Abstract
Electrophysiological changes in basal ganglia neurons are hypothesized to underlie motor dysfunction in Parkinson’s disease (PD). Previous results in head-restrained MPTP-treated non-human primates have suggested that increased bursting within the basal ganglia and related thalamic and cortical areas may be a hallmark of pathophysiological activity. In this study, we investigated whether there is increased bursting in substantia nigra pars reticulata (SNpr) output neurons in anesthetized and awake, head-restrained unilaterally lesioned 6-OHDA mice when compared to control mice. Confirming previous studies, we show that there are significant changes in the firing rate and pattern in SNpr neuron activity under urethane anesthesia. The regular firing pattern of control urethane-anesthetized SNpr neurons was not present in the 6-OHDA-lesioned group, as the latter neurons instead became phase locked with cortical slow wave activity (SWA). Next, we examined whether such robust electrophysiological changes between groups carried over to the awake state. SNpr neurons from both groups fired at much higher frequencies in the awake state than in the anesthetized state and surprisingly showed only modest changes between awake control and 6-OHDA groups. While there were no differences in firing rate between groups in the awake state, an increase in the coefficient of variation (CV) was observed in the 6-OHDA group. Contrary to the bursting hypothesis, this increased CV was not due to changes in bursting but was instead due to a mild increase in pausing. Together, these results suggest that differences in SNpr activity between control and 6-OHDA lesioned mice may be strongly influenced by changes in network activity during different arousal and behavioral states.
Keywords: Substantia nigra pars reticulata, bursts, pauses, 6-OHDA, mouse, beta oscillation, Parkinson’s disease, single unit recording
Introduction
Parkinson’s disease is the second most common neurodegenerative disease, affecting nearly 1% of the population (Huse et al., 2005; Tanner and Goldman, 1996). The disease is typically diagnosed when several dopamine-dependent motor signs are present, such as bradykinesia, muscle rigidity, and resting tremor. Current hypotheses of the neurological mechanisms underlying these motor deficits implicate changes in the electrical activity patterns of basal ganglia neurons after dopamine depletion, specifically increased oscillatory firing in the beta band, increased neural synchronization, and abnormally increased bursting (Galvan and Wichmann, 2008; Rubin et al., 2012).
Electrophysiological studies have suggested that abnormal bursting may play a role in the pathophysiology of PD (reviewed by (Lobb, 2014)). Several studies in MPTP-treated non-human primates have found an increase in the percentage of ‘bursty’ basal ganglia neurons or the proportion of spikes in bursts in the internal segment of the globus pallidus (GPi) and the subthalamic nucleus using a variety of burst detection methods. However, inconclusive results have been found with neurons in the external segment of the globus pallidus (Bergman et al., 1994; Boraud et al., 1998; Boraud et al., 2000; Sanders et al., 2013; Soares et al., 2004; Wichmann et al., 1999). Increased bursting has also been seen in human PD patients during neurosurgical procedures targeting the STN and globus pallidus (Starr et al., 2005; Steigerwald et al., 2008; Tang et al., 2005; Tang et al., 2007) in comparison to recordings from other neurological diseases (e.g. dystonia, essential tremor, and Huntington’s disease).
One of the most widely used animal models of parkinsonism is the unilaterally 6-hydroxydopamine (6-OHDA)-lesioned rodent (Schwarting and Huston, 1996a, b). Basal ganglia neurons recorded in vivo under anesthesia undergo significant changes in firing pattern after a 6-OHDA lesion, developing a strong preference to fire in-phase or anti-phase with cortical SWA (Belluscio et al., 2003; Hollerman and Grace, 1992; MacLeod et al., 1990; Magill et al., 2001; Mallet et al., 2012; Murer et al., 1997; Seeger-Armbruster and von Ameln-Mayerhofer, 2013; Tseng et al., 2005; Tseng et al., 2001a; Tseng et al., 2001b; Walters et al., 2007; Zold et al., 2007). Several studies in awake rodents have demonstrated that the spike trains of SNpr neurons in 6-OHDA-lesioned rats can become entrained to movement-related episodes of high beta in the local field potential (LFP) (Avila et al., 2010; Brazhnik et al., 2012; Brazhnik et al., 2014); however, these studies have not addressed the hypothesis of whether there is an abnormally increased baseline level of bursting in rodent parkinsonism. To investigate this hypothesis, we recorded basal ganglia output neurons in the SNpr in control and unilaterally 6-OHDA-lesioned, behaviorally impaired mice. SNpr neurons were recorded in both the anesthetized and the awake state of head-restrained mice. We found that the SNpr neuron firing in 6-OHDA lesioned but not control anesthetized mice was heavily influenced by cortical SWA activity. In contrast, in awake, head-restrained mice, SNpr neurons had a single-spiking pattern in both control and 6-OHDA groups, with no change in the firing rate, and only subtle changes in the firing pattern including increased coefficient of variation and pausing metrics.
Materials and Methods
All experiments were performed in accordance with protocols reviewed and approved by the Animal Care and Use Committee of Emory University. A full description of many of the methods employed here has been previously published (Lobb et al., 2013). A total of 37 adult mice (14 non-injected controls, 7 saline-injected controls, 16 6-OHDA-injected mice; all C57BL/6J and non-infected Slc32a1tm2(Cre)/Lowl/J on a C57BL/6J background) were used in this study. After accommodating mice to handling, behavioral testing (see below for details) was conducted and then either saline or 6-OHDA administered. The behavior of the mouse was reassessed 4 weeks later. No effect of saline injections was seen when compared to the non-injected hemisphere (0.86 ± 3.11 % loss of striatal TH, n = 6). Therefore, non-injected and saline-injected mice were pooled. Of the 16 6-OHDA-injected mice, 2 died after surgery and one had an insufficient lesion (only 40% striatal TH loss). The remaining animals had successful lesions (> 80%; average of 94.0 ±1.69% loss in striatal TH staining, n = 13, see below). The animals were used either in the anesthetized or in the awake state. Recordings were made acutely from anesthetized (15; 8 controls, 7 6-OHDA) or awake (19; 13 controls, 6 6-OHDA) mice. At the end of the final recording session, mice were perfused transcardially with a 4% paraformaldehyde solution in phosphate-buffered saline, and the brains removed for histological processing.
Saline/6-OHDA Administration
Lesions in the nigrostriatal dopamine system were made using standard methods (Cenci and Lundblad, 2007). Briefly, 1 μl of 6-OHDA (3.0 mg/ml free base 6-OHDA prepared in saline containing 0.02% ascorbic acid) or saline (also containing 0.02% acetic acid) was injected into the medial forebrain bundle (MFB) of the right hemisphere (bregma: −1.2 mm AP, +1.1 mm ML, 4.75 mm DV (Franklin and Paxinos, 2008) of isoflurane-anesthetized mice. Mice were fed chocolate Pediasure daily until they recovered their pre-surgical weight.
Behavioral Testing
Open-Field Test
Mice were placed in a 20 × 12 × 9.5 inch white plastic rectangular box for 5 min and were videotaped throughout. Automated behavioral analysis was carried out with a custom Matlab script (adapted from (Gomez-Marin et al., 2012)). The mouse was detected as an elongated shape using the Matlab ‘regionprops’ command and tracked across successive video frames. The distance traveled and number of rotations in 5 min were measured.
