Abstract
KRAS mutations are the most common mutations in non-small cell lung cancer (NSCLC) with adenocarcinoma histology. KRAS mutations result in the activation of the RAF-MEK-ERK pathway, and agents that target RAF-MEK-ERK pathways have been investigated in KRAS mutant NSCLC. The two agents furthest in development are selumetinib and trametinib. Trametinib has greater binding for the MEK1/2 allosteric site, and generally has superior pharmacokinetics. A randomized phase II trial of docetaxel with and without selumetinib revealed that the combination resulted numerically superior overall survival, and a statistically significant improvement in progression-free survival and objective response rate. However, a concerning rate of hospital admission, grade 3 or 4 neutropenia, and febrile neutropenia was observed with the combination. Trials have investigated MEK inhibitors as single agents, and in combination with erlotinib or chemotherapy. The data do not support the further development of single agent MEK inhibitors or in combination with erlotinib. The activity of MEK inhibitors appears to be similar in patients with KRAS mutant and wild-type NSCLC suggesting KRAS mutation status is not a reliable biomarker for efficacy. It is possible that mutations of genes in addition to KRAS mutations impact the activity of MEK inhibitors, or specific subsets of KRAS mutations may be resistant or susceptible to MEK inhibition. Other potential explanations are gene amplifications, alternative RNA splicing of genes resulting in activation of their protein products, and deregulation of noncoding RNAs and consequent altered protein expression.
Keywords: KRAS mutation, targeted therapy, MEK inhibition
Historically non-small cell lung cancer (NSCLC) was considered a single monolithic disease, and all patients with stage IIIB or IV disease who were eligible for chemotherapy received a platinum doublet regardless of histology or tumor biology. Unfortunately this approach resulted in limited progress, and the vast majority of trials of different platinum-based combinations did not result in an improvement in overall survival (OS).1,2 The identification of the epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements, and the development of EGFR tyrosine kinase inhibitors (TKI) and ALK inhibitors changed the diagnostic and therapeutic approach to the treatment of NSCLC.3–5 These agents resulted in substantial improvement in the treatment of patients with NSCLC with these specific molecular alterations. As tumor gene sequencing became more rapid and affordable, the focus of NSCLC research changed to the identification and development of therapies for NSCLC with specific molecular abnormalities.6 While the majority of the research focus in NSCLC has been on the role of mutation or rearrangements, it is important to note other molecular events such as amplification or overexpression may also be oncogenic drivers.
KRAS mutations are the most common mutations in NSCLC, and unfortunately a targeted therapy currently is currently not available for this patient population. KRAS mutations are associated with adenocarcinoma histology and a history of tobacco use. A recent analysis of resected NSCLC showed the rate of KRAS mutations in adenocarcinoma and squamous NSCLC was 34% and 6%, respectively.7 The rate of KRAS mutations among former or current smokers compared to never smokers in a recent meta-analysis was significantly higher (25% vs. 6%, p<0.01).8 Previous clinical trials investigated agents that target the KRAS pathway by either by directly targeting the RAS protein or by inhibiting downstream proteins in the MEK-ERK or the PI3K-AKT-mTOR pathways.9,10 MEK inhibitors are the most promising targeted therapy for patients with advanced KRAS mutant NSCLC to date. However, the activity of MEK inhibitors may not be limited to KRAS mutant NSCLC and may be synergistic with chemotherapy. To date MEK inhibitors have been investigated as single agents, in combinations with chemotherapy and EGFR TKI therapy, and in KRAS mutant and wild-type NSCLC.
Currently all KRAS mutations are considered to be the same biologically and clinically, but the situation may be more complex. Retrospective studies suggest that patients with a KRAS mutation and history of never smoking are more likely to have transition mutations rather than transversion mutation, but the clinical significance of this is unclear.11 Preclinical evidence suggests that the different KRAS mutations may differentially impact downstream signaling pathways of MEK-ERK or PI3k-AKT-mTOR. Mutant KRAS (G12C) preferentially binds the Ral guanine nucleotide dissociation stimulator whereas KRAS (G12D) has a higher affinity for the PI3K pathway.12 Retrospective data from a four-arm trial of targeted therapies (erlotinib, vandetanib, bexarotene and erlotinib, and sorafenib) revealed that patients with KRAS G12C and G12V mutations (n=24) compared to patients with other KRAS mutations (n=19) or KRAS wild-type tumors (n=172) experience a statistically significant shorter progression-free survival (p=0.046).12 Given the multiple therapies investigated and the small number of patients in each cohort these data are hypothesis generating. However, it raises the possibility that the biology of KRAS mutations may differ, and the efficacy of targeted therapies may be related to the specific KRAS mutation.
