Abstract
Not all cells behave uniformly after treatment in tissue engineering studies. In fact, some treated cells display no signs of treatment or show unique characteristics not consistent with other treated cells. What if the “unique” cells could be isolated from a treated population, and further studied? Photo-convertible reporter proteins, such as Dendra2, allow for the ability to selectively identify unique cells with a secondary label within a primary labeled treated population. In the current study, select cells were identified and labeled through photo-conversion of Dendra2-transfected human Wharton's Jelly cells (hWJCs) for the first time. Robust photo-conversion of green-to-red fluorescence was achieved consistently in arbitrarily selected cells, allowing for precise cell identification of select hWJCs. The current study demonstrates a method that offers investigators the opportunity to selectively label and identify unique cells within a treated population for further study or isolation from the treatment population. Photo-convertible reporter proteins, such as Dendra2, offer the ability over non-photo-convertible reporter proteins, such as green fluorescent protein, to analyze unique individual cells within a treated population, which allows investigators to gain more meaningful information on how a treatment affects all cells within a target population.
Keywords: Human Wharton's jelly cells, Dendra2, Photo-convertible reporter gene, Nucleofection, Cell tracking
Introduction
Fluorescent proteins are often used as reporters for a variety of different biological and chemical applications, including protein tagging, cell structure identification, and cell tracking.3, 12, 14 Green-to-red photo-convertible proteins such as Kaede, KikGR, EosFP, and Dendra2 are incredibly useful for observing cell trafficking and cell tracking, and have been well characterized.23 However, these photo-convertible proteins have been highly under-utilized in tissue engineering. Photo-convertible proteins allow for the potential to create highly specific biochemical assays, and enable for sub-labeling of treated populations. Single fluorescent reporters such as green fluorescent protein (GFP), yellow fluorescent protein (YFP), and red fluorescent protein (RFG) have been popular to label treated cell populations. However, if a sub-population or individual cells within a treated population show unique characteristics different from the treated population, there is no convenient method for isolating or tracking unique cells without adding an additional label, that may affect the already treated cell population. Photo-convertible reporter proteins, such as Dendra2, offer the utility of a secondary label. Photo-convertible proteins are attractive because the reporter protein can be selectively converted from green to red, providing a secondary label that distinguishes unique cells from primary labeled treated cells (Fig. 1). Thus, identification of unique cells within a treated cell population can be isolated via fluorescent activated cell sorting (FACS) for further analysis or unique cells can be observed and temporarily tracked within a treated population of cells. Photo-conversion of primary labeled treated cells provides investigators with a tool to gain more insight into how a specific treatment affects different cells within a target population.18
Fig. 1. Schematic diagram of hWJC transfection and photo-conversion.
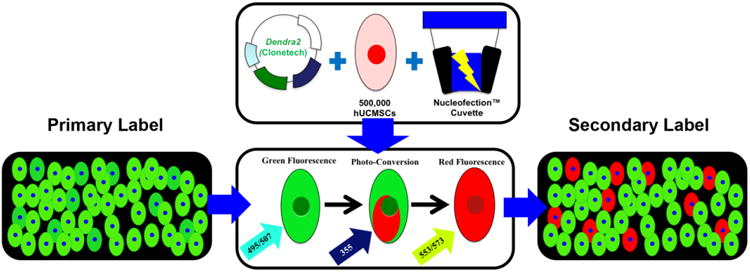
Photo-convertible reporter genes such as Dendra2 enable cells fluoresce green as a primary label for treatment. Dendra2 enables cells that exhibit a unique characteristic or behavior to be photo-converted with blue or UV light to exhibit a secondary label that distinguishes unique cells from all other cells within a treated population for observation. hWJCs were transfected via the electroporative method, Nucleofection™, with pDendra2. Cells were transfected at a density of 5×105 cells per cuvette. After transfection, cells were imaged for green fluorescence, then converted to red fluorescence by exposure to UV light, and imaged for red fluorescence.
In addition, photo-conversion of reporter genes may provide significant insights into the turnover of proteins within an individual cell, protein kinetics and distribution within a cell, and the structural and morphological changes occurring in the nucleus or mitochondria as cells undergo mitosis and differentiation. Gunewardene et al.15 and Wu et al.28 demonstrated that photo-convertible reporter genes can be used to accurately track protein distribution and kinetics in mouse fibroblasts and multiple cell types in Arabidopsis root, respectively. The potential to use photo-convertible proteins in tissue engineering may become a powerful tool to provide fundamental information about proteins and cells while enabling the identification of positively transfected cells in a large population.
Dendra2 has an advantage over other photo-convertible reporter genes in that Dendra2 can be photo-converted with low intensity ultra-violet (UV) (360 – 420 nm) light or high intensity blue light (460 – 500 nm), as opposed to only UV light at varying powers, which makes Dendra2 more versatile in tissue engineering applications, as exposure of cells to UV light can be avoided or drastically reduced.7, 13, 24, 28
Human Wharton's jelly cells (hWJCs) are mesenchymal stromal cells that are an alternative to bone marrow stem cells. hWJCs are an excellent source for tissue engineering applications because they are abundant in supply, cause no donor site morbidity, are highly proliferative, and not ethically controversial.2, 9, 22, 26 However, hWJCs are primary cells, and typically are difficult gene delivery targets unless a viral vector is used. Viral vectors are highly effective at transducing primary cells, stem cells, and progenitor cells, but safety concerns regarding toxicity, immunogenesis, and oncogenesis from insertional mutagenesis still remain.5, 25 Non-viral vectors are able to circumvent many safety concerns associated with viral gene delivery; however, non-viral vectors suffer from low transfection efficiencies, which can make identification of positively transfected cells challenging.21 Nucleofection™ is an electroporative method that has demonstrated a reliable ability to transfect primary cells, stem cells, and progenitor cells non-virally.1, 11, 17 Hence, it was hypothesized that a photo-convertible reporter gene transfected into hWJCs would reliably allow for selection and identification of unique hWJCs through green-to-red photo-conversion enabling the ability to track unique hWJCs over a short period time.
