Abstract
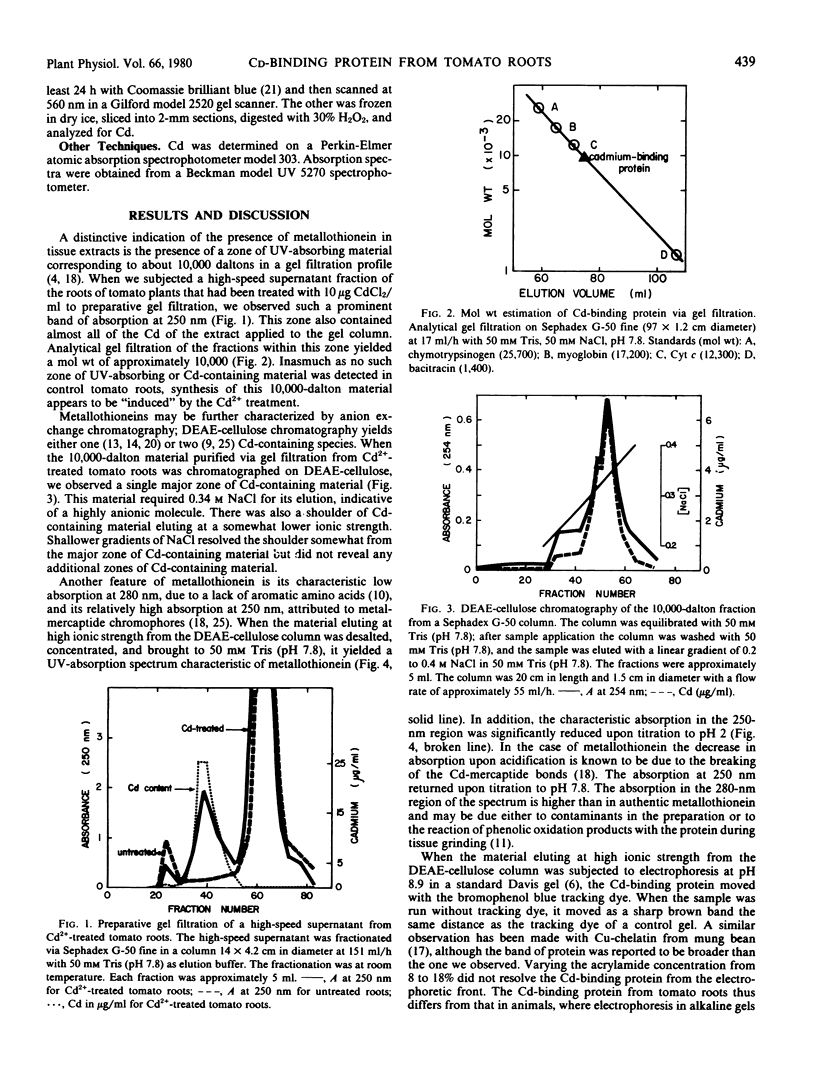
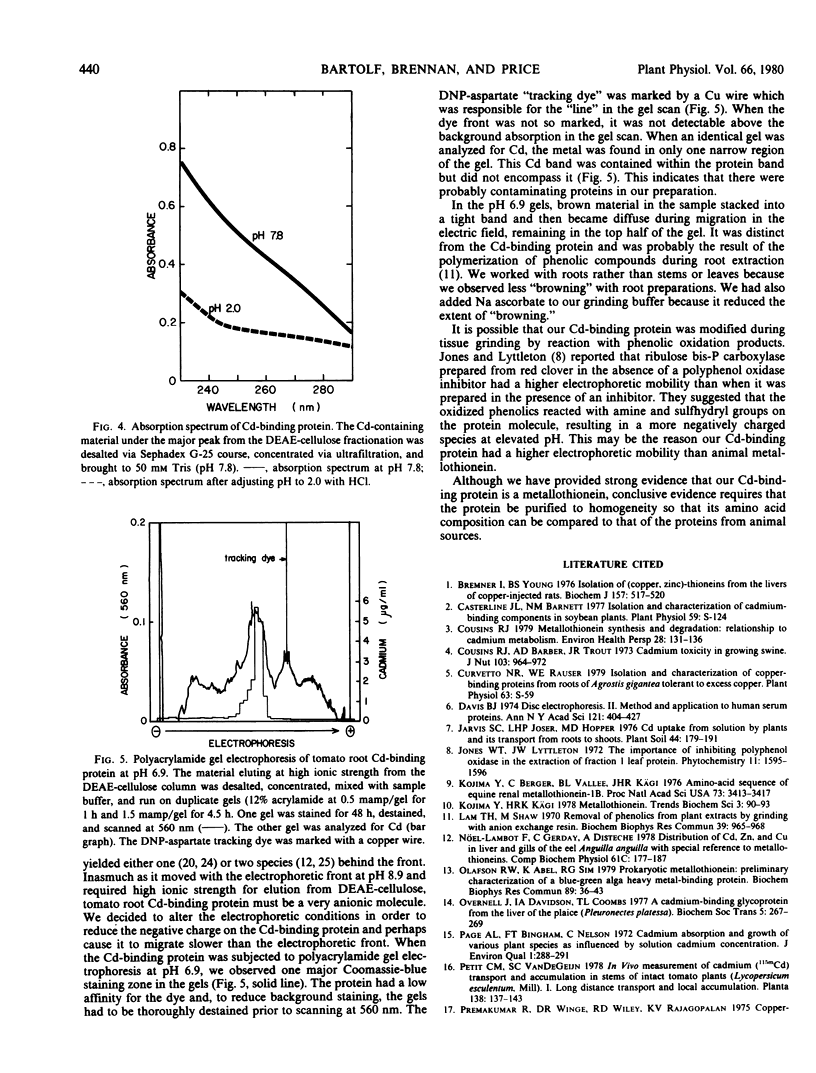
A Cd-binding protein has been isolated from the roots of Cd-treated tomato plants cv. Rutgers. Almost all the Cd from a high-speed supernatant fraction was recovered in a 10,000-dalton fraction from a gel filtration column coincident with 250-nanometer absorbing material. DEAE-cellulose chromatography of this 10,000-dalton material yielded one major component, which eluted at 0.34 molar NaCl, had an absorption spectrum characteristic of metallothionein, and showed absorption changes upon acidification typical of metallothionein. Although the Cd-binding protein did not behave like metallothioneins from animal sources during gel electrophoresis at pH 8.9, a single band containing Cd and staining with Coomassie brilliant blue could be detected following electrophoresis at pH 6.9. Synthesis of the Cd-binding protein appeared to be “induced” by treatment of the plants with Cd2+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremner I., Young B. W. Isolation of (copper, zinc)-thioneins from the livers of copper-injected rats. Biochem J. 1976 Aug 1;157(2):517–520. doi: 10.1042/bj1570517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins R. J., Barber A. K., Trout J. R. Cadmium toxicity in growing swine. J Nutr. 1973 Jul;103(7):964–972. doi: 10.1093/jn/103.7.964. [DOI] [PubMed] [Google Scholar]
- Cousins R. J. Metallothionein synthesis and degradation: relationship to cadmium metabolism. Environ Health Perspect. 1979 Feb;28:131–136. doi: 10.1289/ehp.7928131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Kojima Y., Berger C., Vallee B. L., Kägi J. H. Amino-acid sequence of equine renal metallothionein-1B. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3413–3417. doi: 10.1073/pnas.73.10.