Electrophysiological Recordings
Recordings in the anesthetized state
Recordings were performed as previously described (Lobb et al., 2013). Mice were anesthetized with urethane (1.7 g/kg) and placed in a stereotaxic apparatus. A craniotomy was made over the SNpr recording site (bregma: −3.2 mm AP, 1.6 mm ML). Additionally, a depth LFP wire (100–200 kΩ) was implanted into the primary motor cortex (+1.1 mm AP from bregma, 1.2 mm ML, 0.8 mm DV) to record cortical LFP signals. Next, glass pipettes (tip size, approximately 3 μm) filled with 1 M NaCl and 1% pontamine sky blue were lowered into the SNpr (3.9 – 4.5 mm DV). Monopolar recordings were then made in the SNpr with reference to a silver chloride wire placed under the scalp near the temporal musculature. The final recording site for each mouse was marked by iontophoretic ejection of pontamine sky blue (−10 to −15 μA, 20–25 min).
Awake, head-fixed recordings
During surgical preparation, mice were anesthetized with isoflurane and placed in a stereotaxic apparatus. A craniotomy was made over the future SNpr recording site and covered with Kwik-cast (WPI Inc.). The dura was left intact. A custom headpost weighing 1–2 g was implanted on the skull with dental cement (RelyX Unicem 2, 3M). In some experiments, skull screws were used to enhance the fixation of the implant to the skull. The mice were allowed at least 3 days to recover.
After recovery, the mice were given 3 days to acclimate to being head-fixed (one session per day, 60 min per session) to a custom-made head-fixation apparatus. The apparatus consisted of a plastic hemitube (1 5/8″ OD, 1 3/8″ ID, 3.5″ long) with head holder bars that allowed the wing-like headpost to be secured and a detachable flat top to ensure that the mouse stayed in the tube. The size of the tube was large enough that although the mouse was not immobilized, it could not turn or contort its body. Once the head was stabilized, mice showed no overt signs of stress and appeared relaxed. During recording sessions mice were maintained on a fixed interval reward paradigm (FI 60 s, 10% sucrose via a lick spout) to encourage quiet wakefulness during most of the session. Since mice in both the normal and lesioned groups generally drank almost all of liquid provided, this indicates that mice remained awake for the entire session.
Following acclimation, mice were head-fixed and the Kwik-cast cover over the craniotomy was removed. Glass pipettes (as above) were lowered into the SNpr. We found that the pipettes penetrated the dura without causing damage to the dura or the pipette. Monopolar recordings were then made in the SNpr with reference to a second glass pipette (~20 μm tip size which is too large to pick up single units) placed just underneath the dura in the craniotomy. For final recording sessions, the site of the recordings was marked by iontophoretic injection of pontamine sky blue as before. After each session, the craniotomy was covered with Kwik-cast. After completion of the recording sessions, the mouse was perfused with PBS followed by perfusion with 4% paraformaldehyde and 15% sucrose. The brain was then removed and transferred to a 4% paraformaldehyde / 30% sucrose solution for later histological processing.
Histological Verification, TH Immunohistochemistry and Densitometry
Coronal sections of fixed brains (50 μm thick) were cut on a microtome and mounted onto slides. Sections with blue recording dots were counterstained with Neutral Red (Lobb et al., 2013). Every sixth section was stained for tyrosine hydroxylase (TH), scanned with an Aperio ePathology microscope (Leica Biosystems), and analyzed for TH staining intensity with ImageJ (Lobb et al., 2013).
Data Analysis
Spiking-LFP relationship
To determine if spiking activity occurred at a preferred phase of an oscillation, circular analysis was performed (Lobb et al., 2013). Briefly, the ranked distribution of the Hilbert-transformed instantaneous phase (low-pass filtered at 1.5 Hz) at each spike time was tested for statistical significance using the Omnibus test. Data from individual cells are presented with first-order circular plots using the Matlab package CircStat (Berens, 2009). Group data are presented with second-order circular plots.
Burst/Pause analysis
Bursts were detected with the Poisson (PS) method (Legendy and Salcman, 1985) and the Robust Gaussian Surprise (RGS) method (Ko et al., 2012). The PS method detects bursts as consecutive inter-spike intervals (ISIs) that are highly unexpected from a Poisson process which would produce spikes at a rate that is given by the mean firing rate of a given spike train. The RGS method (similar to the Rank Surprise method of Gourevitch and Eggermont (2007)) allows for non-Poisson spiking behavior, uses a moving window to reflect local changes in rate, and simultaneously detects pauses. Both methods employ the use of seeds to determine prospective bursts and pauses. Burst seeds in the PS method were determined as all ISIs that are 50% of the average ISI of the spike train. Burst/pause seeds in the RGS were determined as outliers (5% / 95%, respectively) of a lognormal ISI distribution based on pooled ISIs from each group. Doublets were excluded. A surprise value of 3 was used with both methods. Surprise is measured as the negative logarithm of the probability of seeing a certain number of spikes in a specified time interval assuming a given ISI distribution (Legendy and Salcman, 1985).
Spectral analysis
Power spectra and spectrograms were created using the Matlab package Chronux (http://chronux.org) using a pass-band of 10–80 Hz and 5 tapers (Mitra and Bokil, 2008). A time window of 0.5 s moved every 0.1 s was used for spectrograms.
Statistics
Statistics were performed in Graphpad Prism 6.0. Changes in behavior metrics were determined by using a 2-way RM ANOVA. Differences in all other metrics between control and 6-OHDA-treated mice were determined by unpaired t-tests or Mann-Whitney U tests, depending on whether the distributions were normal (determined with the D’Agostino-Pearson omnibus normality test). Outliers that were detected with the nonlinear regression method ‘ROUT (Q = 1%) were removed (Motulsky and Brown, 2006). All data are presented as means ± SEM unless otherwise stated. Significance is illustrated with asterisks (*, p < 0.05; **, p < 0.01; ***, p < 0.001). Proportion data was transformed with an arcsin function prior to statistical testing.
Results
Parkinsonism was induced by an acute unilateral injection of 6-OHDA into the MFB (Figure 1). Administration of 6-OHDA produced a near complete lesion of the nigrostriatal dopaminergic system on the injected hemisphere of the brain (saline: 0.86 ± 3.11 % loss, n = 6; 6-OHDA: 94.0 ±1.69 % loss in striatal TH staining, n = 13). An example is shown in Figure 1A–B. This was accompanied by significant changes in the behavior of a sample of 6-OHDA-injected mice when measured 4 weeks post-lesion. 6-OHDA-lesioned mice (n = 6) moved a significantly shorter distance in the arena (2-way RM ANOVA; interaction: F1,11 = 8.71, p = 0.013) with a tendency to rotate ipsiversively (i.e. towards the lesioned hemisphere; 2-way RM ANOVA; interaction: F1,11 = 20.7 p = 0.0008) when compared to control mice (n = 7) (Figure 1C).
Figure 1. Induction of Parkinsonism by intracranial administration of 6-OHDA.