The current study provides a demonstration of how hWJCs transfected with Dendra2 via Nucleofection may be selectively photo-converted to fluoresce red and identified from other green fluorescing hWJCs, which could be highly useful for tissue engineers to gain insight on cells that behave in a unique or unexpected manner in in vitro tissue engineering studies. The current study examined transfection efficiency, photo-conversion kinetics of Dendra2, and the ability to reliably identify select cells within a transfected population of hWJCs.
Materials and Methods
Procurement and expansion of hWJCs
hWJCs were isolated from Wharton's jelly of three human umbilical cords (n = 3) obtained from Lawrence Memorial Hospital (LMH) (LMH IRB approval #LMH 08-2). The three umbilical cords came from males born at full term under normal conditions. hWJCs were isolated according to the previously published protocol.10 hWJCs were cultured in traditional hWJC medium (10% fetal bovine serum (FBS-MSC Qualified) and 1% Penicillin-Streptomycin in low glucose DMEM (Life Technologies, Grand Island, NY)). hWJC medium was changed three times per week, and hWJCs were maintained at 37°C with 5% CO2 in a cell culture grade incubator. hWJCs were trypsinized with 0.05% Trypsin-EDTA (1×) (Life Technologies) at 80 to 90% confluency. All hWJCs were expanded to passage 1 (P1), and flash-frozen until needed for experiments. Cells were thawed and expanded to P4 for all experiments. Three umbilical cords (n = 3) were used in total for the current study. All experiments were performed in triplicate for each cord, unless otherwise noted.
Cell surface marker characterization
At P2, a sub-culture of cells from each cord was characterized through cell surface marker identification via flow cytometry on a MoFlo XDF fluorescent activated cell sorter (FACS) (Beckman Coulter, Brea, CA). hWJCs were characterized using the following antibodies and secondary antibodies: STRO-1 Mouse IgM (2.5:200) (1 mg per mL; R&D Systems, Minneapolis, MN); Alexa Fluor 568® Rabbit Anti-Mouse IgG (2:200) (2 mg per mL; Life Technologies); CD105 Mouse IgG (2.5:200) (1 mg per mL; R&D Systems); Qdot® 525 donkey anti-mouse IgG (2:200) (1 μM; Life Technologies); Human CD45 pre-conjugated to Qdot® 800 (2:200) (Life Technologies); Human CD73 pre-conjugated to FITC (5:200) (BD Biosciences, San Jose, CA); Human CD34 pre-conjugated to Brilliant Violet (5:200) (BD Biosciences); Human CD90 pre-conjugated to APC (5:200) (BD Biosciences). 20,000 events were recorded for each sample. Positive identification of cell markers was defined as fluorescent emission that exceeded the fluorescent threshold of cells stained with corresponding isotype (negative) controls. The isotype controls used in these studies were Rabbit IgG Alexa Fluor 568, Donkey IgG Qdot 525, IgG2 Qdot 800 (all from Life Technologies), and IgG1 FITC, IgG1 Brilliant Violet, and IgG1 APC (all from BD Biosciences). The cell characterization experiments were repeated three times for each cord.
Plasmid
pDendra2-C (Clontech, Mountain View, CA) is a fluorescent fusion protein vector, in which the gene of interest can be fused to the c-terminal of the Dendra2 photo-convertible reporter gene, upstream of a poly-A sequence. Dendra2 is driven by a cytomegalovirus (CMV) promoter, and contains a Kanamycin resistant gene driven by Simian virus 40 (SV40) for bacterial selection. The entire empty vector backbone is 4.7 kb.
Transfection
On the day of transfection, medium from all wells was removed, and cells were washed with phosphate buffered saline (PBS) twice. Afterward, cells were incubated for 1 h at 37°C in traditional hWJC medium with 10 μM of Y-27632-ROCK Inhibitor (Reagents Direct, Encinitas, CA). After 1 h, hWJCs were washed twice with PBS, trypsinized, and then resuspended in either 100 μL 4D Nucleofector™ P1 Primary Solution (P1PS) (Lonza, Basel, Switzerland) (Untreated Control) or 95 μL of P1PS and 5 μL of pDendra2 (1 μg per μL; Clontech). Cells were resuspended at a density of 5×105 cells per 100 μL. Untreated control cells were immediately transferred to 6-well plates (BD Biosciences) containing 1.5 mL traditional hWJC medium and incubated at 37°C. hWJC suspensions containing pDendra2 were immediately transferred to 100-μL 4D Nucleofection™ cuvettes. Each cuvette was gently tapped twice then placed in a 4D Nucleofector™ (Lonza) and nucleofected with the program FF-104. hWJCs were incubated at room temperature (ca. 22°C) for 10 minutes then transferred to a 6-well plate (BD Biosciences) containing 1.5 mL traditional hWJC medium with 10 μM of Y-27632-RI (Reagents Direct) and incubated at 37 °C.
Fluorescent microscopy
Twenty-four hours after transfection, hWJCs were collected for analysis. hWJCs were imaged using a customized Olympus IX81 inverted (Olympus America, Center Valley, PA) microscope, outfitted for both epifluorescence (filters: Semrock, Inc, Rochester, NY, filter wheels: Sutter Instrument, Novato, CA) and Spinning Disk Confocal (Yokogawa, Toyko, Japan) microscopy. The microscope is equipped with a XY stage (Prior, Rockland, MA) and temperature, humidity, and CO2 control for chronic, automated live cell imaging. Images were collected using an Olympus LUCPlanFL 20× 0.4 NA objective and were captured using the acquisition and analysis software, SlideBook (Intelligent imaging Innovations (3i), Denver, CO).
Three experiments were performed in total 24 h after transfection. In the first experiment, all cells within the field of view were exposed to a 100 mW (mercury arc lamp) of UV light (387 ± 11 nm) for photo-conversion, blue light (494 ± 20 nm) for green fluorescent expression, and green light (560 ± 25 nm) for red fluorescent expression. Cells were exposed to UV light at a frequency of 1 Hz every 10 seconds. The experiment was repeated nine times with three sets of cells from 3 different umbilical cords (n = 3).