Many times NSCLC is perceived as having a single oncogenic driver mutation, but concurrent mutations may exist and this may impact the efficacy of targeted therapy.13 Using genetically engineered mouse models to assess the efficacy of selumetinib studies demonstrated the concomitant loss of tumor suppressor genes p53 or Lkb1 reduced the response of KRAS mutant lung cancers to single agent docetaxel.14 Mouse models with KRAS alone and KRAS and p53 mutations revealed significant benefit with the addition selumetinib with docetaxel compared to docetaxel alone; mice with KRAS and Lkb1mutations were resistant to the combination of docetaxel and selumetinib. Thus, the presence of a second mutation may influence the activity of MEK inhibitors.
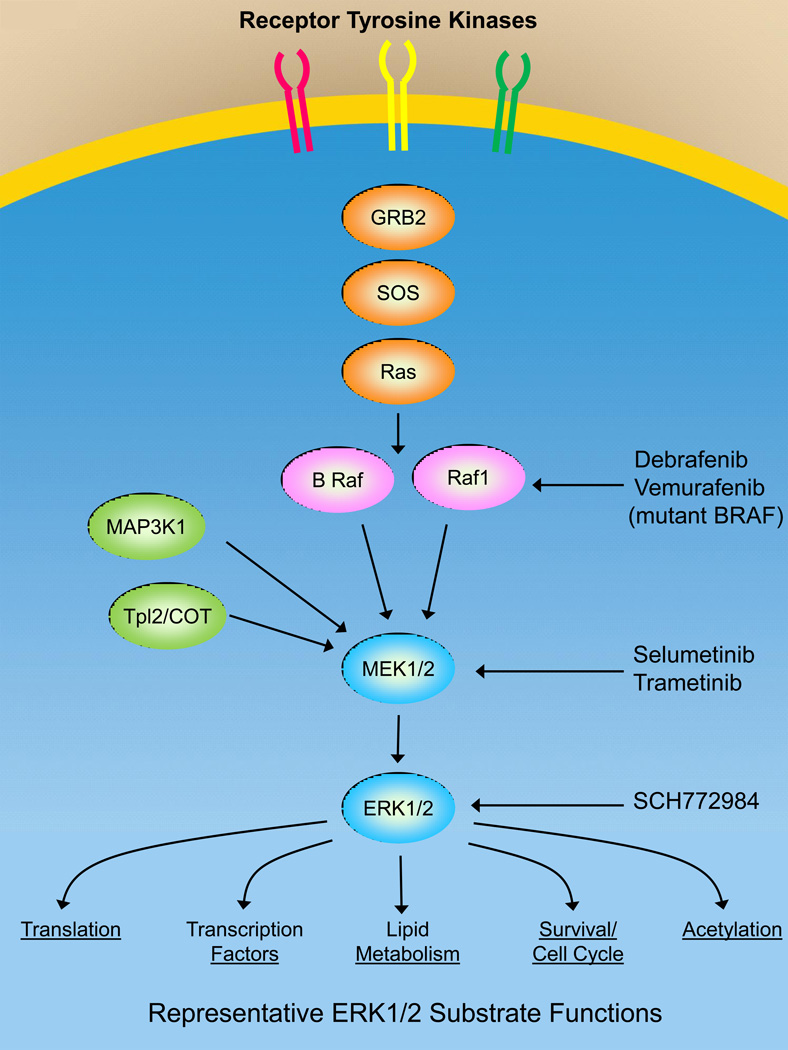
MAPK kinase pathway
Figure 1 shows the three-tiered MAPK pathway that is regulated by RAS (KRAS, HRAS, NRAS) binding to BRAF or RAF1, which are mitogen-activated protein kinases (MAP3Ks). MAP3Ks phosphorylate and activate MAP-ERK kinases 1 and 2 (MEK1/2), both of which phosphorylate and activate extracellular signal-regulated kinases (ERK1 and 2). The importance of the RAF-MEK-ERK pathway is demonstrated by the important functions that ERK1/2 substrates regulate including proliferation, survival, translation, lipid metabolism, transcription and protein acetylation. In addition to RAF proteins there are additional MAP3Ks including MAP3K8 (TPL2/COT) and MAP3K1 (MEKK1) that are able to phosphorylate and activate MEK1/2. MAP3K1 and MAP3K8 are not directly regulated by RAS but are mutated or amplified in different cancers.15 There are two approved RAF inhibitors, vemurafenib and dabrafenib, that are used clinically in cancers with activating BRAF mutations such as malignant melanoma.16,17 BRAF inhibitors are not used to treat tumors with wild-type BRAF because of a paradoxical RAF activation seen with wild-type but not mutant BRAF leading to strong ERK1/2 activation resulting in tumor cell proliferation.18
Figure 1.