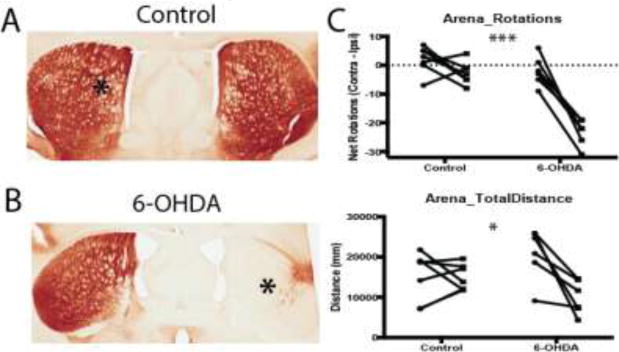
A–B. TH immunostaining in the striatum after unilateral administration (marked with an asterisk) of saline (A) or 6-OHDA (B) into the MFB, demonstrating striatal dopamine depletion on the 6-OHDA-lesioned side. This resulted in significant behavioral deficits as shown in the open-field test (C). 6-OHDA-lesioned mice spontaneously rotated in the ipsiversive direction (side of the lesion) and traveled a shorter distance in the arena as compared to control mice. For each group, behavioral measures collected before and after injection for each mouse are shown as connected data points. *, p < 0.05; ***, p < 0.001
In vivo recordings in the anesthetized state
Under urethane anesthesia, LFPs recorded in the motor cortex and SNpr showed robust SWA around 0.5 Hz (data not shown). There was no significant difference in peak frequency of SWA (Ctrl: 0.488 ± 0.10, 6-OHDA: 0.570 ± 0.07; p > 0.05) between LFPs recorded in control and 6-OHDA-lesioned mice, as determined with spectral analysis. No significant peaks in the beta frequencies were seen in either group (data not shown) during SWA.
During SWA, control SNpr neurons fired with a mean firing rate of 23.6 Hz (mean inter-spike interval (ISI) of 42.4 ± 4.2 ms; median: 41.1 ± 4.0 ms; n = 14) and exhibited a regular firing pattern (coefficient of variation (CV): 0.386 ± 0.043). In contrast, SNpr neurons recorded from the lesioned side in 6-OHDA-lesioned mice showed a severalfold decreased mean firing rate compared to controls (3.5 Hz; mean ISI: 288 ± 55.8 ms, p < 0.001 ; median: 76.9 ± 13.4 ms, p < 0.01 ; n = 15) and had a much less regular firing pattern (CV: 1.51 ± 0.20, p < 0.001). Representative examples of SNpr neurons from control and 6-OHDA-lesioned mice are shown in Figures 2A and 2D, respectively.
Figure 2. Changes in the firing pattern of SNpr neurons in urethane anesthetized 6-OHDA-lesioned mice.
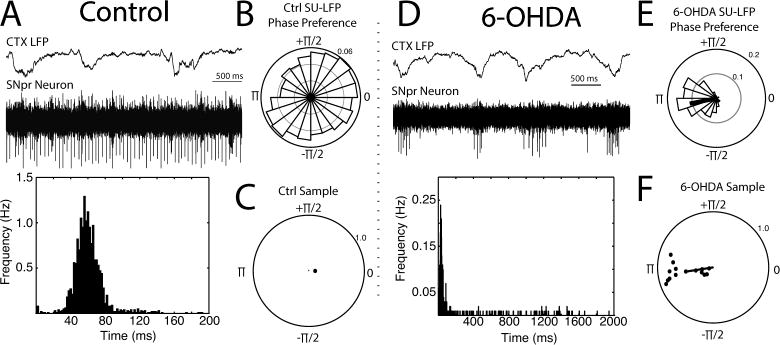
Recordings of SNpr neurons were made in control (A–C) and 6-OHDA-lesioned (D–F) mice. Example recordings along with their respective ISI histograms (bottom, 2 ms bin width; note the different time scales) are shown in A (control mouse) and D (6-OHDA lesioned hemisphere). A simultaneously recorded LFP from the motor cortex at a depth of 0.8 mm is shown above the spiking neuron. SNpr neurons from 6-OHDA-lesioned mice showed a significant firing preference with respect to the cortical SWA phase extracted from the LFP as a Hilbert transform (see Methods). The example in D was chosen to illustrate the periodic nature of the firing; however, cells typically did not fire as robustly on every cycle (e.g. Figure 3A). First-order polar plots of the cells shown in A and D are shown in B and E respectively. Second-order polar plots depicting the mean preferred phase for all SNpr neurons in each group are shown in C and F for control and 6-OHDA-lesioned mice, respectively. A significant grand mean for 6-OHDA cells in F is shown with a black line. Only cells with statistically significant phase preferences (Rayleigh test, p < 0.05) are shown.
Additionally, all 6-OHDA SNpr neurons had a significant preference to fire in-phase with cortical SWA (i.e. the cortical UP state; n = 15, p < 0.05, Rayleigh test; sample grand mean 1.05π where π corresponds to the peak negativity of the LFP at this depth), whereas this was seen in only 1 SNpr neuron in the control group of animals (n =12; Figure 2B–C,E–F), consistent with previous studies (Belluscio et al., 2003; MacLeod et al., 1990; Magill et al., 2001; Mallet et al., 2012; Sanderson et al., 1986; Walters et al., 2007).
An analysis of bursts (transient increases in spiking) and pauses/decelerations (transient decreases in spiking) was then performed on control (example in Figure 3A) and 6-OHDA (example in Figure 3B) SNpr spike trains using the RGS and PS methods. The profound difference in the ISI distributions of the two groups (Figure 2A vs. 2D) had a significant impact on burst detection results with the Poisson method finding that the interburst rate was significantly increased but bursts were weaker in intensity (intraburst ISI) in the 6-OHDA-lesioned group and the RGS method finding that the interburst rate significantly decreased while the intensity (intraburst ISI) remained unchanged (Figure 3C–D). However, both methods found that the number of spikes per burst increased (Figure 3E). These differences predominately arise from the strong impact of the long ISIs (often pause spikes) on the definition of a burst in the mean ISI-based PS method but not in the median ISI-based RGS method. These findings demonstrate that methodological details of burst detection can have a significant qualitative impact on the results and should be carefully considered to avoid conflicting interpretations (see Discussion).
Figure 3. Bursting in the SNpr in anesthetized control and 6-OHDA-lesioned mice.
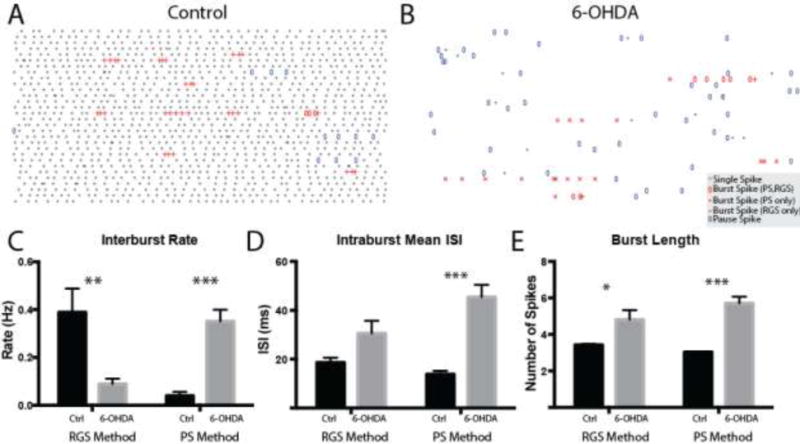
Representative raster plots (30 s total, 1 s/row) of control and 6-OHDA SNpr neurons are shown in A and B, respectively. Single spiking events are shown as gray bars. Burst spikes detected by both the PS and RGS methods are depicted with open red ovals. Burst spikes detected by the PS method but not the RGS method are depicted with red crosses. Burst spikes detected by the RGS method but not the PS method are depicted with red pluses. C–E Summary data for the burst rate (C, number of bursts divided by the total duration; RGS: control: 0.389 ± 0.099 Hz, n = 12 6-OHDA: 0.089 ± 0.022 Hz, n = 15, p = 0.003 unpaired t-test (UTT); PS: control: 0.027 ± 0.011, 6-OHDA: 0.351 ± 0.049, p < 0.0001 Mann-Whitney U test (MWU)), mean intraburst ISI or intensity (D, mean ISI in a burst averaged across bursts; RGS: control: 18.6 ± 2.03 ms, 6-OHDA: 30.52 ± 5.167 ms, p = 0.075 MWU; PS: control: 13.8 ± 1.38 ms, 6-OHDA: 45.4 ± 5.05 ms, p < 0.0001 UTT) and burst length, (E, number of spikes per burst; RGS: control: 3.43 ± 0.051, 6-OHDA: 4.82 ± 0.515, p = 0.022 UTT; PS: control: 3.04 ± 0.023, 6-OHDA: 5.71 ± 0.350, p < 0.0001 UTT) are shown. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Pauses detected by the RGS method were also analyzed. Neither the interpause rate (control: 0.282 ± 0.070 Hz, 6-OHDA: 0.302 ± 0.063 Hz, p = 0.831 UTT) or spikes per pause (control: 2.56 ± 0.139, 6-OHDA: 2.71 ± 0.173, p = 0.526 UTT) were changed in the 6-OHDA-lesioned group; however, the mean ISI of the pause was more than 10 times longer (control: 98.8 ± 12.0 ms, 6-OHDA: 1150 ± 0.238 ms, p < 0.0001 MWU).