In the second experiment, the UV light was confined to a diameter of 15 – 20 μm to limit UV exposure to one or two transfected cells. The UV light output was reduced to 20 mW (mercury arc lamp), and exposure was set to 1 Hz every 20 s. To minimize photo-conversion from blue light, excitation of green and red fluorescent expression were done using 488-nm and 561-nm solid-state lasers, respectively, through a Yokogawa Spinning Disk. The experiment was repeated nine times with three sets of cells from three different umbilical cords (n = 3).
In the third experiment, a single hWJC within a population of positively transfected cells was photo-converted by exposure to UV light at 100 mW (mercury arc lamp) for a duration of 10 s at a frequency of 1 Hz. After photo-conversion, cells within the entire field of view were exposed to 488-nm and 561-nm solid-state lasers for green and red fluorescent expression respectively every 30 min at a frequency of 1 Hz over the time period of 48 h.
To determine how long Dendra2 expression (both green and red fluorescence) persisted, a fourth experiment was conducted where select hWJCs within a population of positively transfected cells were photo-converted by exposure to UV light at 100 mW (mercury arc lamp) for a duration of 10 s at a frequency of 1 Hz. After photo-conversion, cells within the entire field of view were exposed to 488-nm and 561-nm solid-state lasers for green and red fluorescent expression respectively every 24 h at a frequency of 1 Hz over the time period of 10 d.
Results
Cell surface marker characterization
hWJCs from three different umbilical cords (n = 3) were analyzed for cell surface markers found on mesenchymal stem cells via flow cytometry (Table 1). hWJCs from all three cords were non-hematopoietic with cells showing little to no presentation of CD34 (97.2 ± 1.6% negative) (all values are reported as means ± standard deviations), and little to no presentation of CD45 (81 ± 13% negative). Cells from each umbilical cord displayed varying degrees of presentation of CD73 (12.8 ± 5.3% positive), CD90 (97.1 ± 4.3% positive), CD105 (21.6 ± 9.7% positive), and STRO-1 (6.2 ± 3.8% positive), which are all found on mesenchymal stem cells.
Table 1. Cell Characterization of Stem Cell Markers at Passage 2.
| Umbilical Cord | Sample | Events | Gated Cells | CD34 (-) | CD45 (-) | CD73 (+) | CD90 (+) | CD105 (+) | STRO-1 (+) |
|---|---|---|---|---|---|---|---|---|---|
| A | 1 | 20,000 | 19,555 | 97.70% | 75.13% | 16.68% | 90.52% | 19.71% | 6.67% |
| 2 | 20,000 | 455 | 97.58% | 67.47% | 18.90% | 88.57% | 19.12% | 4.40% | |
| 3 | 20,000 | 6,870 | 97.15% | 60.45% | 20.87% | 99.14% | 19.33% | 5.97% | |
|
| |||||||||
| Mean | 9,000 ± 9700 | 97.48 ± 0.29% | 67.7 ± 7.3% | 18.8 ± 2.1 | 92.7 ± 5.6 | 19.39 ± 0.30% | 5.7 ± 1.2% | ||
|
| |||||||||
| B | 1 | 20,000 | 19,717 | 94.86% | 70.76% | 15.24% | 98.29% | 33.89% | 10.90% |
| 2 | 20,000 | 19,783 | 95.69% | 88.34% | 10.21% | 99.29% | 30.13% | 9.63% | |
| 3 | 20,000 | 19,783 | 95.17% | 89.00% | 9.15% | 99.82% | 36.56% | 10.83% | |
|
| |||||||||
| Mean | 19,761 ± 38 | 95.24 ± 0.42% | 83 ± 10% | 11.5 ± 3.4% | 99.13 ± 0.78% | 33.5 ± 3.2% | 10.45 ± 0.71% | ||
|
| |||||||||
| C | 1 | 20,000 | 19,815 | 98.88% | 87.61% | 10.10% | 98.79% | 10.10% | 2.29% |
| 2 | 20,000 | 19,853 | 98.76% | 94.66% | 6.65% | 99.80% | 11.78% | 2.60% | |
| 3 | 20,000 | 19,871 | 99.11% | 96.40% | 7.08% | 99.74% | 13.91% | 2.37% | |
|
| |||||||||
| Mean | 19,846 ± 29% | 98.92 ± 0.18% | 92.9 ± 4.7% | 7.9 ± 1.9% | 99.44 ± 0.57% | 11.9 ± 1.9% | 2.42 ± 0.16% | ||
Transfection efficiency and cell density
hWJCs were transfected from three different umbilical cords (n = 3) with 5 μg of pDendra2 per 500,000 cells, and exhibited a transfection efficiency of 31 ± 7.4% with a cell density of 3,200 ± 1,900 per randomized field of view.
Fluorescence Microscopy
Multiple cell photo-conversion
hWJCs transfected with pDendra2 showed reliable and consistent photo-conversion of all cells within the field of view. The kinetics of photo-conversion were measured in randomized fields of view from hWJCs from three different umbilical cords (n = 3), as cells were exposed to UV light (384 nm) for a duration of 300 s (Fig. 2). The intensity of the green fluorescence dropped dramatically from 5,300 ± 1900 Arbitrary Units (AU) to under 1,200 ± 360 AU in 300 s, while the intensity of the red fluorescence started at 750 ± 130 AU and increased to 4,200 ± 2,400 AU in 300 s. The critical point at which the intensity of red fluorescence overtook green fluorescence occurred at 125 ± 60 s. There was a marked visual change in the overall fluorescent color of the hWJCs that underwent photo-conversion, which is displayed in a time-lapse video from one recording that is representative of all attempts (Supplementary Fig. 1). The experiment was repeated nine times, with three attempts from a different set of cells from each umbilical cord. Each experiment yielded reproducible results.
Fig. 2. Full photo-conversion of hWJCs.