The ERK1/2 MAPK signaling network includes a MAP3K including BRAF and RAF1 that are activated by RAS. Additional MAP3Ks can activate the MAPK signaling network including MAP3K8 (TPL2/COT) and MAP3K1 (MEKK1). MAP3Ks phosphorylate and activate MEK1/2 which phosphorylates and activates ERK1/2. ERK1/2 phosphorylate and regulate the activity of many different proteins controlling important cellular functions required for cell survival and proliferation. Dabrafenib and vemurafenib are inhibitors targeting activated BRAF. Selumetinib and trametinib are allosteric inhibitors of MEK1/2. SCH771984 is an ERK1/2 inhibitor in early stage clinical trials.
There are currently two MEK1/2 inhibitors, selumetinib and trametinib, that have been tested in many different cancer types including NSCLC with several more MEK inhibitors at different stages of clinical testing. Trametinib has been approved in combination with dabrafenib for melanoma. ERK1/2 inhibitors such as SCH772984 are also in development and have shown efficacy for BRAF inhibitor resistant melanoma.19 Whereas the BRAF and ERK1/2 inhibitors compete with ATP at the active site of the kinase, MEK1/2 inhibitors such as selumetinib and trametinib bind to an allosteric regulatory site independent of the ATP binding site. Trametinib has a greater affinity than selumetinib for the MEK1/2 allosteric regulatory site and generally has superior pharmacokinetics. A single oral dose of trametinib has a long serum half-life and is able to inhibit ERK1/2 activity in tumors for more than 24 hours.20
MEK1/2 inhibitors have their greatest antitumor effects with tumors harboring RAS or BRAF activating mutations because the RAF-MEK-ERK pathway is generally activated and contributes significantly to tumor cell proliferation and survival. However, there are examples where MEK inhibitors have significant efficacy with tumors that do not have activating RAS or BRAF mutations. For example, in basal-like triple negative breast cancer where activating RAS or BRAF mutations are rare; however, the RAF-MEK-ERK pathway is often activated due to genomic amplification of EGFR, KRAS or BRAF.21 In fact, EGFR, KRAS and BRAF were amplified 22%, 32% and 31%, respectively, in basal-like triple negative breast cancers. This is an excellent example of the importance of understanding the genomic landscape of an individual’s tumor where amplification and not activating mutations are driving a critical signaling pathway in tumor cells. In addition to gene amplification alternative RNA splicing has been shown to result in BRAF activation.22 Deregulation of noncoding RNAs may also alter expression of proteins that could result in the activation of the MAPK kinase pathway resulting in the tumor being vulnerable to MEK inhibition.