Awake In Vivo Recordings
Single-unit and LFP recordings were made in the SNpr from awake, head-restrained control and 6-OHDA-lesioned mice (from the lesioned hemisphere) during periods of quiet rest between free rewards spaced at 60s intervals (see Methods). Periods in which the mouse shifted its body around inside the tube could not be analyzed due to the presence of large artifacts and were excluded. A spectral analysis performed on LFPs revealed no significant differences between control and 6-OHDA-lesioned mice, including a lack of a clear peak in the beta range (Figure 4; total power, control: 6.16 × 10−8 ± 1.03 × 10−8 V2/Hz, n = 9; 6-OHDA: 6.08 × 10−8 ± 6.47 × 10−9 V2/Hz, n = 5; p > 0.05, Mann-Whitney U test).
Figure 4. Lack of increased beta oscillations in the SNpr in the awake, head-restrained mouse.
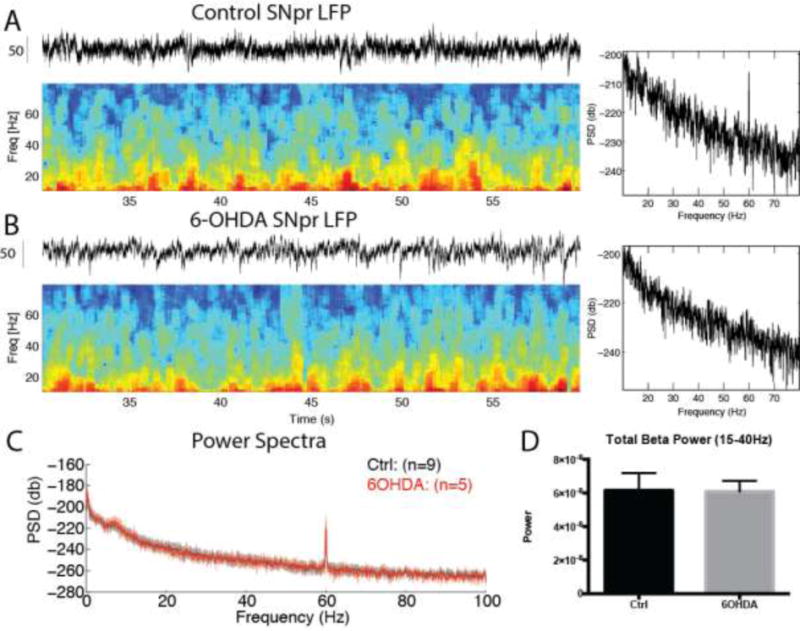
A–B. Spectrograms of the holding period between rewards (at t = 10s) recorded in the control (A) and 6-OHDA (B) SNpr. C. Summary power spectra for control and 6-OHDA SNpr recordings. No difference in beta power was seen between groups (D, p > 0.05, Mann-Whitney U test). Peak at 60 Hz was due to AC line artifacts.
Next, the spiking activity of SNpr neurons was analyzed in both control and 6-OHDA groups (Figure 5). Representative examples are shown in Figure 5A and 5B, respectively. There was no difference in the mean firing rate of SNpr neurons between groups (57.1 Hz in controls and 47.4 Hz in 6-OHDA-lesioned mice; mean ISI control: 17.5 ± 0.879 ms, n = 30, 6-OHDA: 21.1 ± 1.52 ms, n = 32, p = 0.0752, Mann-Whitney U test; median control 16.7 ± 0.806 ms, 6-OHDA: 18.2 ± 1.14 ms, p > 0.05, Mann-Whitney U). However, there was a modest but significant increase in the CV in the 6-OHDA group as compared to controls (control: 0.456 ± 0.015, 6-OHDA: 0.572 ± 0.038, p = 0.019, Mann-Whitney U).
Figure 5. SNpr neurons in the awake, head-restrained mice.
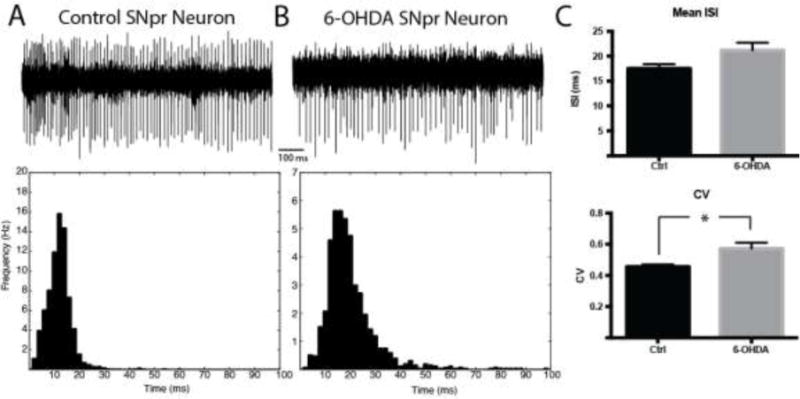
A–B. Representative 1s traces of single SNpr neurons recorded in the awake state of a control mouse (A, mean ISI = 13.0 ms, CV = 0.395; 2 ms bin width) or from the lesioned hemisphere of a 6-OHDA-lesioned mice (B, mean ISI = 21.8 ms, CV = 0.643; 2 ms bin width). C. There was no significant change in mean ISI between groups (p > 0.05, Mann-Whitney U test); however, there was a significant increase in CV (p = 0.019, Mann-Whitney U test).
We next analyzed whether the higher CV in the 6-OHDA treated animals could be due to changes in increased bursting/pausing (Figure 6) as found above in the anesthetized state. We found that bursting of SNpr neurons was largely unchanged in control and 6-OHDA-lesioned mice, with the only significant result being that the bursts detected by the Poisson Surprise method were weaker, not stronger, in intensity as measured by mean intraburst ISI duration (control: 6.90 ± 0.295 ms, n = 26, 6-OHDA: 8.75 ± 0.550 ms, n = 29, p < 0.008, Mann-Whitney U). No significant effects were found with the RGS method.
Figure 6. Bursts and pauses of SNpr neurons in awake, head-restrained mice.