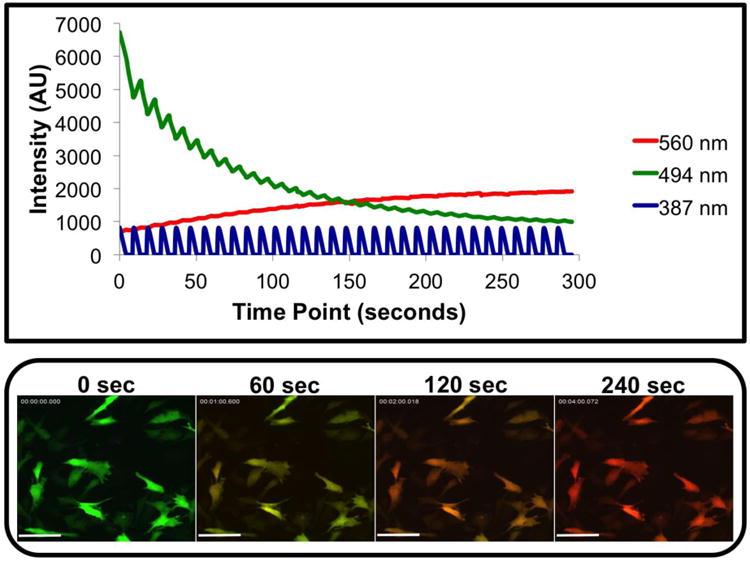
Photo-conversion of hWJCs from green fluorescence to red fluorescence was very robust. hWJCs were gently exposed to UV light at 100 mW every 10 s at a frequency of 1 Hz for 300 s. Green fluorescence intensity and red fluorescence intensity were measured in real-time as hWJCs were photo-converted. There was a rapid decrease in green fluorescence intensity as red fluorescence intensity increased. The critical moment where red fluorescence eclipsed green fluorescence occurred at 125 ± 60 s. Please refer to Supplementary Figure 1 to view the full time-lapse video of a single recording of the photo-conversion that is representative of all attempts. The results displayed were fully consistent with nine attempts to photo-convert cells from three different umbilical cords. AU = Arbitrary Unit; Scale Bar = 100 μm
Selected cell photo-conversion
Selected hWJCs were photo-converted from green to red fluorescence while minimizing photo-conversion of surrounding hWJCs. Photo-conversion of individual cells was accomplished by narrowing the UV beam to a diameter of 15 – 20 μm (Fig. 3). Targeted cells were photo-converted completely with minimal photo-conversion of surrounding cells in the measured time period (Fig. 4). The procedure was repeated nine times with three different sets of cells used from each of three different umbilical cords (n =3). In each procedure, one of the cells targeted for photo-conversion was labeled “Target 1” while the adjacent (non-targeted) cell was labeled “Target 2”. The green fluorescent intensity of Target 1 started out at 2,655 ± 700 AU and decreased to 900 ± 260 AU over 720 s, whereas the red fluorescent intensity started at 480.5 ± 4.9 AU and increased to 1,470 ± 290 AU over 720 s. The critical point at which red fluorescent intensity overtook green fluorescent intensity occurred at 197.0 ± 4.2 s. The green fluorescent intensity in Target 2 started at 3,700 ± 2,800 AU and was recorded at 2,800 ± 1,700 AU at the end of 720 s, whereas the red fluorescent intensity of Target 2 started at 480 ± 7.8 AU and was recorded at 1,200 ± 750 AU at the end of 720 s. The starting and ending green and red fluorescent intensities varied between each set of cells; however, no photo-conversion occurred in cells not directly targeted for photo-conversion within the recorded time. Each experiment yielded reproducible results. A time-lapse video from one recording that is representative of all attempts clearly shows Target 1 photo-converts from green to red, while Target 2 and all surrounding cells remain green (Supplementary Fig. 2).
Figure 3. Photo-conversion of single cells.
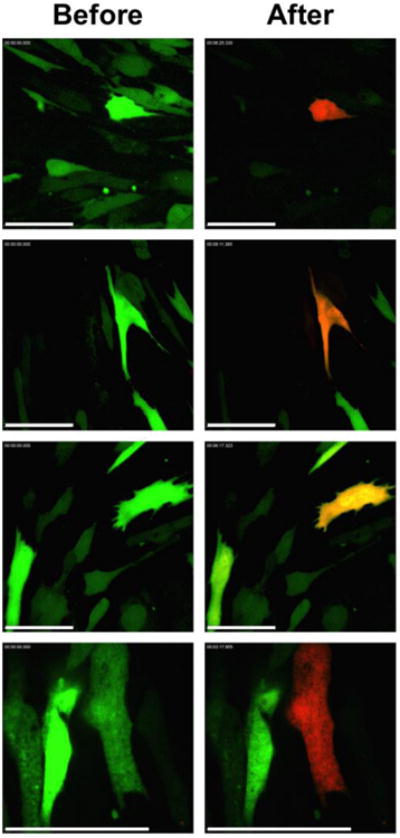
Individual hWJCs were targeted for photo-conversion. The left column displays the fluorescence of cells before photo-conversion. The right column displays cells after photo-conversion. The amount of Dendra2 produced in each cell varied, thus some targeted cells displayed a strong expression of red fluorescence while other cells display a weak expression of red fluorescence after photo-conversion. The images displayed are a random collection of images taken from three different (n = 3) different populations of hWJCs transfected with Dendra2. Scale Bar = 100 μm.
Fig. 4. Kinetics of targeted and non-targeted cells for photo-conversion.