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T. H., Shaw M. Removal of phenolics from plant extracts by grinding with anion exchange resin. Biochem Biophys Res Commun. 1970 Jun 5;39(5):965–968. doi: 10.1016/0006-291x(70)90418-3. [DOI] [PubMed] [Google Scholar]
- Olafson R. W., Abel K., Sim R. G. Prokaryotic metallothionein: preliminary characterization of a blue-green alga heavy metal-binding protein. Biochem Biophys Res Commun. 1979 Jul 12;89(1):36–43. doi: 10.1016/0006-291x(79)90939-2. [DOI] [PubMed] [Google Scholar]
- Overnell J., Davidson I. A., Coombs T. L. A cadmium-binding glycoprotein from the liver of the plaice (Pleuronectes platessa). Biochem Soc Trans. 1977;5(1):267–269. doi: 10.1042/bst0050267. [DOI] [PubMed] [Google Scholar]
- Pulido P., Kägi J. H., Vallee B. L. Isolation and some properties of human metallothionein. Biochemistry. 1966 May;5(5):1768–1777. doi: 10.1021/bi00869a046. [DOI] [PubMed] [Google Scholar]
- Sokolowski G., Weser U. Formation, circular dichroism and x-ray photoelectron spectroscopy of hepatic Zn-thionein. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1715–1726. doi: 10.1515/bchm2.1975.356.2.1715. [DOI] [PubMed] [Google Scholar]
- Weser U., Rupp H., Donay F., Linnemann F., Voelter W., Voetsch W., Jung G. Characterization of Cd, Zn-thionein (metallothionein) isolated from rat and chicken liver. Eur J Biochem. 1973 Nov 1;39(1):127–140. doi: 10.1111/j.1432-1033.1973.tb03111.x. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Premakumar R., Rajagopalan K. V. Metal-induced formation of metallothionein in rat liver. Arch Biochem Biophys. 1975 Sep;170(1):242–252. doi: 10.1016/0003-9861(75)90115-0. [DOI] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]