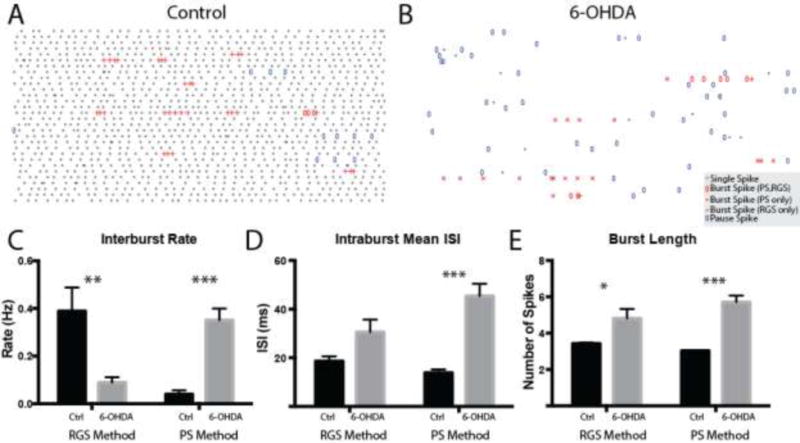
Representative raster plots (1 s per row) of control and 6-OHDA SNpr neurons are shown in A and B, respectively. Single spikes are colored gray. Burst spikes detected by both the PS and RGS methods are depicted with open red ovals. Burst spikes detected by the PS method but not the RGS method are depicted with red crosses. Burst spikes detected by the RGS method but not the PS method are depicted with red pluses. C–F Summary data for the interburst rate (C, RGS: control: 1.06 ± 0.103 Hz, 6-OHDA: 0.862 ± 0.080 Hz, p = 0.135 UTT; PS: control: 0.607 ± 0.079, 6-OHDA: 0.804 ± 0.110, p = 0.158 UTT), mean intraburst ISI (D, RGS: control: 7.19 ± 0.417 ms, 6-OHDA: 7.69 ± 0.360 ms, p = 0.260 MWU; PS: control: 6.90 ± 0.295, 6-OHDA: 8.75 ± 0.550, p = 0.008 MWU), pause rate (E, control: 0.708 ± 0.063 Hz, 6-OHDA: 1.00 ± 0.125 Hz, p = 0.041 UTT) and mean intrapause ISI (F, control: 42.8 ± 1.99 ms, 6-OHDA: 55.4 ± 5.33 ms, p = 0.243 MWU) are shown.*, p < 0.05; **, p < 0.01.
However, significant changes were noted in pausing with the RGS method. The number of pauses per second (interpause rate) was increased by 41% in 6-OHDA-lesioned mice (control pauses/s: 0.708 ± 0.063, 6-OHDA: 1.00 ± 0.125, p = 0.041, unpaired t-test). While there was no significant change in the intrapause ISI, pauses were significantly longer (control spikes/pause: 3.07 ± 0.130, 6-OHDA: 3.69 ± 0.205, p = 0.014, unpaired t-test). These results suggest that the increased irregularity of spiking in the SNpr spike trained from the 6-OHDA-lesioned animals was due to increased pausing.
Discussion
We investigated the electrical activity of SNpr neurons in control and unilateral 6-OHDA-lesioned mice, in both the urethane-anesthetized and awake, head-restrained states. In anesthetized mice during < 1 Hz SWA, we confirm and extend previous observations that there is a fundamental change in spiking after 6-OHDA lesioning: the regular activity of SNpr neurons becomes highly irregular and phase-locked to cortical SWA (Belluscio et al., 2003; MacLeod et al., 1990; Magill et al., 2001; Mallet et al., 2012; Sanderson et al., 1986; Walters et al., 2007). In the awake, head-restrained state where cortical SWA is absent, SNpr neurons fired at a much higher firing rate than during SWA in the anesthetized state. However, there was no difference in rate between control and 6-OHDA SNpr neurons. There was an increase in the irregularity of spiking as measured from the CV in the 6-OHDA treated animals, which was likely due to an increase in pausing. Contrary to the increased bursting hypothesis, bursting appeared to be largely similar in both groups in the awake state.
The effect of anesthesia on basic SNpr spiking properties
Our results agree with previous studies that anesthesia can have a strong impact on SNpr spiking dynamics. In this study, we only investigated SNpr spiking during < 1 Hz cortical SWA. We did not look at any other brain states that can be found at different stages of urethane-anesthetized rats (Friedberg et al., 1999), although the firing rate and pattern of SNpr neurons may change in such different brain states (Belluscio et al., 2003). During SWA, we found that accompanying this dramatic change in the firing pattern was a reduction in the mean firing rate of the neuron. Previous studies in 6-OHDA lesioned rats only showed non-significant trends towards a reduced firing rate (Belluscio et al., 2003; MacLeod et al., 1990; Walters et al., 2007). This may be due to long pauses in our spike train data and their effect on the mean rate calculation (see below), which may be a result of anesthetic depth or species differences.
Two observations from our data regarding the effect of anesthesia on SNpr spiking are readily apparent. First, the firing rate of SNpr neurons was increased two-fold in the awake, head-restrained state (control mean ISI of 17.5 ms or 57 Hz vs. 6-OHDA mean ISI of 21.1 ms or 47 Hz; note that there was no significant difference between groups) when compared to urethane anesthesia (control mean ISI of 42.4 ms or 24 Hz vs. 6-OHDA mean ISI of 288 ms or 3.5 Hz). The in vivo firing rates seen here are faster than the ~10–20 Hz firing rate which SNpr neurons display in isolated brain slices (Atherton and Bevan, 2005; Lee and Tepper, 2007; Lee et al., 2011; Zhou et al., 2009; Zhou et al., 2006) suggesting that afferent input is responsible for these increased rates. Furthermore, the firing rate of SNpr neurons may depend on the behavioral state or recording technique, as previous recordings of rodent SNpr neurons with chronically implanted microwires have observed a substantially lower firing rate (Avila et al., 2010; Barter et al., 2014; Chang et al., 2006). Our finding that SNpr neurons recorded from head-fixed quietly attentive mice fire near 50 Hz is more in line with studies in head-fixed monkeys or humans, where electrodes are also acutely advanced to the target (Benedetti et al., 2009; Boraud et al., 1996; Cleary et al., 2013; Wichmann et al., 1999). These differences point out that the detailed impact of head-fixation and electrode types on recorded firing rates should be carefully assessed in future studies.
Secondly, non-surprisingly the activity pattern of SNpr neurons after 6-OHDA was markedly different under cortical slow-wave anesthesia than in the awake state. During cortical SWA, SNpr neurons receive convergent in-phase inhibitory inputs from the striatum, anti-phase inhibitory inputs from the globus pallidus, and in-phase excitatory inputs from the subthalamic nucleus (Walters et al., 2007). Since lesion of the STN regularizes SNpr spiking (Burbaud et al., 1995; Murer et al., 1997), it is likely that the in-phase activity of the SNpr may be caused by excitatory input from the STN, although this input may be depressed after dopamine depletion (Yamawaki et al., 2012). This agrees with in vivo studies that show that after nigrostriatal dopamine depletion phasic STN activity is enhanced (Magill et al., 2001; Walters et al., 2007) and in vitro studies showing a loss of long-term depression at corticostriatal (Calabresi et al., 1992) and subthalamonigral synapses (Dupuis et al., 2013). In the awake state when the cortex is relatively desynchronized and SWA is suppressed, these changes in synaptic plasticity may be expressed in a less time-locked fashion and therefore expressed more through an increase in CV as observed here. A similar increase in irregularity was recently described as an increase in entropy in SNpr recordings from awake rats after unilateral 6-OHDA lesioning, and a regularization of spiking was observed with STN-DBS, possibly contributing to the therapeutic effect (Dorval and Grill, 2014). These findings can be explained by a constant synaptic high-conductance state in awake conditions (Destexhe et al., 2003) that is based on network interactions that dominate over the intrinsic activity of participating neurons. Under a barrage of excitatory and inhibitory inputs increased irregularity of spiking is observed when more synchronicity is present between inputs (Gauck and Jaeger, 2003) while at the same time an increased background of synaptic activity partly shunts the effect of any one specific pathway (Chance et al., 2002). The synaptic conductances controlling spiking will be strongly varying with behavioral conditions and critically depend on the strength of participating synapses, which is shaped by plasticity.