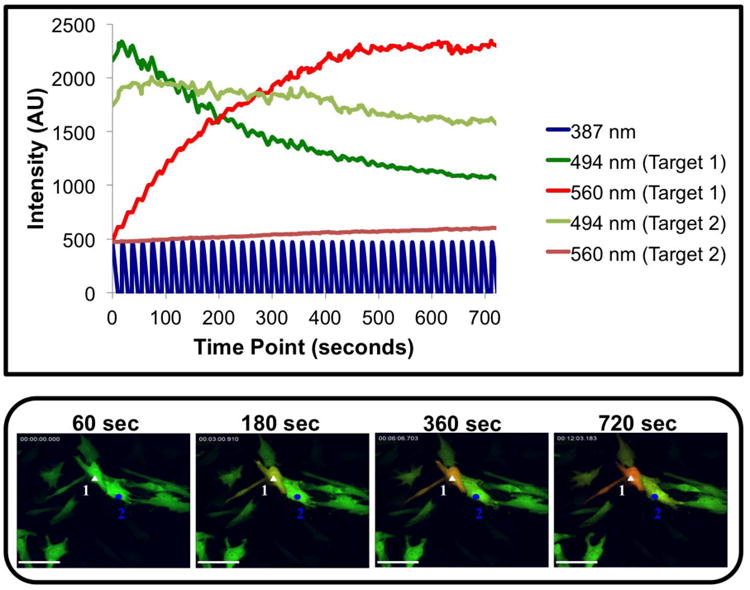
The UV light beam was confined to a 15 – 20 μm diameter to restrict UV light exposure to a selection of one to two hWJCs. Changes in fluorescent intensity for both green and red fluorescence were measured in two adjacent cells over the course of 720 s. The cell designated “Target 1” is labeled with a white triangle and was exposed directly to the UV light beam, whereas the cell designated “Target 2” is labeled with a blue circle. Target 1 was exposed to UV light at 20 mW every 20 seconds at a frequency of 1 Hz. Over the course of 720 s, the green fluorescence rapidly decreased while the red fluorescence steadily increased in Target 1. Green fluorescence decreased slightly, and red fluorescence increased slightly in Target 2, but photo conversion did not occur within the measured time period, demonstrating that select cells can be targeted in culture. Please refer to Supplementary Figure 2 to view the full time-lapse video a single recording representative of all attempts to demonstrate isolated photo-conversion. The results displayed were fully consistent with nine attempts to photo-convert individual cells from three different umbilical cords. AU = Arbitrary Unit; Scale Bar = 100 μm.
Tracking cell movement of photo-converted cells
A diagram of how cell tracking could work is illustrated in Figure 5. Two hWJCs were photo-converted within a population of hWJCs positively transfected with pDendra2. The two photo-converted cells were tracked over 48 h and monitored to observe whether long-term exposure of a photo-converted cell induced photo-conversion of surrounding cells. The experimental procedure was repeated twice with two different sets of hWJCs (n = 2), yielding reproducible results. The photo-converted hWJCs displayed a red fluorescence that changed to an orange fluorescence over 48 h in both experiments; while surrounding cells remained firmly green (Fig. 6). In both experiments the photo-converted cell moved in a circular pattern, and touched several cells over the duration of 48 h. At the end of 48 h, the photo-converted cell from one experiment appeared to be undergoing cell division, and is represented in Figure 6. None of the cells that came in contact with the photo-converted cell in each experiment showed any visual signs of photo-conversion. The entire time-lapse video shows the movement of the photo-converted cell and is representative of both experiments (Supplementary Fig. 3).
Fig. 5. Schematic diagram of cell tracking in tissue culture.
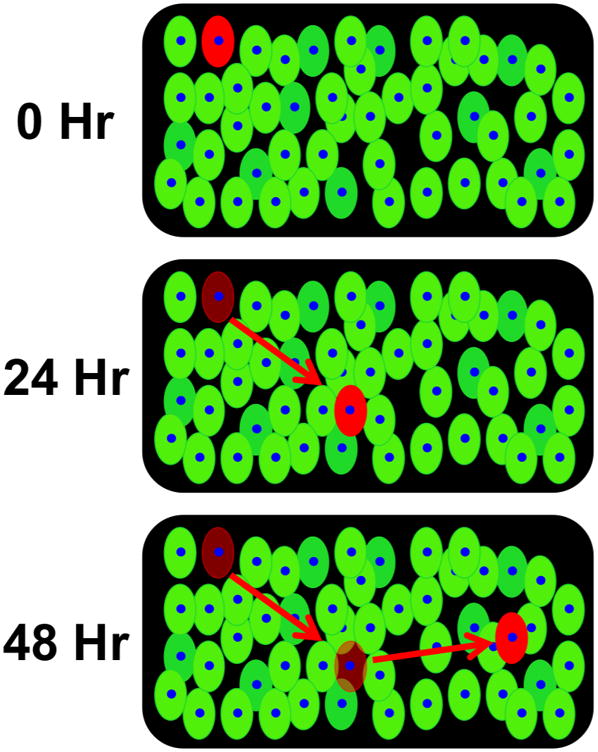
The diagram illustrates how cell tracking could work in a tissue culture if a single transfected cell is photo-converted. The photo-converted cell would appear red against cells that are green, which would allow for precise tracking of cell movement and observation of morphological changes in tissue culture.
Fig. 6. Cell tracking of a single photo-converted hWJCs over the course of 36 h.
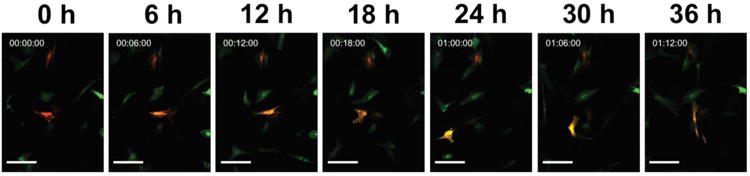
Two hWJCs were photo-converted with UV light at an intensity of 100 mW for 10 s at a frequency of 1 Hz. The single cell was tracked for a total of 48 h. The panels show the movement and changes in the photo-converted cell every 6 h for 36 h. Please refer to Supplementary Figure 3 to view the full 48 h time-lapse video of the movement of the two photo-converted hWJCs. The experiment was run twice, showing the same results both times. Scale Bar = 100 μm
Duration of Dendra2 expression
Select hWJCs were photo-converted within a population of positively transfected cells to monitor the duration of both green and red fluorescence simultaneously. Cells were monitored over the duration of 10 d. The experimental procedure was repeated twice for three different sets of hWJCs (n = 3). Positively transfected hWJCs expressed green fluorescence 7 d after transfection in all tested samples; however, green fluorescent expression persisted until day 9 in one set of transfected hWJCs. The intensity of green fluorescence peaked 2 d after transfection, and continuously diminished over the following days until completely disappearing after 7 d post transfection. Red fluorescent expression was detectable up to 3 d after photo-conversion in cells from all three umbilical cords as is illustrated in Figure 7. After red fluorescence faded, photo-conversion was re-attempted, but cells did not express any further red fluorescence or green fluorescence.