MEK inhibitors in combination with chemotherapy
Selumetinib is an oral, selective inhibitor of the MEK1/MEK2 kinases, and preclinical data using KRAS mutant NSCLC xenograft models indicated that selumetinib significantly inhibited tumor growth.23 The most common toxicities observed in the phase I and phase II trials of single agent selumetinib were rash, mild to moderate diarrhea, fatigue and edema.24,25 In the phase I and II single agent trials no episodes of grade 3 or 4 neutropenia were observed. A prospective phase II trial of docetaxel (75 mg/m2 every 3 weeks) with and without selumetinib in patients with KRAS mutant NSCLC who had progressed after first-line chemotherapy was performed.26 The primary end-point was OS, and secondary end-points were PFS and objective response rate (ORR). Patients assigned to the selumetinib and docetaxel (n=44) compared to the docetaxel alone arm (n=43) experienced a statistically non-significant improvement in OS (hazard ratio (HR) of 0.80, 80% confidence interval (CI) 0.56 to 1.14; p=0.21; median 9.4 and 5.2 months, respectively), but a statistically significant improvement in PFS (HR of 0.58, 80% CI, 0.42 to 0.79; p =0.014, median 5.3 and 2.1 months, respectively) and ORR (37% vs. 0%, p<0.0001). The rates of adverse events leading to hospital admission in the selumetinib and docetaxel and the docetaxel alone arms were 48% and 19%, respectively. The rates of febrile neutropenia in the selumetinib and docetaxel and docetaxel alone arms were 18% and 0%, respectively.
A phase III trial of docetaxel (75 mg/m2 every 3 weeks) with selumetinib or placebo in patients with KRAS mutant NSCLC is ongoing (NCT01933932).27 The primary end-point is PFS, and secondary endpoints are OS, ORR and symptom improvement rate and time to symptom progression. Patients in both arms receive prophylactic pegylated granulocyte colony stimulating factor (GCSF). A phase II three arm trial is investigating selumetinib with docetaxel 75 mg/m2 or 60 mg/m2 every 3 weeks and docetaxel alone at 75 mg/m2 every 3 weeks in patients with KRAS wild-type NSCLC; the primary end-point is PFS (NCT01750281).27
Trametinib is an oral, selective inhibitor of MEK1/MEK2 kinases, and in cell line and xenograft models it demonstrated activity in RAS mutant models.28 A phase I trial revealed acceptable toxicity, and the doselimiting toxicities were rash, diarrhea, and central serous retinopathy.29 RAS mutant cell line data suggested synergism between trametinib and docetaxel.30 A phase I/Ib trial investigated the combination of trametinib with docetaxel, and patients with KRAS mutant (Table 1) and KRAS wild-type NSCLC (Table 2) were enrolled.31 The phase I trial design was dose escalation of trametinib and docetaxel, and the second part of the trial was an expansion cohort with the recommended dose for phase II trials in the KRAS mutant and wild-type cohorts. GCSF support was mandatory. The most common non-hematological grade 3 or 4 adverse events observed were diarrhea (11%), fatigue (4%), asthenia (4%), dyspnea (4%), mucosal inflammation (4%), and increased liver tests (4%). The hematologic grade 3 or 4 toxicities observed were anemia (15%), neutropenia (19%), and febrile neutropenia (4%). The ORR (as assessed by the investigator) observed in evaluable patients with KRAS mutant (n=21) and KRAS wild-type (n=19) was 28%, and 32%, respectively. A subset analysis of the patients with KRAS G12C mutation (n=8) revealed a response rate of 40% and a disease control rate of 80%. A similar phase I/Ib trial investigated trametinib in combination with pemetrexed in KRAS mutant and wild-type NSCLC.32 The trial design was similar, but GCSF were not required. The most common grade 3 or 4 non-hematological adverse events observed were asthenia (10%), nausea (7%), dyspnea (7%), diarrhea (5%), and decreased appetite (5%). The grade 3 or 4 hematological events were anemia (14%) and neutropenia (21%). The ORR (as assessed by the investigator) in evaluable patients with KRAS mutant (n=22) and wild-type (n=15) NSCLC was 17% and 15%, respectively. The median PFS in the KRAS mutant and wild-type patients were similar (Tables 1 and 2).
