):895–906. [PubMed] [Google Scholar]
- Terada H. Some biochemical and physiochemical properties of the potent uncoupler SF 6847 (3,5-di-tert-butyl-4-hydroxybenzylidenemalononitrile). Biochim Biophys Acta. 1975 Jun 17;387(3):519–532. doi: 10.1016/0005-2728(75)90090-0. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochim Biophys Acta. 1972 Dec 14;283(3):430–441. doi: 10.1016/0005-2728(72)90260-5. [DOI] [PubMed] [Google Scholar]