Fig. 7. Tracking the expression of Dendra2 over 7 d.
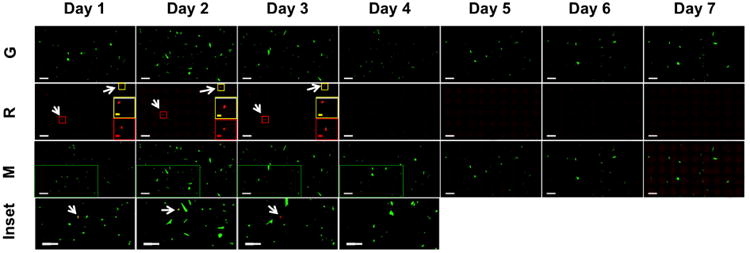
Select hWJCs were photo-converted with UV light at an intensity of 100 mW for 10 s at a frequency of 1 Hz within a population of positively transfected cells. Green fluorescence was monitored for 10 d; however, green fluorescence and red fluorescence were not detectable beyond 7 d after transfection. Images are composite image montages composed of 45 neighboring fields of view stitched together into a 5 × 9 image. The top row of images shows only the green fluorescence channel. The second row down shows only the red fluorescence channel. A red inset box and yellow inset box display an enlarged image of the photo-converted hWJC. The third row down displays composite images where the green fluorescent and red fluorescent channels have been merged. Green inset boxes are displayed in images corresponding to days 1 – 4, and are enlarged in the fourth row down. White arrows indicate the location of hWJCs that were photo-converted and express red fluorescence. No red fluorescence was detected in days 4 – 7 from cells that were photo-converted 1 d after transfection. The experiment was run twice with cells from three different umbilical cords (n = 3). The images are representative of all samples run. G = Green fluorescence channel. R = Red fluorescence channel. M = Merged green and red fluorescence channel. Inset corresponds to the green inset box in the “M” row. White Scale Bar = 400 μm. Yellow Scale Bar = 200 μm. Red Scale Bar = 200 μm.
Discussion
Dendra2 is a monomeric photo-convertible protein that was engineered by Gurskaya et al.16 Dendra2 is highly photo-stable, and matures at 37°C, which makes Dendra2 an attractive candidate for labeling proteins or structures within cells. Chudakov et al.6, 8 have conducted extensive research on using photo-convertible proteins, such as Dendra2 for studying and tracking protein movement in cells. Kaede is a popular rival photo-convertible protein used in many experiments to track cells; however, Dendra2 has rarely been utilized for labeling cells. Dendra2 has been extensively used for molecule identification and tracking in cells, and offers the advantage of being able to be photo-converted with blue light instead of UV, and can be precisely photo-converted.19, 20, 27 Thus, the objective of the current study was to demonstrate the reliability of selectively labeling positively transfected green fluorescent hWJCs using Dendra2 via photo-conversion of green fluorescence to red fluorescence.
For the first time, it was demonstrated that hWJCs responded well to photo-conversion when exposed to UV light (384 nm) at 100 mW output every 10 s for 1 Hz. hWJCs displayed a robust gene expression of Dendra2 after nucleofection, and healthy proliferation, suggesting that the transfection efficiency of 31% is not a limitation for tissue engineering studies where transfected cells need to divide to regenerate tissue. The lack of presentation of hematopoietic markers and the varying presentation CD73, CD90, CD105, and STRO-1 suggest that subpopulations may exist within the isolated hWJC population that display surface markers consistent with mesenchymal stem cell markers as reviewed by Bongso et al.4
Select hWJCs were photo-converted successfully multiple times while minimizing photo-conversion of surrounding cells when exposed to UV light for an isolated period of time, which is consistent with studies that have precisely tracked intercellular proteins within much smaller spatial areas than cells.6, 20, 23 However, the ability to close an aperture and narrow the diameter of the beam of light exposed to a field of view is a key limitation in targeting individual cells for photo-conversion. The ability to selectively photo-convert individual cells transfected with Dendra2 is contingent on the ability to narrow the UV light beam onto a single cell. It should be noted that all cells will eventually photo-convert from green to red fluorescence as a result of scattering light if the well plate is continuously exposed to the pulsing UV light. Tracking of photo-converted cells in tissue culture revealed that photo-converted cells did not trigger photo-conversion events in cells that came in contact with the photo-converted cell. Interestingly, the photo-converted cell changed from red to orange over the course of 48 h. The shift from red fluorescence to orange fluorescence may have been the result of de-localization of protein or protein turnover in the cell as the cell stretched and migrated over the course of 48 h. The expression of Dendra2 was detected up to 7 d after transfection, and red fluorescent expression was detected up to 3 d after photo-conversion. In the current study, “re-photo-conversion” of cells was found not to be possible after green and red fluorescence had diminished. Dendra2 displayed robust green and red fluorescent expression, and may be highly useful for short-term tissue engineering studies where rapid differentiation occurs within a 7 d time period. Dendra2 may additionally be beneficial in studies that focus on transient gene expression or transient protein expression. The use of Dendra2 in stable transfection systems may enable a prolonged expression of Dendra2; increasing the utility of Dendra2. Future studies that use Dendra2 in stable gene expression systems where DNA integrates into the genome through zinc finger nucleases, transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPRs) could be highly valuable for tracking cells, monitoring the generation and destruction of intracellular transcripts, and examining the turn-over of target proteins and growth factors within a cell or tissue.