Table 1. Select studies of MEK inhibitors in KRAS mutant NSCLC.
| First author | Treatment | Number of patients | ORR | PFS (median) |
OS (median) |
|---|---|---|---|---|---|
| Janne26 | Docetaxel/selumetinib vs. docetaxel/placebo | 87 | 37% 0% P<0.0001 |
5.3 months 2.1 months HR=0.58, p=0.014 |
9.4 months 5.2 months HR=0.80, p=0.21 |
| Gandara31 | Trametinib/docetaxel | 25 | 28% | Not available | Not available |
| Kelly32 | Trametinib/pemetrexed | 22 | 17% | 4.1 months | Not available |
| Blumenschein35 | Trametinib vs. docetaxel | 129 | 12% 12% |
11.7 weeks 11.4 weeks HR=1.14, p=0.5197 |
Not available Not available HR=0.97, p=0.934 |
| Carter33 | Selumetinib/erlotinib Selumetinib | 30 9 |
10% 0% P=1.0 |
2.3 months 3.8 months P=0.61 |
Not reached 12.8 months P=0.86 |
Abbreviations: ORR: objective response rate; PFS: progression-free survival; OS; overall survival; NSCLC: non-small cell lung cancer
Table 2. Select trials of MEK inhibitors in KRAS wild-type NSCLC.
| First author | Treatment | Number of patients | ORR | PFS (median) |
OS (median) |
|---|---|---|---|---|---|
| Kelly32 | Trametinib/Pemetrexed | 15 | 16% | 5.8 months | Not available |
| Gandara31 | Trametinib/Pemetrexed | 19 | 32% | Not available | Not available |
| Carter33 | Erlotinib Erlotinib/selumetinib | 19 19 |
11% 5% P=1.0 |
2.1 months 2.1 months P=0.98 |
12.9 months 13.7 months P=0.65 |
Abbreviations: ORR: objective response rate; PFS: progression-free survival; OS; overall survival; NSCLC: non-small cell lung cancer
MEK inhibitors in combination with erlotinib
The combination of selumetinib and erlotinib was investigated in two parallel randomized phase II trials.33 Patients with KRAS mutant NSCLC were randomized to selumetinib alone or erlotinib and selumetinib and the primary end-point was ORR; patients with KRAS wild-type NSCLC were randomized to erlotinib alone or erlotinib and selumetinib and the primary end-point was PFS. Among patients with the KRAS mutant the ORR was higher in the combination arm than in the single arm, but the median progression free survival was similar in the two arms. In the KRAS wild-type NSCLC the ORR and the median PFS were similar on the two treatment arms (Table 2). The limited activity of single agent erlotinib in patients with KRAS mutant NSCLC raises concerns about the pairing of MEK inhibitors with EGFR TKI’s.34 At this time the data do not support further investigation of the combination of MEK inhibitors and EGFR TKI therapy.
Single agent MEK inhibitors
Several trials have investigated single agent MEK inhibitors. A randomized phase II trial of single agent selumetinib compared to pemetrexed in patients with advanced NSCLC who had progressed after one or two lines of therapy revealed limited single agent activity of selumetinib (n=84). Patients were not selected based on KRAS status or for non-squamous histology.25 The PFS observed in the selumetinib and pemetrexed arms was similar (HR of 1.08, 80% CI, 0.75 to 1.54; p=0.79), and two patients in each arm experienced a response. More recently single agent trametinib was compared to docetaxel in randomized phase II trial in patients with KRAS mutant NSCLC (n=129). Patients assigned to trametinib compared to docetaxel experienced a similar PFS (HR of 1.14, 95% CI, 0.75–1.75; p=0.5197), and the ORR was the same in both arms (12%).35 The data do not suggest a role for single agent MEK inhibitors in KRAS mutant or unselected patients with advanced NSCLC.
A phase I trial with an expansion cohort investigated the MEK inhibitor RO2987655 (CH4987655) in patients with advanced cancer and RAS-RAF mutations.36,37 The treatment-related adverse events observed were asymptomatic increase in creatinine phosphokinase (17%), rash (16%), diarrhea (8%), folliculitis (7%), and serous retinal detachment (6%). Of the 95 patients enrolled 24 patients had KRAS mutant NSCLC, and 18 patients were evaluable for response. The ORR observed among patients with KRAS mutant NSCLC was 11% (2 of 18 patients) and 44% of patients had stable disease for ≥ 8 weeks (8 patients). Post-treatment biopsies on cycle 1 day 15 of NSCLC tumors revealed a significant down-regulation of phosphorylated ERK expression was observed (p<0.009); however, a significant reduction of Ki-67 expression was not observed.