Beta Oscillations and Bursting
In this study, we did not find an increase in the beta power in the SNpr LFP in the lesioned hemisphere of the 6-OHDA-lesioned mouse. This was surprising considering that it has been observed in recent studies in the freely moving, 6-OHDA-lesioned rat (Avila et al., 2010; Brazhnik et al., 2012; Brazhnik et al., 2014). The lack of beta oscillations in our awake mice could be due to differences in recording techniques, species (rat vs. mice), or behavioral state. With respect to differences in the recording technique, we recorded LFPs with a glass electrode located in the SNpr with respect to a second glass electrode located just under the dura in the visual cortex. In the experiments of Walters and colleagues, the LFP was recorded by one microwire with reference to a low-impedance 9th wire located in the same bundle penetrating the substantia nigra. Thus, the LFP recorded in those experiments is much more localized. Although we cannot with certainty identify the origin of a given LFP signal (i.e. cortex or nigra), a beta signal should have been seen with our setup. However, it is possible, although unlikely, that a synchronous beta oscillation in the visual cortex and nigra would have been canceled by the recording method employed here. With this caveat in mind, we do not believe that the absence of an increase in beta band power is due to differences in recording methodology.
Another possibility is that beta band oscillations are not prominent in the head-restrained state in rodents. Enhanced high-frequency beta within the SNpr has recently been shown to be highly dependent on behavioral state and can be linked to individual steps in locomotor movements (Brazhnik et al., 2014). It is possible that the similar but weaker high-beta frequency oscillation observed in alert non-locomoting rats was dependent on the training history of the rat, and may reflect cognitive processes related to task memory and would thus not be observed in naïve rats. This would be analogous to the replay of neural activity due to training that has been seen in the hippocampus in the quiet awake state (Jadhav et al., 2012; Wikenheiser and David Redish, 2013) and also has been found in the ventral striatum (Pennartz et al., 2004). Head-fixation per-se would not be expected to abolish beta band oscillations, since enhanced beta oscillations can be seen in head-restrained monkeys and human patients placed in a stereotaxic frame during surgical procedures, although the peak of these oscillations does vary with species (Avila et al., 2010; Brown et al., 2001; Kuhn et al., 2009; Mallet et al., 2008; Raz et al., 2000).
Finally, these results do not support the idea that the presence of beta is simply antikinetic (Brown, 2006), since we would expect activity related purely to the inhibition of movement to be enhanced in the head-restrained state. It may be that beta may have more of a role in changes in movement or flexible behaviors (Leventhal et al., 2012) that were discouraged by our interval-based free-reward paradigm promoting habitual licking with each reward.
Bursts in Rodent Parkinsonism
This study tested the hypothesis that bursting was increased in rodent parkinsonism, as modeled with unilateral nigrostriatal dopamine depletion from 6-OHDA administration to the MFB. It is worth noting that a variety of burst metrics exist and, unfortunately, there is no consensus on what counts and what does not count as a burst, particularly when neural activity is strongly modulated by an oscillatory input (Lobb, 2014). This can be seen in our anesthetized recordings which clearly showed a significant change in the firing pattern but generated conflicting results between burst detection methods: the commonly used PS method finding a significantly increased rate of weaker intensity bursting and the RGS method finding a significantly decreased rate of bursting of a similar intensity. Why do these surprise methods disagree with one another? In control conditions, the RGS method detects more bursts than the PS method (e.g., Figure 3A), possibly due to its greater sensitivity (Ko et al., 2012). In the 6-OHDA group, the ISI distribution is significantly positively skewed due to the presence of many long ISIs (many of which are pauses). These long ISIs can have a substantial impact on burst determination. Both methods employ a threshold ISI (or seed) to signal that a prospective burst may have occurred. For the PS method, this value is set based on the mean ISI, which is sensitive to these long ISI outliers. The presence of these long ISIs in the PS method caused the ISI burst seed threshold used to identify burst intervals (set to twice the mean ISI in the PS method) to be much longer (17.9 ms in control to 65.0 ms in 6-OHDA). This explains why the method detects many more, albeit weaker intensity bursts. One of the statistical advances of the RGS method is that bursts and pauses are detected simultaneously as outliers from the median of the lognormal ISI distribution. Thus for the RGS method, the threshold ISI value is less sensitive to these long ISI outliers since it is closer to the mode of the ISI distribution. Fewer but longer bursts are then detected. Furthermore, results using a median-based threshold rather than a mean-based threshold in the PS method qualitatively mirror the RGS method (i.e. interburst rate decreases with no change in the mean intraburst ISI; data not shown). Thus, in general, we recommend the use of median-based statistics rather than mean-based statistics when describing and detecting events from ISI distributions (which are typically positively-skewed with a longer tail to the right). Furthermore, these results illustrate the need for burst detection methods that explicitly account for the presence of an oscillation and associated ISI variability. Given the statistical caveats of different burst detection methods, a more rigorous approach towards a consistent definition of bursts and bursting needs to be pursued in order to avoid conflicting results just based on burst detection methods used.
Using a variety of burst methodologies (Lobb, 2014), previous studies in MPTP-treated monkeys have shown that bursting is enhanced in the traditional basal ganglia output nuclei of the globus pallidus internal segment and the SNpr (Bergman et al., 1994; Boraud et al., 1998; Wichmann et al., 1999; Wichmann and Soares, 2006) suggesting that abnormally increased bursting could drive motor dysfunction in Parkinson’s disease. In our study, SNpr neuron activity in awake, head-restrained mice was significantly more regular than in the anesthetized 6-OHDA condition. Under these awake conditions, neither the PS nor the RGS method detected a significant change in bursting activity, with the exception of a weakening intensity of PS-detected bursts. However, it must be emphasized that synaptic inputs to SNpr neurons (reflected in the LFP) may change significantly depending on the behavioral state of the rodent (Brazhnik et al., 2014), especially during task initiation and execution (Leventhal et al., 2012). For example, an increase in the number of epochs of oscillatory inputs such as what would be produced by increased beta or gamma band oscillations that can entrain SNpr neuron firing may enhance or decrease bursts or pauses in activity. This suggests that bursting may be a highly behavioral state-dependent aspect of basal ganglia activity.
Furthermore, it is also likely that different neural dynamics will occur in parkinsonian mice with a bilateral dopamine neuron lesion since those mice would display a non-circling behavioral phenotype and both hemispheres would be affected. Because neural dynamics could be influenced by symmetric or asymmetric interhemispheric interactions, it is unclear whether bursting or pausing would be similarly changed with a bilateral lesion. It will be important in future work to establish a high-quality bilateral dopamine lesion model of mice for a more direct comparison with PD patients.
In this study, we confirm previous studies showing that there are robust changes in rodent parkinsonism in the firing rate and pattern in SNpr neuron activity under urethane anesthesia. A significant difference in firing rate was seen in the awake state when compared to the anesthetized state. Comparing control and 6-OHDA groups within the awake state the only significant difference consisted of a modest increase in CV. Contrary to the bursting hypothesis, this increase in CV was due to a mild increase in pausing not bursting. Overall, our results suggest that bursting and other changes in the firing pattern in SNpr neurons may be strongly influenced by the arousal and behavioral states of the mouse.