The current study demonstrated that Dendra2 could be a highly effective tool for gene delivery and tissue engineering studies in vitro, and future investigation of Dendra2 in ex vivo transfection experiments may yield fascinating results in co-culture studies. However, it remains to be seen whether Dendra2 has any effect on proliferation, stemness, and differentiation, or vice-versa, which will be an important area of future investigation. Dendra2-transfected cells could be highly valuable in tissue engineering experiments that examine how cells interact in a co-culture system, how cells interact with a biomaterial, or two various cell culturing environments. Select cells on a biomaterial scaffold or co-cultured with non-transfected cells could be photo-converted from green fluorescence to red fluorescence for further observation on how cells migrate, behave, and interact with a biomaterial or different cell type. In some tissue engineering applications, unique cells that are selectively photo-converted by the investigator could be isolated via FACS, which would enable an investigator to further analyze the isolated cells. Furthermore, Dendra2 could be strategically used in differentiation studies where Dendra2 is fused to a differentiation gene, and photo-conversion could be used to identify cells that display changes in morphology (or a lack there of). The use of a photo-convertible reporter gene has been highly underutilized in tissue engineering, and could be an extremely effective tool in tissue engineering experiments by allowing real-time observation of cells in materials and tissues for short periods of time immediately after transfection. Photo-convertible reporter proteins, such as Dendra2, provide an opportunity for investigators to gain insightful information into how cells respond to a treatment by affording the ability to secondary label select cells within an already primary labeled population without disrupting a treated cell population.
In summary, Dendra2 is a robust non-toxic photo-convertible protein that expressed well in hWJCs, and allowed for precise control of photo-conversion from green to red in arbitrarily selected cells over the time scale of 120 s. For the first time, a photo-convertible reporter gene was transfected into hWJCs, and the current study demonstrates how photo-convertible reporter genes, such as Dendra2, can be reliably and robustly photo-converted. Photo-convertible proteins such as Dendra2 enable strategic photo-conversion of select cells without photo-converting surrounding cells, which could be highly beneficial for in vitro and ex vivo studies. Thus, the potential to use a photo-convertible protein in tissue engineering applications could provide new insights regarding cell behavior in tissues and biomaterials, which could provide means to create new therapies in regenerative medicine.
Supplementary Material
Supplementary Fig. 1. Time-lapse video of full photo-conversion of hWJCs. All hWJCs converted from green to red fluorescence with gentle exposure to UV light. Duration = 00:58. Scale Bar = 100 μm.
Supplementary Fig. 2. Time-lapse video of isolated photo-conversion of hWJC. A single hWJC was photo-converted from green to red fluorescence with targeted UV light exposure, while surrounding cells did not photo-convert. Duration = 01:41. Scale Bar = 100 μm.
Supplementary Fig. 3. 48 h time-lapse video of the movement of the single photo-converted hWJC. A single hWJC was photo-converted, and was tracked over the course of 48 h. No cells that came into contact with the photo-converted cell displayed any signs of photo-conversion. Duration = 00:30. Scale Bar = 100 μm.
Acknowledgments
This project was funded by the NIH (R01 AR056347) and the State of Kansas. We would like to acknowledge the efforts of the nursing staff at Lawrence Memorial Hospital (Lawrence, KS) for assisting us in obtaining human umbilical cords with the informed consent of patients. Furthermore, we would like to acknowledge the efforts of Peggy Keefe, Austin Smith, and Mackenzie Bloom for their assistance on this project.
Footnotes
Conflicts of Interest: Adam J. Mellott, Heather E. Shinogle, David S. Moore, and Michael S. Detamore declare no conflicts of interest.
Ethical Standards: All human subjects research were carried out in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
No animal studies were carried out by the authors for this article.
Author Disclosure: The authors have no financial or intellectual property conflicts to disclose.
Author Contribution Statement: The idea to examine the use of Dendra2 for tissue engineering was conceived by A.J.M, H.E.S, and D.S.M. The initial experimental design was developed by A.J.M, H.E.S, and D.S.M. The experiment was executed by A.J.M. and H.E.S. Advice and technical support was provided by H.E.S., D.S.M., and M.S.D. The manuscript was written by A.J.M., and revised and edited by H.E.S., D.S.M., and M.S.D.
References
- 1.Aluigi M, Fogli M, Curti A, Isidori E, Gruppioni C, Chiodoni M, Colombo P, Versura A, D'Errico Grigioni A, Ferri E. Nucleofection Is an Efficient Nonviral Transfection Technique for Human Bone Marrowñ Derived Mesenchymal Stem Cells. Stem Cells. 2006;24:454–461. doi: 10.1634/stemcells.2005-0198. [DOI] [PubMed] [Google Scholar]
- 2.Bailey M, Wang L, Bode C, Mitchell K, Detamore M. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue engineering. 2007;13:2003–2010. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 3.Baker SM, Buckheit RW, 3rd, Falk MM. Green-to-red photoconvertible fluorescent proteins: tracking cell and protein dynamics on standard wide-field mercury arc-based microscopes. BMC Cell Biol. 2010;11:15. doi: 10.1186/1471-2121-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton's jelly of the human umbilical cord. Stem Cell Rev. 2013;9:226–40. doi: 10.1007/s12015-012-9418-z. [DOI] [PubMed] [Google Scholar]
- 5.Check E. Gene therapy put on hold as third child develops cancer. Nature. 2005;433:561. doi: 10.1038/433561a. [DOI] [PubMed] [Google Scholar]