Conclusions
While the preliminary clinical trials of MEK inhibitors in NSCLC have been focused on patients with KRAS mutant NSCLC the preclinical data and clinical data suggests that the activity of MEK inhibitors is not limited to the KRAS mutant subtype of NSCLC. The data suggest further development of MEK inhibitors should be in combination with chemotherapy rather than in combination with erlotinib or as single agents. Selumetinib is the agent furthest in development, and a randomized phase II trial revealed promising activity but a concerning rate of hospitalization, grade 3 or 4 neutropenia, and febrile neutropenia. The ongoing trials investigating selumetinib and docetaxel include prophylactic GCSF or investigate a reduced dose of docetaxel to reduce the rate of grade 3 or 4 neutropenia and febrile neutropenia. These trials should define the role of selumetinib in the KRAS mutant and wild-type patient populations. Selumetinib is further in development, but trametinib has greater affinity for MEK1/2 allosteric regulatory site, and warrants further investigation. Several other MEK inhibitors are in development and will be investigated in clinical trials as well. While the primary focus of developing MEK inhibitors in NSCLC has been based on KRAS mutation status it is important to recognize that this pathway can be activated by multiple mechanisms. There are also potential clinical differences in the type of KRAS mutations, and it possible that additional mutations (e.g. Lkb1 mutations) or molecular events may influence the activity of MEK inhibitors. The similar activity of MEK inhibitors in KRAS mutant and wild-type NSCLC suggest KRAS mutation status alone is not the optimal predictive biomarker for further development of MEK inhibitors. The development of a predictive biomarker for MEK inhibitors will be critical to further development of this class of agents.
Highlights.
MEK inhibitors have been investigated in NSCLC, predominantly in patients with KRAS mutant NSCLC
The type of KRAS mutations or other mutations may influence the activity of MEK inhibitors.
Mechanisms other than KRAS mutations may activated this the MAPK kinase pathway
MEK inhibitors in combination with chemotherapy have revealed the most promising activity.
KRAS mutation status alone does not appear to be a biomarker of MEK inhibitor efficacy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement
The authors have no conflict of interest to declare, and this manuscript was written without the assistance of medical writer or editor.
References
- 1.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 2.Breathnach O, Freidlin B, Conley B, et al. Twenty-two years of phase III trials for patients with advanced non-small cell lung cancer: sobering results. J Clin Oncol. 2001;19:1734–1742. doi: 10.1200/JCO.2001.19.6.1734. [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALKpositive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 5.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pareek CS, Smoczynski R, Tretyn A. Sequencing technologies and genome sequencing. J Appl Genet. 2011;52:413–435. doi: 10.1007/s13353-011-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shepherd FA, Domerg C, Hainaut P, et al. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol. 2013;31:2173–2181. doi: 10.1200/JCO.2012.48.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao C, Qiu LX, Liao RY, et al. KRAS mutations and resistance to EGFR-TKIs treatment in patients with non-small cell lung cancer: a meta-analysis of 22 studies. Lung Cancer. 2010;69:272–278. doi: 10.1016/j.lungcan.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Riely GJ, Johnson ML, Medina C, et al. A phase II trial of Salirasib in patients with lung adenocarcinomas with KRAS mutations. J Thorac Oncol. 2011;6:1435–1437. doi: 10.1097/JTO.0b013e318223c099. [DOI] [PubMed] [Google Scholar]
- 10.Riely GJ, Brahmer JR, Planchard D, et al. A randomized discontinuation phase II trial of ridaforolimus in non-small cell lung cancer (NSCLC) patients with KRAS mutations. Journal of Clinical Oncology. 2012;2012 abstract 7531, [Google Scholar]