Highlights.
Changes in rate and pattern of SNpr spiking in anesthetized, 6-OHDA-treated mice
No significant change in bursting in awake, head-restrained 6-OHDA-treated mice
Enhanced beta were not seen in either anesthetized or awake states in 6-OHDA mice
Statistical assumptions of burst detection methods can impact burst identification
Acknowledgments
We would like to thank the laboratory of Dr. Yoland Smith for providing histological assistance. We also thank Dr. Thomas Wichmann for providing the Matlab code for the Poisson Surprise method and comments on the manuscript. This work was funded by the Morris K. Udall Center of Excellence for Parkinson’s Disease Research (NIH grant number NS071669) and the Ruth L. Kirschstein National Research Service Award (NS080589).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- Atherton JF, Bevan MD. Ionic mechanisms underlying autonomous action potential generation in the somata and dendrites of GABAergic substantia nigra pars reticulata neurons in vitro. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:8272–8281. doi: 10.1523/JNEUROSCI.1475-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila I, Parr-Brownlie LC, Brazhnik E, Castaneda E, Bergstrom DA, Walters JR. Beta frequency synchronization in basal ganglia output during rest and walk in a hemiparkinsonian rat. Experimental neurology. 2010;221:307–319. doi: 10.1016/j.expneurol.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barter JW, Castro S, Sukharnikova T, Rossi MA, Yin HH. The role of the substantia nigra in posture control. The European journal of neuroscience. 2014;39:1465–1473. doi: 10.1111/ejn.12540. [DOI] [PubMed] [Google Scholar]
- Belluscio MA, Kasanetz F, Riquelme LA, Murer MG. Spreading of slow cortical rhythms to the basal ganglia output nuclei in rats with nigrostriatal lesions. The European journal of neuroscience. 2003;17:1046–1052. doi: 10.1046/j.1460-9568.2003.02543.x. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Lanotte M, Colloca L, Ducati A, Zibetti M, Lopiano L. Electrophysiological properties of thalamic, subthalamic and nigral neurons during the anti-parkinsonian placebo response. The Journal of physiology. 2009;587:3869–3883. doi: 10.1113/jphysiol.2009.169425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P. CircStat: A Matlab Toolbox for Circular Statistics. Journal of Statistical Software. 2009;31:1–21. [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalami nucleus. II. Neuronal activity in the MPTP model of parkinsonism. Journal of neurophysiology. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross C. High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neuroscienc letters. 1996;215:17–20. doi: 10.1016/s0304-3940(96)12943-8. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Guehl D, Bioulac B, Gross C. Effects of L-DOPA on neuronal activity of the globus pallidus externalis (GPe) and globus pallidus internalis (GPi) in the MPTP-treated monkey. Brain research. 1998;787:157–160. doi: 10.1016/s0006-8993(97)01563-1. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Stutzmann JM, Bioulac B, Gross CE. Effects of riluzole on the electrophysiological activity of pallidal neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkey. Neuroscience letters. 2000;281:75–78. doi: 10.1016/s0304-3940(00)00780-1. [DOI] [PubMed] [Google Scholar]
- Brazhnik E, Cruz AV, Avila I, Wahba MI, Novikov N, Ilieva NM, McCoy AJ, Gerber C, Walters JR. State-dependent spike and local field synchronization between motor cortex and substantia nigra in hemiparkinsonian rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:7869–7880. doi: 10.1523/JNEUROSCI.0943-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazhnik E, Novikov N, McCoy AJ, Cruz AV, Walters JR. Functional correlates of exaggerated oscillatory activity in basal ganglia output in hemiparkinsonian rats. Experimental neurology. 2014;261C:563–577. doi: 10.1016/j.expneurol.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. Bad oscillations in Parkinson’s disease. Journal of neural transmission Supplementum. 2006:27–30. doi: 10.1007/978-3-211-45295-0_6. [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbaud P, Gross C, Benazzouz A, Coussemacq M, Bioulac B. Reduction of apomorphine-induced rotational behaviour by subthalamic lesion in 6-OHDA lesioned rats is associated with a normalization of firing rate and discharge pattern of pars reticulata neurons. Experimental brain research. 1995;105:48–58. doi: 10.1007/BF00242181. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1992;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson’s disease in rats and mice. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience. Chapter 9. 2007. p. 25. Unit 9. [DOI] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- Chang JY, Shi LH, Luo F, Woodward DJ. Neural responses in multiple basal ganglia regions following unilateral dopamine depletion in behaving rats performing a treadmill locomotion task. Experimental brain research. 2006;172:193–207. doi: 10.1007/s00221-005-0312-7. [DOI] [PubMed] [Google Scholar]
- Cleary DR, Raslan AM, Rubin JE, Bahgat D, Viswanathan A, Heinricher MM, Burchiel KJ. Deep brain stimulation entrains local neuronal firing in human globus pallidus internus. Journal of neurophysiology. 2013;109:978–987. doi: 10.1152/jn.00420.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4:739–751. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- Dorval AD, Grill WM. Deep brain stimulation of the subthalamic nucleus reestablishes neuronal information transmission in the 6-OHDA rat model of parkinsonism. Journal of neurophysiology. 2014;111:1949–1959. doi: 10.1152/jn.00713.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis JP, Feyder M, Miguelez C, Garcia L, Morin S, Choquet D, Hosy E, Bezard E, Fisone G, Bioulac BH, Baufreton J. Dopamine-dependent long-term depression at subthalamo-nigral synapses is lost in experimental parkinsonism. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:14331–14341. doi: 10.1523/JNEUROSCI.1681-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3 2008. [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. Journal of neurophysiology. 1999;81:2243–2252. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauck V, Jaeger D. The contribution of NMDA and AMPA conductances to the control of spiking in neurons of the deep cerebellar nuclei. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:8109–8118. doi: 10.1523/JNEUROSCI.23-22-08109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Marin A, Partoune N, Stephens GJ, Louis M, Brembs B. Automated tracking of animal posture and movement during exploration and sensory orientation behaviors. PloS one. 2012;7:e41642. doi: 10.1371/journal.pone.0041642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourevitch B, Eggermont JJ. A nonparametric approach for detection of bursts in spike trains. Journal of neuroscience methods. 2007;160:349–358. doi: 10.1016/j.jneumeth.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Grace AA. Subthalamic nucleus cell firing in the 6-OHDA-treated rat: basal activity and response to haloperidol. Brain research. 1992;590:291–299. doi: 10.1016/0006-8993(92)91108-q. [DOI] [PubMed] [Google Scholar]