- 6.Chudakov DM, Lukyanov S, Lukyanov KA. Tracking intracellular protein movements using photoswitchable fluorescent proteins PS-CFP2 and Dendra2. Nat Protoc. 2007;2:2024–32. doi: 10.1038/nprot.2007.291. [DOI] [PubMed] [Google Scholar]
- 7.Chudakov DM, Lukyanov S, Lukyanov KA. Using photoactivatable fluorescent protein Dendra2 to track protein movement. Biotechniques. 2007;42:553, 555. doi: 10.2144/000112470. 557 passim. [DOI] [PubMed] [Google Scholar]
- 8.Chudakov DM, Verkhusha VV, Staroverov DB, Souslova EA, Lukyanov S, Lukyanov KA. Photoswitchable cyan fluorescent protein for protein tracking. Nat Biotechnol. 2004;22:1435–9. doi: 10.1038/nbt1025. [DOI] [PubMed] [Google Scholar]
- 9.Detamore MS. Human umbilical cord mesenchymal stromal cells in regenerative medicine. Stem Cell Res Ther. 2013;4:142. doi: 10.1186/scrt353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devarajan K, Forrest ML, Detamore MS, Staecker H. Adenovector-mediated gene delivery to human umbilical cord mesenchymal stromal cells induces inner ear cell phenotype. Cell Reprogram. 2013;15:43–54. doi: 10.1089/cell.2011.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy GP, D'Arcy S, Ahsan T, Nerem RM, O'Brien T, Barry F. Mesenchymal stem cells overexpressing ephrin-b2 rapidly adopt an early endothelial phenotype with simultaneous reduction of osteogenic potential. Tissue Eng Part A. 2010;16:2755–68. doi: 10.1089/ten.tea.2009.0623. [DOI] [PubMed] [Google Scholar]
- 12.Durisic N, Laparra-Cuervo L, Sandoval-Alvarez A, Borbely JS, Lakadamyali M. Single-molecule evaluation of fluorescent protein photoactivation efficiency using an in vivo nanotemplate. Nat Methods. 2014;11:156–62. doi: 10.1038/nmeth.2784. [DOI] [PubMed] [Google Scholar]
- 13.Fron E, Van der Auweraer M, Moeyaert B, Michiels J, Mizuno H, Hofkens J, Adam V. Revealing the Excited-State Dynamics of the Fluorescent Protein Dendra2. J Phys Chem B. 2013 doi: 10.1021/jp309219m. [DOI] [PubMed] [Google Scholar]
- 14.Gould TJ, Gunewardene MS, Gudheti MV, Verkhusha VV, Yin SR, Gosse JA, Hess ST. Nanoscale imaging of molecular positions and anisotropies. Nat Methods. 2008;5:1027–30. doi: 10.1038/nmeth.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunewardene MS, Subach FV, Gould TJ, Penoncello GP, Gudheti MV, Verkhusha VV, Hess ST. Superresolution imaging of multiple fluorescent proteins with highly overlapping emission spectra in living cells. Biophys J. 2011;101:1522–8. doi: 10.1016/j.bpj.2011.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurskaya NG, Verkhusha VV, Shcheglov AS, Staroverov DB, Chepurnykh TV, Fradkov AF, Lukyanov S, Lukyanov KA. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat Biotechnol. 2006;24:461–5. doi: 10.1038/nbt1191. [DOI] [PubMed] [Google Scholar]
- 17.Hamm A, Krott N, Breibach I, Blindt R, Bosserhoff A. Efficient transfection method for primary cells. Tissue Engineering. 2002;8:235–245. doi: 10.1089/107632702753725003. [DOI] [PubMed] [Google Scholar]
- 18.Hatta K, Tsujii H, Omura T. Cell tracking using a photoconvertible fluorescent protein. Nat Protoc. 2006;1:960–7. doi: 10.1038/nprot.2006.96. [DOI] [PubMed] [Google Scholar]
- 19.Kaya AI, Ugur O, Altuntas O, Sayar K, Onaran HO. Long and short distance movements of beta(2)-adrenoceptor in cell membrane assessed by photoconvertible fluorescent protein Dendra2-beta(2)-adrenoceptor fusion. Biochim Biophys Acta. 2011;1813:1511–24. doi: 10.1016/j.bbamcr.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa M, Fujita T. Quantitative imaging of directional transport through plasmodesmata in moss protonemata via single-cell photoconversion of Dendra2. J PlantRes. 2013 doi: 10.1007/s10265-013-0547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mellott AJ, Forrest ML, Detamore MS. Physical Non-Viral Gene Delivery Methods for Tissue Engineering. Annals of Biomedical Engineering. 2013:1–23. doi: 10.1007/s10439-012-0678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell K, Weiss M, Mitchell B, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou Easa K, Hildreth T. Matrix cells from Wharton's jelly form neurons and glia. Stem cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 23.Nowotschin S, Hadjantonakis AK. Photomodulatable fluorescent proteins for imaging cell dynamics and cell fate. Organogenesis. 2009;5:217–26. doi: 10.4161/org.5.4.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tell G, Di Piazza M, Kamocka MM, Vascotto C. Combining RNAi and in vivo confocal microscopy analysis of the photoconvertible fluorescent protein Dendra2 to study a DNA repair protein. Biotechniques. 2013;55:198–203. doi: 10.2144/000114088. [DOI] [PubMed] [Google Scholar]
- 25.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–58. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Ott L, Seshareddy K, Weiss ML, Detamore MS. Musculoskeletal tissue engineering with human umbilical cord mesenchymal stromal cells. Regen Med. 2011;6:95–109. doi: 10.2217/rme.10.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf H, Barisas BG, Dietz KJ, Seidel T. Kaede for detection of protein oligomerization. Mol Plant. 2013;6:1453–62. doi: 10.1093/mp/sst039. [DOI] [PubMed] [Google Scholar]
- 28.Wu S, Koizumi K, Macrae-Crerar A, Gallagher KL. Assessing the utility of photoswitchable fluorescent proteins for tracking intercellular protein movement in the Arabidopsis root. PloS one. 2011;6:e27536. doi: 10.1371/journal.pone.0027536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Time-lapse video of full photo-conversion of hWJCs. All hWJCs converted from green to red fluorescence with gentle exposure to UV light. Duration = 00:58. Scale Bar = 100 μm.
Supplementary Fig. 2. Time-lapse video of isolated photo-conversion of hWJC. A single hWJC was photo-converted from green to red fluorescence with targeted UV light exposure, while surrounding cells did not photo-convert. Duration = 01:41. Scale Bar = 100 μm.
Supplementary Fig. 3. 48 h time-lapse video of the movement of the single photo-converted hWJC. A single hWJC was photo-converted, and was tracked over the course of 48 h. No cells that came into contact with the photo-converted cell displayed any signs of photo-conversion. Duration = 00:30. Scale Bar = 100 μm.