- 11.Kim HR, Ahn JR, Lee JG, et al. The impact of cigarette smoking on the frequency of and qualitative differences in KRAS mutations in Korean patients with lung adenocarcinoma. Yonsei Med J. 2013;54:865–874. doi: 10.3349/ymj.2013.54.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ihle NT, Byers LA, Kim ES, et al. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst. 2012;104:228–239. doi: 10.1093/jnci/djr523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Cheng K, Walton Z, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483:613–617. doi: 10.1038/nature10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Cancer Genome Atlas Data Portal. [acccessed 7/9/2014]; https://tcga-data.nci.nih.gov/tcga/
- 16.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 18.Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 19.Morris EJ, Jha S, Restaino CR, et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov. 2013;3:742–750. doi: 10.1158/2159-8290.CD-13-0070. [DOI] [PubMed] [Google Scholar]
- 20.Gilmartin AG, Bleam MR, Groy A, et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res. 2011;17:989–1000. doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas N: Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies BR, Logie A, McKay JS, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6:2209–2219. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 24.Adjei AA, Cohen RB, Franklin W, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–2146. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hainsworth JD, Cebotaru CL, Kanarev V, et al. A phase II, open-label, randomized study to assess the efficacy and safety of AZD6244 (ARRY-142886) versus pemetrexed in patients with non-small cell lung cancer who have failed one or two prior chemotherapeutic regimens. J Thorac Oncol. 2010;5:1630–1636. doi: 10.1097/JTO.0b013e3181e8b3a3. [DOI] [PubMed] [Google Scholar]
- 26.Janne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 27. [2/18/2014]; www.clinicaltrials.gov-accessed.
- 28.Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:773–781. doi: 10.1016/S1470-2045(12)70270-X. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Hong S, Zhang V, et al. Identification of molecular determinants of response to GSK1120212B, a potent and selective MEK inhibitor, as a single agent and in combination in RAS/RAF mutant non-small cell lung carcinoma cells. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research; 2011 Apr 2–6; Orlando, Florida. Philadelphia (PA): AACR; 2011. abstract 4394, [Google Scholar]
- 31.Gandara DR, Hiret S, Blumenschein GR, et al. Oral MEK1/MEK2 inhibitor trametinib (GSK1120212) in combination with docetaxel in KRAS-mutant and wild-type (WT) advanced non-small cell lung cancer (NSCLC): A phase I/Ib trial. Journal of Clinical Oncology. 2013;31 abstract 8028, [Google Scholar]
- 32.Kelly K, Mazieres J, Leighl NB, et al. Oral MEK1/MEK2 inhibitor trametinib (GSK1120212) in combination with pemetrexed for KRAS-mutant and wild-type (WT) advanced non-small cell lung cancer (NSCLC): A phase I/Ib trial. Journal of Clinical Oncology. 2013;31 abstract 8027, [Google Scholar]
- 33.Carter CA, Rajan A, Szabo E, et al. Two parallel randomized phase II studies of selumetinib (S) and erlotinib (E) in advanced non-small cell lung cancer selected by KRAS mutations. Journal of Clinical Oncology. 2013;31 abstract 8026, [Google Scholar]
- 34.Roberts PJ, Stinchcombe TE. KRAS mutation: should we test for it, does it matter? J Clin Oncol. 2013;31:1112–1121. doi: 10.1200/JCO.2012.43.0454. [DOI] [PubMed] [Google Scholar]
- 35.Blumenschein GR, Smit EF, Planchard D, et al. MEK114653: A randomized, multicenter, phase II study to assess efficacy and safety of trametinib (T) compared with docetaxel (D) in KRAS-mutant advanced non–small cell lung cancer (NSCLC) Journal of Clinical Oncology. 2013;31 abstract 8029, [Google Scholar]
- 36.Leijen S, Middleton MR, Tresca P, et al. Phase I dose-escalation study of the safety, pharmacokinetics, and pharmacodynamics of the MEK inhibitor RO4987655 (CH4987655) in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4794–4805. doi: 10.1158/1078-0432.CCR-12-0868. [DOI] [PubMed] [Google Scholar]
- 37.Zimmer L, Barlesi F, Martinez-Garcia M, et al. Phase I expansion and pharmacodynamic study of the oral MEK inhibitor RO4987655 (CH4987655) in selected advanced cancer patients with RAS-RAF mutations. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-0341. [DOI] [PubMed] [Google Scholar]