- Huse DM, Schulman K, Orsini L, Castelli-Haley J, Kennedy S, Lenhart G. Burden of illness in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2005;20:1449–1454. doi: 10.1002/mds.20609. [DOI] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko D, Wilson CJ, Lobb CJ, Paladini CA. Detection of bursts and pauses in spike trains. Journal of neuroscience methods. 2012;211:145–158. doi: 10.1016/j.jneumeth.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Tsui A, Aziz T, Ray N, Brucke C, Kupsch A, Schneider GH, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Experimental neurology. 2009;215:380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Lee CR, Tepper JM. A calcium-activated nonselective cation conductance underlies the plateau potential in rat substantia nigra GABAergic neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:6531–6541. doi: 10.1523/JNEUROSCI.1678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Witkovsky P, Rice ME. Regulation of Substantia Nigra Pars Reticulata GABAergic Neuron Activity by H(2)O(2) via Flufenamic Acid-Sensitive Channels and K(ATP) Channels. Frontiers in systems neuroscience. 2011;5:14. doi: 10.3389/fnsys.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. Journal of neurophysiology. 1985;53:926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- Leventhal DK, Gage GJ, Schmidt R, Pettibone JR, Case AC, Berke JD. Basal ganglia beta oscillations accompany cue utilization. Neuron. 2012;73:523–536. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb C. Abnormal Bursting as a Pathophysiological Mechanism in Parkinson’s Disease. Basal ganglia. 2014;3:187–195. doi: 10.1016/j.baga.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb CJ, Zaheer AK, Smith Y, Jaeger D. In vivo electrophysiology of nigral and thalamic neurons in alpha-synuclein-overexpressing mice highlights differences from toxin-based models of parkinsonism. Journal of neurophysiology. 2013;110:2792–2805. doi: 10.1152/jn.00441.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod NK, Ryman A, Arbuthnott GW. Electrophysiological properties of nigrothalamic neurons after 6-hydroxydopamine lesions in the rat. Neuroscience. 1990;38:447–456. doi: 10.1016/0306-4522(90)90041-2. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience. 2001;106:313–330. doi: 10.1016/s0306-4522(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Mallet N, Micklem BR, Henny P, Brown MT, Williams C, Bolam JP, Nakamura KC, Magill PJ. Dichotomous organization of the external globus pallidus. Neuron. 2012;74:1075–1086. doi: 10.1016/j.neuron.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Bokil H. Observed brain dynamics. Oxford University Press; Oxford, New York: 2008. [Google Scholar]
- Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression – a new method based on robust nonlinear regression and the false discovery rate. BMC bioinformatics. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer MG, Riquelme LA, Tseng KY, Pazo JH. Substantia nigra pars reticulata single unit activity in normal and 60HDA-lesioned rats: effects of intrastriatal apomorphine and subthalamic lesions. Synapse. 1997;27:278–293. doi: 10.1002/(SICI)1098-2396(199712)27:4<278::AID-SYN2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Lee E, Verheul J, Lipa P, Barnes CA, McNaughton BL. The ventral striatum in off-line processing: ensemble reactivation during sleep and modulation by hippocampal ripples. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:6446–6456. doi: 10.1523/JNEUROSCI.0575-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JE, McIntyre CC, Turner RS, Wichmann T. Basal ganglia activity patterns in parkinsonism and computational modeling of their downstream effects. The European journal of neuroscience. 2012;36:2213–2228. doi: 10.1111/j.1460-9568.2012.08108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders TH, Clements MA, Wichmann T. Parkinsonism-related features of neuronal discharge in primates. Journal of neurophysiology. 2013;110:720–731. doi: 10.1152/jn.00672.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson P, Mavoungou R, Albe-Fessard D. Changes in substantia nigra pars reticulata activity following lesions of the substantia nigra pars compacta. Neuroscience letters. 1986;67:25–30. doi: 10.1016/0304-3940(86)90202-8. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Progress in neurobiology. 1996a;50:275–331. doi: 10.1016/s0301-0082(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP. Unilateral 6-hydroxydopamine lesions of meso-striatal dopamine neurons and their physiological sequelae. Progress in neurobiology. 1996b;49:215–266. doi: 10.1016/s0301-0082(96)00015-9. [DOI] [PubMed] [Google Scholar]
- Seeger-Armbruster S, von Ameln-Mayerhofer A. Short- and long-term unilateral 6-hydroxydopamine lesions in rats show different changes in characteristics of spontaneous firing of substantia nigra pars reticulata neurons. Experimental brain research. 2013;224:15–24. doi: 10.1007/s00221-012-3285-3. [DOI] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism and lesions of the external pallidal segment. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:6417–6426. doi: 10.1523/JNEUROSCI.0836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr PA, Rau GM, Davis V, Marks WJ, Jr, Ostrem JL, Simmons D, Lindsey N, Turner RS. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. Journal of neurophysiology. 2005;93:3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Potter M, Herzog J, Pinsker M, Kopper F, Mehdorn H, Deuschl G, Volkmann J. Neuronal activity of the human subthalamic nucleus in the parkinsonian and nonparkinsonian state. Journal of neurophysiology. 2008;100:2515–2524. doi: 10.1152/jn.90574.2008. [DOI] [PubMed] [Google Scholar]
- Tang JK, Moro E, Lozano AM, Lang AE, Hutchison WD, Mahant N, Dostrovsky JO. Firing rates of pallidal neurons are similar in Huntington’s and Parkinson’s disease patients. Experimental brain research. 2005;166:230–236. doi: 10.1007/s00221-005-2359-x. [DOI] [PubMed] [Google Scholar]
- Tang JK, Moro E, Mahant N, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Neuronal firing rates and patterns in the globus pallidus internus of patients with cervical dystonia differ from those with Parkinson’s disease. Journal of neurophysiology. 2007;98:720–729. doi: 10.1152/jn.01107.2006. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Goldman SM. Epidemiology of Parkinson’s disease. Neurologic clinics. 1996;14:317–335. doi: 10.1016/S0733-8619(05)70259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Kargieman L, Gacio S, Riquelme LA, Murer MG. Consequences of partial and severe dopaminergic lesion on basal ganglia oscillatory activity and akinesia. The European journal of neuroscience. 2005;22:2579–2586. doi: 10.1111/j.1460-9568.2005.04456.x. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Kasanetz F, Kargieman L, Pazo JH, Murer MG, Riquelme LA. Subthalamic nucleus lesions reduce low frequency oscillatory firing of substantia nigra pars reticulata neurons in a rat model of Parkinson’s disease. Brain research. 2001a;904:93–103. doi: 10.1016/s0006-8993(01)02489-1. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Kasanetz F, Kargieman L, Riquelme LA, Murer MG. Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001b;21:6430–6439. doi: 10.1523/JNEUROSCI.21-16-06430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JR, Hu D, Itoga CA, Parr-Brownlie LC, Bergstrom DA. Phase relationships support a role for coordinated activity in the indirect pathway in organizing slow oscillations in basal ganglia output after loss of dopamine. Neuroscience. 2007;144:762–776. doi: 10.1016/j.neuroscience.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Experimental brain research. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. Journal of neurophysiology. 2006;95:2120–2133. doi: 10.1152/jn.01013.2005. [DOI] [PubMed] [Google Scholar]
- Wikenheiser AM, David Redish A. The balance of forward and backward hippocampal sequences shifts across behavioral states. Hippocampus. 2013;23:22–29. doi: 10.1002/hipo.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki N, Magill PJ, Woodhall GL, Hall SD, Stanford IM. Frequency selectivity and dopamine-dependence of plasticity at glutamatergic synapses in the subthalamic nucleus. Neuroscience. 2012;203:1–11. doi: 10.1016/j.neuroscience.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Jin Y, Matta SG, Xu M, Zhou FM. An ultra-short dopamine pathway regulates basal ganglia output. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:10424–10435. doi: 10.1523/JNEUROSCI.4402-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Xu JJ, Zhao Y, LeDoux MS, Zhou FM. Opposite functions of histamine H1 and H2 receptors and H3 receptor in substantia nigra pars reticulata. Journal of neurophysiology. 2006;96:1581–1591. doi: 10.1152/jn.00148.2006. [DOI] [PubMed] [Google Scholar]
- Zold CL, Ballion B, Riquelme LA, Gonon F, Murer MG. Nigrostriatal lesion induces D2-modulated phase-locked activity in the basal ganglia of rats. The European journal of neuroscience. 2007;25:2131–2144. doi: 10.1111/j.1460-9568.2007.05475.x. [DOI] [PubMed] [Google Scholar]


